- Biomedical Research (2006) Volume 17, Issue 3
Intercellular Communications within the rat anterior pituitary. XIII: An immunohistochmical and physiological study of the anterior pituitary gland of the rat
akustO asihihsoY1*, Hikaru Hashitani2, Nobuyuki Shirasawa4, Eisuke Sakuma1, Hikaru Suzuki2, Takanobu Otsuka3, Damon C. Herbert5, Tsuyoshi Soji11Functional Morphology, Nagoya City University Medical Science, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya City, Aichi, 467-8601, Japan
2Regulatory Cell Physiology, Nagoya City University Medical Science, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya City, Aichi, 467-8601, Japan
3Department of Musculoscheltal Medicine, Nagoya City University Medical Science, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya City, Aichi, 467-8601, Japan
4Department of Anatomy and Structural Science, Yamagata University School of Medicine, 2-2-2 Iida-nishi, Yamagata 990-9585, Japan
5Department of Cellular and Structural Biology, Mail Code 7762, The University of Texas Health Science Center at San Antonio, San Antonio, Texas 78229-3900 USA
- Corresponding Author:
- Yoshihisa Otsuka
Functional Morphology
Nagoya City University Medical Science 1 Kawasumi
Mizuho-cho, Mizuho-ku, Nagoya City Aichi, 467-8601, Japan
Tel: 81-52-853-8121
Fax: 81-52-842-3210
E-mail: soji_t@med.nagoya-cu.ac.jp; yotsuka@med.nagoya-cu.ac.jp
Accepted Date: July 03 2006
Abstract
Since the first report of folliculo-stellate cells as corticotrophs by Farquhar [1], their histological characteristics have been investigated by many researchers. However, the physiological significance of folliculo-stellate cells has not been clearly elucidated so far. The present study is designed to investigate the function of folliculo-stellate cells, particularly focusing on their responses to LH-RH. Changes in the intracellular concentration of calcium ions ([Ca2+]i) were measured from folliculo-stellate cells in the “transitional zone” of the anterior pituitary gland. The anterior pituitary glands were removed from 7 weeks old male Wistar rats weighing 250 - 300 g. One group was used for immunohistochemical study to detect the distribution of S- 100 protein and LH-RH protein, and the other group was employed for electron microscopic examination. The remaining tissues were used for Ca measurements. Under Nomarski optics, areas of cells which corresponded to either granular cells or agranular, folliculo-stellate cells were marked. Changes in [Ca2+]i from selected areas were then measured using fura-2 loaded preparations. Bath-applied LH-RH (100 μg/ml) caused transient increases in [Ca2+]i which were followed by sustained increase in [Ca2+]i either with or without Ca oscillations. These results demonstrated that not only granular but also agranular folliculo-stellate cells, may be capable of responding to LH-RH by increasing [Ca2+]i, and thus intracellular Ca may play an important role in regulating the function of folliculo-stellate cells upon LH-RH stimulation. We hypothesize that the LH-RH message from the brain entered the anterior lobe via the “transitional zone” and then spread throughout the gland mediated via by a network system of folliculo-stellate cells as well as the portal vascular system.
Keywords
Gap junction, calcium, anterior pituitary, transitional zone, rat
Introduction
In the anterior pituitary gland, granulated pituitary cells producing six kinds of hormones are present along with agranular folliculo-stellate cells. Since the folliculo- stellate cells were first reported as corticotrophs by Farquhar their histological characters have been investigated by many researchers. However, the physiological role of these cells still remains to be established. They have been reported as supporting cells [2,3,4,5], scavengers stem cells [6,7,8] or regulators of hormone secretion [9,10,11] based on their immunocytochemical and fine structural characteristics.
Sato et al. [12] and Sato et al. [13] have recently reported the role of the folliculo-stellate cells as physiological regulators for hormone secretion based on their electrophysiological characteristics. S-100 protein is a marker of folliculo-stellate cells in the rat pituitary gland [14]. Sato et al. [12] reported the three dimensional distribution of S-100 protein cells as folliculo-stellate cells. Moreover, Sato and colleagues [13] emphasized that these cells were localized in the peripheral area of the anterior pituitary gland, especially in the so-called “transitional zone.” The importance of the network system of folliculo-stellate cells as well as the portal vascular system in the regulation of hormone secretion has been pointed out from histological findings focusing on gap junctions of the folliculo- stellate cells [11,14,22,24,29].
Connexins provide a direct pathway for electrical and metabolic signaling between cells to make the gap junctions There are a few reports about the gap junctions in endocrine glands especially pancreatic islets In the hypophysis, Shirasawa et al. [22] showed that co-localization of S-100 protein and connexins indicated that folliculo-stellate cells were communicated with each other through gap junctions. Mabuchi et al. [11] and Kurita et al. [8] reported that the folliculostellate cells had LH-RH receptors and that LH-RH neurons came in contact with the S-100 protein cells in the hind part of the pars tuberalis, suggesting that folliculostellate cells may respond to LH-RH. We hypothesized that the LH-RH message from the brain came into the anterior lobe via the “transitional zone” and then spread throughout the gland via a network system of folliculostellate cells as well as through the portal vascular system. The present study is designed to investigate the physiological function of folliculo-stellate cells, particularly focusing on their responses to LH-RH. Changes in [Ca2+]i in the folliculo-stellate cells in the “transitional zone” of the anterior pituitary gland were measured.
Materials and Methods
Animals
Sixteen male Wistar rats, aged 7 weeks weighing 250-300 g, were anesthetized with ethyl ether (Maruishi Pharmacy Co. Ltd., Osaka, Japan) prior to decapitation. All animals were treated ethically in accordance with the principles for the care and use of animals in the field of physiological sciences, approved by The Animal Experiments Committee of the Nagoya City University Medical School. Morphological examination
For immunocytochemical identification of the folliculostellate cells as S-100 protein-positive cells, and for the study on the distribution of LH-RH receptor positive cells, we separated the animals into two groups. One was for S-100 protein immunohistochemistry using midsagital sections and the second for immunostaining using both the S-100 protein antibody and an anti-LH antibody.
Group1: For the immunocytochemical identification of the S-100 protein-positive cells, the pituitary gland was perfused with sodium phosphate buffered saline, pH 7.2, for 10 min through the left ventricle of the rat, and then fixed for 5 min via perfusion of Bouin‟s solution without acetic acid. Each pituitary gland connected to the pituitary stalk was carefully removed along with the median eminence, and subsequently fixed overnight at 4oC in the same fixative. The tissues were routinely dehydrated in a graded ethanol series and embedded in Paraplast embedding media (Sigma Chem. Co., St. Louis, MO, U.S.A.). Sagittal sections, 8μm in thickness, were prepared and mounted onto poly-L-lysine-coated glass slides, and processed for immunostaining using an S-100 protein antibody
Group2: For immunocytochemical identification of the S-100 protein and LH-RH-positive cells, the sections were dewaxed in xelene and dehydrated in etanol to water. They were then placed in a microwave oven in a 10 mM citric acid solution, pH 6.0, for 3 min to expose the antigens. A mixture of rabbit antibovine S-100 protein serum at a dilution of 1:8000; (LSL Lab Tokyo, Japan) and mouse monoclonal antibody against mammalian LHRH at a concentration of 0.5μg/ml; (Biogenesis Ltd., Poole, England, UK) were used as the primary antibodies. Alexa Fluor-568 labeled anti-rabbit IgG, and Alexa Fluor- 488 labeled anti-mouse IgG (Molecular Probes, Eugene, OR, USA) were employed as the second antibodies. The specificity of the reaction was checked by substituting the first antibodies (anti-LH-RH and anti-S-100 proteins) with PBS. No specific reactions were observed on the control specimens. After immunostaining, photomicrographs were taken at 40-fold magnification using a light microscope equipped with a CCD camera and a computer of Olympus Opt. Co., Tokyo, Japan [22,23].
Electron microscopy
For the electron microscopic observations, the pituitary gland was initially perfused with sodium phosphate buffered saline, pH 7.2, for 10 min through the left ventricle of the heart, and then fixed by perfusing 2.5% glutaraldehyde containing 2% sucrose in 0.05M sodium cacodylate buffer, pH 7.4 into the animals. After fixing, the pituitary glands attached to the brain were removed from the animals and separated into two halves at the sagittal center. For conventional transmission electron microscopy, each specimen was then sectioned into blocks, approximately 1×1x1 mm, and refixed for an additional 20 min in the same fixative as used for perfusion. The specimens were then washed for 30 min with the same buffer and postfixed for two hours with a 1% osmium tetroxide fixative containing 2% sucrose in 0.05M sodium cacodylate buffer, pH 7.4. After postfixation, the tissues were subsequently dehydrated in a graded series of ethanol for 10 min each, immersed twice in propylene oxide for 15 min, and then embedded in epoxy resin [15]. The ultrathin sections were stained with uranyl acetate and lead citrate, and then observed using a Hitachi H-7000 transmission electron microscope.
Intracellular calcium measurements
The intermediate and posterior lobes were turned over at the Rathke‟s residual pouch to expose the junctional area (the „transitional zone‟) of the anterior lobe, pars tuberalis and intermediate lobe (Fig. 1). The „transi tional zone‟, approximately 0.5 mm long and 0.3 mm wide, was then removed from pituitary gland.For measurements of changes in the concentration of intracellular calcium ([Ca2+]i), preparations were pinned out on a block of Sylgard plate (silicone elastomer, Dow Corning Corporation, Midland, Michigan, U.S.A.) which had a window of approximately 0.4 mm x 0.2 mm in the center. The Sylgard block was turned over and then was placed at the bottom of the recording chamber so that the preparation faced a cover glass. After 30 minincubation with warmed (35 °C) physiological saline, the preparations were loaded with fluorescent dye, fura-2, by incubation in nominally Ca free physiological saline containing 10 μM fura-2 AM (FluoroPureTM grade special packaging, Molecular Probes, OR, USA) for 90 min at room temperature. After loading, preparations were superfused with dye-free, warmed (35 °C) physiological saline at a constant flow (about 2 ml/min) for 30 min.
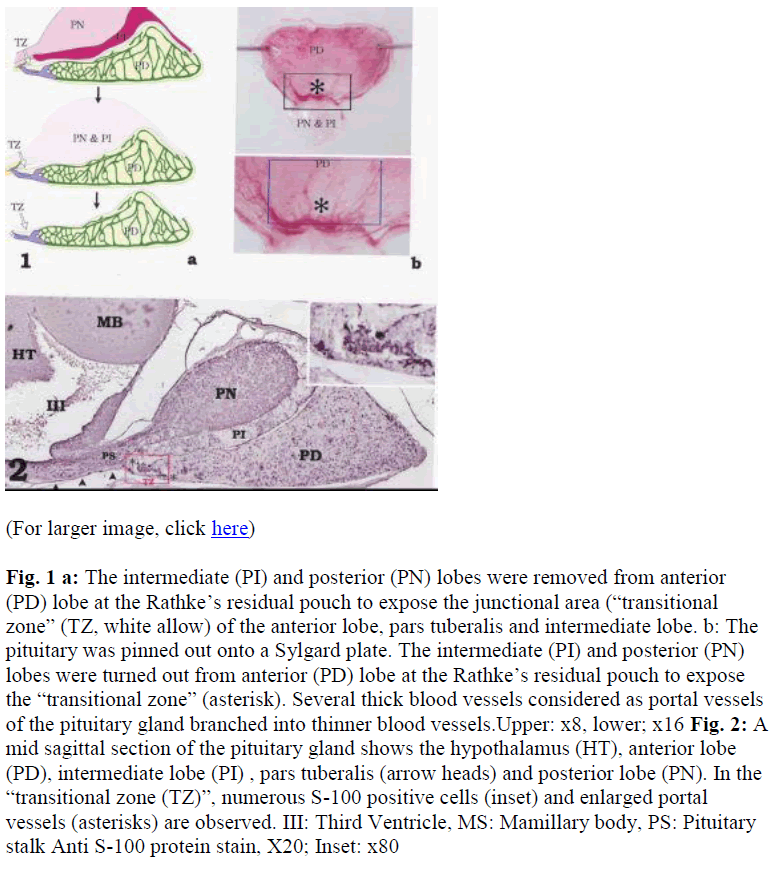
Fig. 1a: The intermediate (PI) and posterior (PN) lobes were removed from anterior (PD) lobe at the Rathke‟s residual pouch to expose the junctional area (“transitional zone” (TZ, white allow) of the anterior lobe, pars tuberalis and intermediate lobe. b: The pituitary was pinned out onto a Sylgard plate. The intermediate (PI) and posterior (PN) lobes were turned out from anterior (PD) lobe at the Rathke‟s residual pouch to expose the “transitional zone” (asterisk). Several thick blood vessels considered as portal vessels of the pituitary gland branched into thinner blood vessels.Upper: x8, lower; x16
Fig. 2: A mid sagittal section of the pituitary gland shows the hypothalamus (HT), anterior lobe (PD), intermediate lobe (PI) , pars tuberalis (arrow heads) and posterior lobe (PN). In the “transitional zone (TZ)”, numerous S-100 positive cells (inset) and enlarged portal vessels (asterisks) are observed. III: Third Ventricle, MS: Mamillary body, PS: Pituitary stalk Anti S-100 protein stain, X20; Inset: x80
Preparations, loaded with fura-2, were first viewed under a water–immersion objective (UPlanApo 60, Olympus) for phase contrast image to select recording areas. To identify cell types, i.e. granular cells and agranular, folliculo- stellate cells, Nomarski optics was used. The preparations were then alternately illuminated with ultraviolet light, wave lengths 340 and 380 nm, alter nating at a frequency of higher than 40 Hz. The ratio of the emission fluorescence (R340/380) in a desired size of rectangular window was measured through a barrier filter (peak transmission, 510 nm; sampling time, 70-200 ms), using a microphotolumi nescence measurement system (ARGUS/ HiSCA, Hamamatsu Photonics, Hamamatsu, Japan), and was taken as an index of [Ca2+]i. The LH-RH (Gonadorelin Tanabe Seiyaku Co., Ltd., Tokyo 100ug/ml, flow rate 0.1ml/min) was perfused for 2 min with Krebs solution. After the perfusion, preparations were washed out with Krebs solution for 1hr. After Ca experiments, preparations were fixed with Bouin‟s solution without acetic acid, and were double stained with anti-S100 protein and LH [22,23].
Solution and drugs
The composition of physiological saline was (in mM): NaCl, 120; KCl, 4.7; MgCl2, 1.2; CaCl2, 2.5; NaHCO3, 15.5; KH2PO4, 1.2 and glucose, 11.5. The solution was bubbled with 95% O2 and 5% CO2, pH 7.2 to 7.3. Nominally Ca++ free solution was prepared by reducing omitting CaCl2.
Results
A mid sagittal section of the pituitary gland shows the hypothalamus with third ventricle, pituitary stalk, and pituitary (Fig. 2). Beneath the hypothalamus and the pituitary stalk, the pars tuberalis was also observed. S-100 protein-positive cells, which are the chromophobic cells in the rat pituitary gland, were rich at a region of the anterior lobe named “transitional zone” (Fig. 2).
After turning over the intermediate and posterior lobes at anterior lobe, the “transitional zone” was clearly observed (Figs. 3a, b). Using double immunohistochemistry of S- 100 (green) and LH (red) (Fig. 3d), almost all of the cells in this area were green fluorescing S-100 positive cells (Figs. 3c,d) with a scattering of red LH positive cells (Fig. 3c). The LH-positive cells were larger than the S-100- positive cells. The stained cells were located between dark areas that were probably blood vessels (Figs. 3c, d). The portal vessels were clearly observed along the basal surface of the “transitional zone” and used for physiological examination.
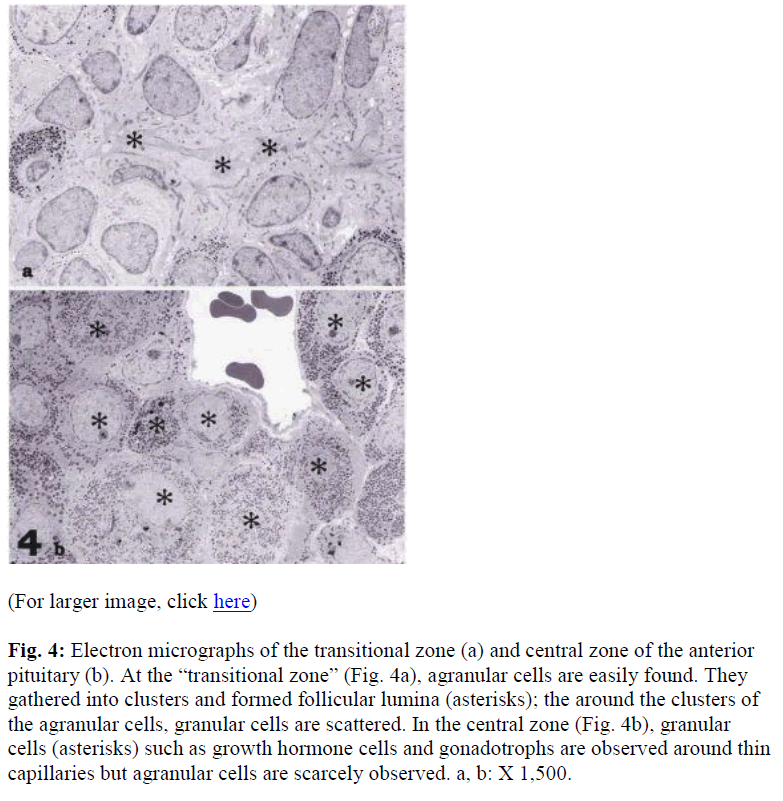
Electron microscopic observations showed that the majority of cells in the “transitional zone” were agranular (Fig 4a), an area rich in S100 positive cells by light microscopic observation. The agranular cells were connected with the junctional complexes; clusters of the cells were surrounded by follicular lumina (Fig. 4a). In contrast in the anterior lobe, there numerous granulated cells were observed. The granulated cells were easily identified as the growth hormone and gonadotrophic cells. Few agranulated cells were also observed (Fig. 4b).
Fig. 4: Electron micrographs of the transitional zone (a) and central zone of the anterior pituitary (b). At the “transitional zone” (Fig. 4a), agranular cells are easily found. They gathered into clusters and formed follicular lumina (asterisks); the around the clusters of the agranular cells, granular cells are scattered. In the central zone (Fig. 4b), granular cells (asterisks) such as growth hormone cells and gonadotrophs are observed around thin capillaries but agranular cells are scarcely observed. a, b: X 1,500.
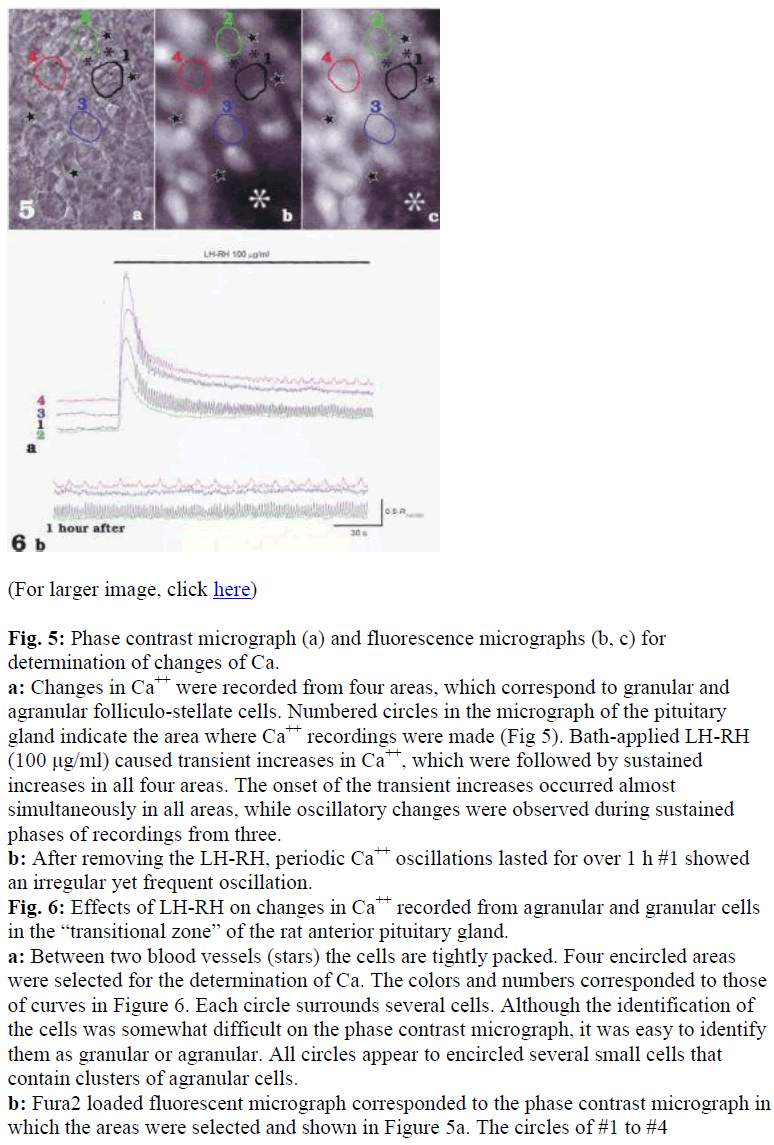
To investigate LH-RH induced signal transmissions in both granular and agranular folliculo-stellate cells, changes in [Ca2+]i were recorded from selected areas which corresponded to either of the cell types in the anterior pituitary gland of the rat (Fig. 5). Bath-applied LHRH (100 μg/ml) caused transient increases in [Ca2+]i which were followed by sustained increases [Ca2+]i in all areas (Fig. 6a). The onset of the transient increases occurred almost simultaneously in all areas, and oscillatory changes in [Ca2+]i were observed during sustained phases in some areas (Fig 6a). Note that different patterns of Ca oscillations were induced by the application of LHRH in different areas (see #1 and #4 in Fig. 6a and b). After removal of the LH-RH, Ca oscillation lasted for over 1 hr (Fig. 6b), and subsequent application of LH-RH invariably failed to cause increases in [Ca2+]i.
Fig. 5: Phase contrast micrograph (a) and fluorescence micrographs (b, c) for determination of changes of Ca.
a: Changes in Ca++ were recorded from four areas, which correspond to granular and agranular folliculo-stellate cells. Numbered circles in the micrograph of the pituitary gland indicate the area where Ca++ recordings were made (Fig 5). Bath-applied LH-RH (100 μg/ml) caused transient increases in Ca++, which were followed by sustained increases in all four areas. The onset of the transient increases occurred almost simultaneously in all areas, while oscillatory changes were observed during sustained phases of recordings from three.
b: After removing the LH-RH, periodic Ca++ oscillations lasted for over 1 h #1 showed an irregular yet frequent oscillation.
Fig. 6: Effects of LH-RH on changes in Ca++ recorded from agranular and granular cells in the “transitional zone” of the rat anterior pituitary gland.
a: Between two blood vessels (stars) the cells are tightly packed. Four encircled areas were selected for the determination of Ca. The colors and numbers corresponded to those of curves in Figure 6. Each circle surrounds several cells. Although the identification of the cells was somewhat difficult on the phase contrast micrograph, it was easy to identify them as granular or agranular. All circles appear to encircled several small cells that contain clusters of agranular cells.
b: Fura2 loaded fluorescent micrograph corresponded to the phase contrast micrograph in which the areas were selected and shown in Figure 5a. The circles of #1 to #4
corresponded to #1 to #4 in figure 5b and c, respectively, and all showed weak basal fluorescence. c: Fluorescent micrograph of the same area of figure 5a and b just after LH-RH perfusion. The almost all of the cells were stimulated, especially the area indicated by the white asterisk. Athough no fluorescence was observed in Figure 5b, slight fluorescence dots can be seen in the Figure 5c. a, b, c:X 200.
The transitional zone was set on the silicon plate by the small pins (a). A rectangle part is shown in b. Several strands of cells can be observed (directions of arrows show the strands). S-100 protein positive cells are mainly associated with strands©. Between the strands of the S-100 positive cells (c; green color), blood vessels are seen as negative areas (white asterisks). Double immunohistochemistry using antibodies to S-100 and LH, LH positive sites (red color) are scattered among S-100 positive site (d). It is clear that the S-100 positive sites are the cellular main component of the “transitional zone.”a:x4, b,c,d: x8
Discussion
The present study showed that the intracellular concentration of calcium ions displayed drastic increases in the region of the “transitional zone” of the pituitary gland following LH-RH stimulation.
A numerous supposing functions were proposed the other hand, Shirasawa et al. [23] showed that S-100 protein was a marker of folliculostellate cell of the rat. Soji and his co-workers further investigated the distribution of the folliculo-stellate cell (S-100 positive cells), and found an S-100 positive cell rich area within the pars tuberalis and pars distalis (about 80 to 90% of cells in the transitional zone) [18]. This area was tentatively named as “transitional zone” [8,11].
The gap junctional connections of the folliculo-stellate cells were noted by electron microscopy [24] and immunohistochemistry against connexin 43 [22]. The gap junctions were clustered on the S-100 positive cells, and their distributing density was greatest in the “transitional zone” in the pars distalis.. Sato et al. [19]) noted gap junctional coupling between the folliculo-stellate cells especially in the “transitional zone”. This was a good agreement with the previous finding in which propagations of evoked Ca transients between folliculo-stellate cells was prevented Intercellular Communications within the rat anterior pituitary by carbenoxolone. Thus Soji and co-workers strongly suggested that these cells participated in the regulation of hormone secretion, at least LH secretion, parallel with the hypophyseal portal vessel. S-100 protein is known as the calcium binding protein and the marker protein of the folliculo-stellate cell [13,23]. Thus it is reasonable to think that communication through the gap junctions use s Ca in a similar way to the way it occurs in heart muscles. Mabuchi [11] and Kurita [8] showed the distribution of LH-RH positive fibers in transitional zone by immunocytochemistry.
In the “transitional zone,” Kurita et al. [8] also reported that LH-RH receptors were more frequently found on the folliculo-stellate cells than in the central area of the anterior pituitary. Mabuchi et al. [11] found LHRH neurons coursing from the bottom of the 3rd ventricle zone into the pars tuberalis. These results suggested that folliculo-stellate cells might respond to LH-RH and then transmit physiological signals to neighboring cells through gap junction communication.
In the present study, [Ca2+]i in not only granular cells but also agranular folliculo-stellate cells in the transitional zone” were increased on LH-RH stimulations. Upon LHRH stimulation intracellular calcium rapidly increased and reached its peak within 1-2 seconds in all areas. The initial Ca transients were then gradually declined, and were continued to a sustained phase of Ca increases. These processes were classified into two patterns; one showed no or slight oscillations (#2 and #3 in Fig. 5a) and the other showed more prominent oscillations (#1 and #4 in Fig. 5a). Since granular cells respond to LH-RH by generating biphasic Ca transients, and also exhibit spontanoues Ca oscillations, cells which generated prominent oscillations (#1 and #4 in Fig. 5a) are assumed to be granular cells. Based on phase contrast images, no- or slight-oscillations (#2 and #3 in Fig. 5a) occurred in clusters of small cells, presumably folliculo-stellate cells Consitently, intracellular recordings from cells in the „transitional zone‟ showed that all cells were electrically quiescent. This observation indicated that the folliculo-stellate cells in the “transitional zone” could respond to LH-RH by increasing [Ca2+]i, and this Ca signal could be propagated to neighboring cells via the gap junctions.
In the present study, in selected cells, transient increases in response to LH-RH were observed. The main cells in this region were folliculo-stellate cells (80-90 % of parenchymal ells) [18]. It was rather reasonable to understand from statistical result in the “transitional zone” [18] that almost all of reacted cells to LH-RH were folliculostellate cells. It is generally considered that the calcium ions carry certain “messages” and they are thought to be important regulators of organ physiology. Therefore, it is natural to consider, in the pituitary gland, calcium ions participate in information transport perhaps in conjunction with the portal vessel system.
Both granular cells and agranular cells were selected in this study and results showed that both reacted to LH-RH. It was natural to concern that the agranulated cell included the folliculo-stellate cells and cells discharged of granules or immature granulated cells. We already showed that S-100 positive folliculo-stellate cells are the main constituent of the “transitional zone”. The granulated cells, not only immature but also discharged, did not display any S-100 positive reaction [23]. Previous studies showed the existence of LH-RH receptors on the folliculo- stellate cells in the “transitional zone” [8,11,22].
These reactions could be a route for the LH-RH message to be transmitted from the hypothalamus to the pituitary gland. We also conclude that calcium is a messenger for LH-RH in this area, and that together with portal system of vessels serves as the main route for hormonal transport. In addition to the main system of hormone transport by the portal vessels, Soji and co-workers have been proposed that the S-100 protein positive, folliculo-stellate cells substantially participate in the regulation system for the hormone secretion in the anterior pituitary [8,11,14,18,19,22,24,27,28,30]. The present studies provide evidence in support of this theory from a physiological aspect.
References
- Farquhar MG. “Corticotrophs” of the rat adenohypophysis as revealed by electron microscopy. Anat Rec 1957; 127: 291.
- Candol RR. The ultra structure of stellate cells in the pars distalis of the salamander pituitary gland. Am J Anat 1969; 126: 429-456.
- Kagayama M. The follicular cells in the pars distalis of the dog pituitary gland: An electron microscopic study. Endocrinology 1965; 77: 1053-1060.
- Salazer H. Ultrastructural evidence for the existence of a non-secretory sustentacular cell in the human adenohypophysis. Anat Rec 1968; 160: 419-420.
- Shiotani Y. An electron microscopic study on stellate cells in the rabbit adenohypophysis under various endocrine conditions. Cell Tissue Res 1980; 213: 237- 249.
- Kurosumi K, Kobayashi Y. Corticotrophs in the anterior pituitary glands of normal and adrenalectomized rats as revealed by electron microscopy. Endocrinology 1966; 78: 745-758.
- Rennels EG: Electron microscopic alterations in the rat hypophysis after scalding. Am J Anat 1964; 114: 71- 91.
- Yoshimura F, Sato S, Soji T, Yokoyama M. Development and differentiation of rat pituitary follicular cells under normal and some experimental conditions with special reference to an interpretation of renewal cell system. Endocrinol Jpn 1977; 24: 435-449.
- Mabuchi Y, Shirasawa N, Sakuma E., Hashimoto Y, Kuno M, Coombs RJ, Herbert DC, Soji T. Intercellular Otsuka/ Hashitani/ Shirasawa/ Sakuma/ Suzuki/ Otsuka/ Herbert/ Soji communication within the rat anterior pituitary: relationship between LH-RH neurons and folliculo-stellate cells in the pars tuberalis. Cell Tissue Res 2004; 317: 79-90.
- Shirasawa N, Mabuchi Y, Sakuma E, Yashiro T, Kikuchi Y, Hashimoto Y, Horiuchi O, Tsuruo Y, Herbert DC, Soji T. Intercellular communication within the rat anterior pituitary gland. X. Immunohistocytochemistry of S-100 and connexin 43 of folliculo-stellate cells in the rat anterior pituitary gland. Anat Rec 2004; 278A: 462-473.
- Soji T, Herbert DC: Intercellular communication within the rat anterior pituitary gland. Anat Rec 1989; 224: 523-533.
- Sato G, Shirasawa N, Sakuma E, Sato Y, Asai Y, Wada I, Horiuchi O, Herbert DC, Soji T: Intercellular Communications within the Rat Anterior Pituitary. XI: An Immunohistochemical Study of Distributions of S-100 Positive Cells in the Anterior Pituitary of the Rat. Tissue Cell 2005; 37: 269-280.
- Sato Y, Hashitani H, Shirasawa N, Sakuma E, Naito A, Suzuki H, Asai Y, Sato G, Wada I, Herbert DC, Soji T. Intercellular Communications within the Rat Anterior Pituitary. XII: An Immunohistochemical and physiological evidences for the gap junctional coupling of the folliculo-stellate cells in the rat pituitary. Tissue Cell 2005; 37: 281-291.
- Nakajima T, Yamaguchi H, Takahashi K. S100 protein in folliculo-stellate cells of the rat pituitary anterior lobe. Brain Res 1980; 191: 523-531.
- Nishizono H, Soji T, Herbert DC. Intercellular communication within the rat anterior pituitary gland. V. Changes in cell-to-cell communications as a function of timing of castration in male rats. Anat Rec 1993; 235: 577-582.
- Soji T, Herbert DC. Intercellular communication within the rat anterior pituitary gland. II. Castration effects and changes after injection of luteinizing hormonereleasing hormone (LH-RH) or testosterone. Anat Rec 1990; 226: 342-346.
- Soji T, Nishizono H, Yashiro T, Herbert DC: Intercellular communication within the rat anterior pituitary gland. III. Postnatal development and periodic changes of cell-to-cell communications in female rats. Anat Rec 1991; 231: 351-357.
- Soji T, Yashiro T, Herbert DC. Intercellular communication within the rat anterior pituitary gland. I. Postnatal development and changes after injection of luteinizing hormone-releasing hormone (LH-RH) or testosterone. Anat Rec 1990; 226: 337-341.
- Soji T, Yashiro T, Herbert DC. Intercellular communication within the rat anterior pituitary gland. IV. Changes in cell-to-cell communications during pregnancy. Anat Rec 1992; 233: 97-102.
- Wang HJ. Immunoelectron microscopic study for the influence of LH-RH by interconnected folliculo-stellate cells. Nagoya Med J 2000; 44: 81-91
- Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta 2004; 1662: 42-60
- Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol 1995; 129: 805-817.
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem 1996; 65: 475-502.
- Dean PM, Matthews EK, Sakamoto Y. Pancreatic islet cells: Effects of monosaccharide, glycolytic intermediates and metabolic inhibitors on membrane potential and electrical activity. J Physiol 1974; 246: 459-478.
- Matthews EK and Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol 1975; 246:421- 437.
- Sakamoto Y. 1980, Electrical activity of the pancreatic islet cells. Biomedical Res 1: 135-139.
- Seino S. ATP-sensitive potassium channels: A model of heteromultimeric potassium channel/ receptor assemblies. Annu Rev Physiol 1999; 61: 337-362.
- Kurita J, Shirasawa N, Mabuchi Y, Sakuma E, Sato Y, Sato G, Coombs RJ, Herbert DC, Soji T. Intercellular communication within the rat anterior pituitary. Immunohistochemical study on the relationship between n the hypothalamus and anterior pituitary gland with special reference to agranular cells and GnRH neurons in the pars tuberalis. Acta Histochem Cytochem 2004; 37: 227-239.
- Shirasawa N, Yoshimura F, Miyashita E, Yashiro T, Sumi Y, Suzuki, T. Quantification of immunohistochemical model sections. Cell Mol Biol 1983; 29: 327-329.
- Luft JH: Improvement in epoxy resin embedding methods. J. Biophys Biochem Cytol 1961; 9: 409-414.
- Yoshida M, Coombs RJ, Kuno M, Wada I, Horiuchi O, Herbert DC, Soji T, Mabuchi Y. Are portal vessels present in the pituitary gland „veins‟? A histological study, Biomedical Research 2004; 15: 160-168.