Research Article - Annals of Cardiovascular and Thoracic Surgery (2018) Volume 1, Issue 2
Innovative mirror image transatrial techniques for biventricular repair in inverted dextrocardia
Gananjay G Salve1*, Shreepal A Jain1, Manglesh S Nimbalkar1, Sandip S Katkade1, Jeril Kurien1, Himanshu Choudhury2, Bharat V Dalvi1, Raman Krishna Kumar3 and Krishnanaik Shivaprakash11Department of Paediatric Cardiac Sciences, Sir H.N. Reliance Foundation Hospital, Raja Ram Mohan Roy Road, Near Prarthana Samaj, Girgaum- 400004, Mumbai, Maharashtra, India
2Department of Radiodiagnosis, Sir H.N. Reliance Foundation Hospital, Raja Ram Mohan Roy Road, Near Prarthana Samaj, Girgaum- 400004, Mumbai, Maharashtra, India
3Department of Paediatric Cardiology, Amrita Institute of Medical Sciences and Research Centre, Peeliyadu Road, Ponekkara, Edapally, Ernakulam, Kerala- 682041, India
- *Corresponding Author:
- Dr. Gananjay G. Salve
Deputy Consultant, Paediatric Cardiothoracic Surgery
201, 2nd floor, Building no.2
Vaishali-nagar, K.K. Marg
Jacob Circle, Mahalaxmi- 400011
Mumbai, Maharashtra, India
Phone: +91-9880063080
E-mail: gananjay.salve@gmail.com
Accepted on July 11, 2018
Citation: Salve GG, Jain SA, Nimbalkar MS, et al. Innovative mirror image transatrial techniques for biventricular repair in inverted dextrocardia. Ann Cardiovasc Thorac Surg 2018;1(2):30-38.
DOI: 10.35841/cardiovascular-surgery.1.2.30-38
Visit for more related articles at Annals of Cardiovascular and Thoracic SurgeryAbstract
Background: Correction of hearts with situs inversus dextrocardia represent technical challenges due to mirror image anatomy and altered conduction pathway. Ventriculotomy has been the traditional approach for biventricular repair. We report our experience of trans-atrial approach in these patients employing an easily reproducible preoperative delineation of the anatomy. In addition we also discuss our way of surgical execution for these complex subsets.
Methods and Findings: 15 patients (M:F=7:8) with situs inversus dextrocardia with diverse congenital cardiac anomalies underwent biventricular repair through our trans-atrial approach from left side of patient with detailed pre-operative evaluation of the anomalies. Orientation of intra-cardiac anatomy was obtained pre-operatively by rendering standard illustrations in reversed and inverted way. Median age and weight were 12 months (4.5 months-31 years) and 5.8 kg (3.4-59 kg) respectively.
The surgical spectrum included closure of ventricular septal defects (n=6), repair of double outlet right ventricle (n=3), repair of tetralogy of Fallot (n=5) and double switch operation for congenitally corrected transposition of great arteries, routable ventricular septal defect and pulmonary stenosis. Tricuspid valve leaflet detachment was performed frequently to aid the repair (n=7).
Mean hospital stay was 12.4 ± 2.6 days with no hospital mortality. On follow-up, all patients remained in sinus rhythm and in New York Heart Association class I. One patient had a small residual ventricular septal defect with insignificant shunt. Another patient with tetralogy of Fallot correction underwent right pulmonary artery balloon plasty. Patient with double switch operation needed re-admission for supraventricular tachycardia and medical management. Another patient of tetralogy of Fallot re-developed infundibular gradient, awaiting intervention.
Conclusions: Trans-atrial biventricular repair is feasible and reproducible in these subsets. The inverted illustrations facilitate in enabling successful repairs while avoiding heart blocks and ventriculotomy. Short-term results are satisfactory.
Keywords
Situs inversus, Dextrocardia, Trans-atrial, Biventricular repair
Introduction
Incidence of situs inversus totalis is very rare, approximately being 1:10000 [1]. Situs inversus with dextrocardia mostly exists with structurally normal heart [2]. The common congenital cardiac anomalies associated with situs inversus and dextrocardia are atrial septal defect (93%), discordant atrioventricular connection (44%) and discordant ventriculo-arterial connection (30%) [3].
Biventricular repair for complex congenital heart defects in situs solitus hearts itself is challenging [4-7]. Very few articles in the literature have reported biventricular repair of inverted hearts with dextrocardia, most of them are for corrected transposition [7,8]. Ventriculotomy has been the standard approach for all of them.
Biventricular repair of congenital cardiac anomalies with situs inversus and dextrocardia becomes technically challenging due to the complex positioning of conduction system. This is probably the reason why historically these subset of patients underwent univentricular repair [5,6], or biventricular repair through ventriculotomy [7,8]. There have been no reports of trans-atrial biventricular repair till date.
Biventricular repair is possible when the two ventricles with adequate volumes are present and the anatomic relationship between the ventricles and the atrio-ventricular valves is favourable [9,10]. We believe strongly in this concept and hence attempted trans-atrial biventricular repair of inverted hearts with dextrocardia using innovative mirror image techniques.
Methods
From March 2011 to December 2016, fifteen patients with situs inversus and dextrocardia (Table 1) associated with diverse congenital cardiac anomalies underwent trans-atrial biventricular repair. There were 7 males and 8 females. The median age was 12 months (range, 4.5 months to 31 years) and the median weight was 5.8 kg (range, 3.4 to 59 kg).
| S.No | Age | Sex | Wt. in kg | Diagnosis | Previous surgery | Surgical treatment (Trans-atrial) | STL detachment |
|---|---|---|---|---|---|---|---|
| 1 | 8m | M | 3.5 | PM VSD, PDA | - | VSD closure, PDA ligation | + |
| 2 | 1y | F | 5.1 | DORV,Inlet VSD, Ap VSD, PDA | - | VSD tunneling(LV-Ao), PDA ligation | + |
| 3 | 5m | F | 5.8 | PM VSD, PDA | - | VSD closure, PDA ligation | - |
| 4 | 1.5y | M | 7.7 | TOF, conal branch crossing RVOT | - | VSD closure, infundibular resection, RVOT patch | + |
| 5 | 4y | M | 9.9 | TOF | Lt.thoracotomy MBT shunt nonfunctioning | VSD closure, infundibular resection, TAP patch | - |
| 6 | 11m | F | 5 | DORV, SA VSD, PDA | - | VSD tunneling(LV-Ao), PDA ligation | + |
| 7 | 4.5m | F | 5.7 | PM VSD, PDA | - | VSD closure, PDA ligation | - |
| 8 | 9m | M | 3.7 | PM VSD, PDA | - | VSD closure, PDA ligation | - |
| 9 | 5y | M | 10.2 | TOF | Lt.thoracotomy MBT shunt functioning | VSD closure, infundibular resection, TAP patch, shunt interruption | - |
| 10 | 1y | M | 7.2 | TOF, coronary crossing RVOT | Sternotomy Central shunt functioning | Redo-sternotomy, VSD closure, infundibular resection, Double barrel, shunt interruption | + |
| 11 | 6m | F | 5.9 | PM VSD, PDA | - | VSD closure, PDA ligation | - |
| 12 | 31y | F | 59 | ccTGA, VSD, PS | Lt.thoracotomy MBT shunt nonfunctioning | Double Switch Operation= Rastelli + Hemi-mustard + BDGS | - |
| 13 | 1.1y | F | 5.3 | DORV, SA VSD, PDA | - | VSD tunneling(LV-Ao), PDA ligation | + |
| 14 | 8y | F | 15 | TOF, coronary crossing RVOT | - | VSD closure, infundibular resection, RV-PA conduit | - |
| 15 | 7m | M | 3.4 | PM VSD, PDA | - | VSD closure, PDA ligation | + |
Ao- aorta, Ap- apical, BDGS- bidirectional glenn shunt, ccTGA- congenitally corrected transposition of great arteries, DORV- double outlet right ventricle, F- female, LV- left ventricle, M- male, m- months, MBT- modified Blalock-Taussig, PA- pulmonary artery, PDA- patent ductus arteriosus, PM- perimembranous, PS- pulmonary stenosis, RV- right ventricle, RVOT- right ventricular outflow tract, SA- subaortic, STL- septal tricuspid leaflet, TAP- trans-annular patch, TOF- tetralogy of Fallot, VSD- ventricular septal defect, y- years
Table 1. Details of patients with situs inversus and dextrocardia.
The brief echocardiographic diagnosis of all the patients has been mentioned in Table 1 including previous surgeries if any. The surgical spectrum included closure of ventricular septal defects (n=6), complex intra-ventricular tunneling for double outlet right ventricle (n=3) and repair of tetralogy of Fallot (n=5). As the anatomy dictated, the chief surgeon stood on the left side of the patient, to operate through the left sided right atrium. Various techniques were performed to address the narrow right ventricular outflow tract which included transannular patch (n=2), right ventricular outflow tract patch (n=1), right ventricle to pulmonary artery conduit (n=1) and double barrel technique [11] for coronary artery crossing the right ventricular outflow tract.
One adult female with situs inversus, dextrocardia, congenitally corrected transposition of great arteries, routable ventricular septal defect, and severe pulmonary stenosis underwent double switch operation, viz. Rastelli procedure with hemi-Mustard baffle and bidirectional Glenn shunt [12,13].
Pre-operative evaluation
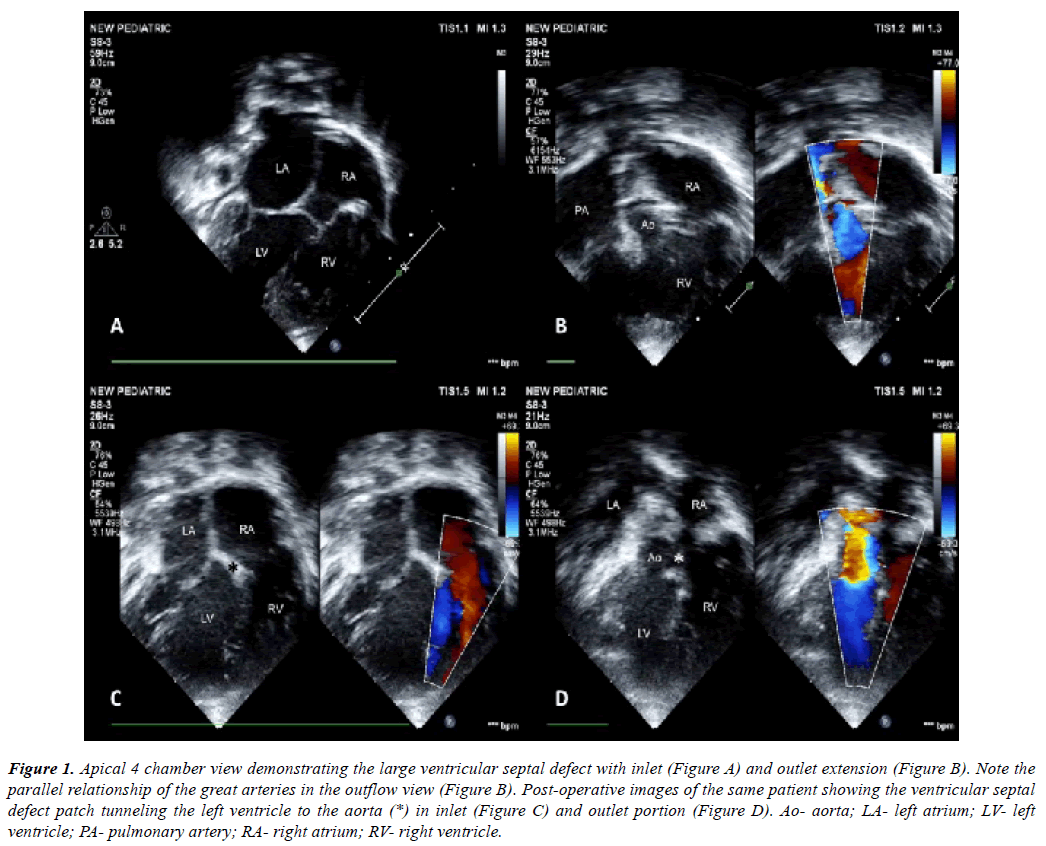
A detailed two-dimensional echocardiography was performed to decide on feasibility of biventricular repair (Figure 1A, Figure 1B). This included adequacy of ventricular volumes, presence or absence of straddling atrio-ventricular valve chordae, degree of atrio-ventricular valve regurgitation, position of atrial appendages and size and routability of ventricular septal defect. En face reconstruction of the right ventricular side of the septum [14] was obtained in case of multiple ventricular septal defects (n=1) and in cases of doubtful routability of the ventricular septal defect (n=2, both double outlet right ventricle cases).
Figure 1: Apical 4 chamber view demonstrating the large ventricular septal defect with inlet (Figure A) and outlet extension (Figure B). Note the parallel relationship of the great arteries in the outflow view (Figure B). Post-operative images of the same patient showing the ventricular septal defect patch tunneling the left ventricle to the aorta (*) in inlet (Figure C) and outlet portion (Figure D). Ao- aorta; LA- left atrium; LV- left ventricle; PA- pulmonary artery; RA- right atrium; RV- right ventricle.
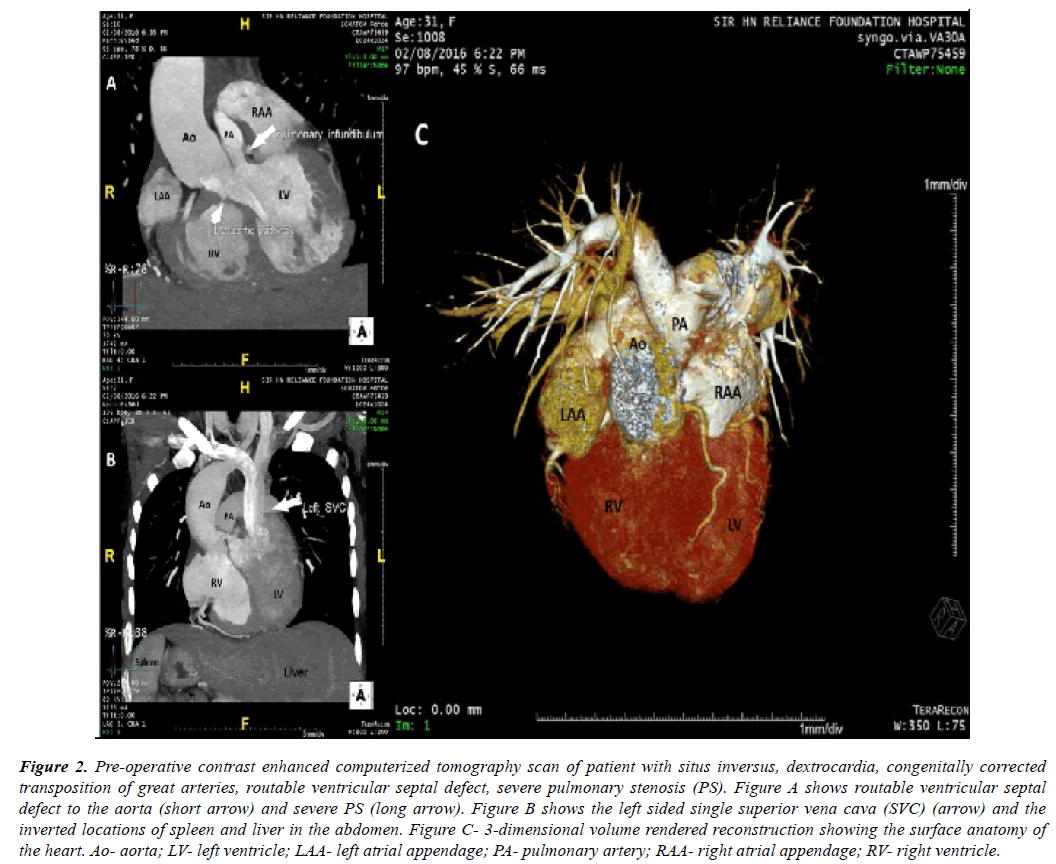
Contrast enhanced computerized tomography scan was performed for the patient with congenitally corrected transposition of great arteries (Figure 2A, Figure 2B, Figure 2C).
Figure 2: Pre-operative contrast enhanced computerized tomography scan of patient with situs inversus, dextrocardia, congenitally corrected transposition of great arteries, routable ventricular septal defect, severe pulmonary stenosis (PS). Figure A shows routable ventricular septal defect to the aorta (short arrow) and severe PS (long arrow). Figure B shows the left sided single superior vena cava (SVC) (arrow) and the inverted locations of spleen and liver in the abdomen. Figure C- 3-dimensional volume rendered reconstruction showing the surface anatomy of the heart. Ao- aorta; LV- left ventricle; LAA- left atrial appendage; PA- pulmonary artery; RAA- right atrial appendage; RV- right ventricle.
All cases were discussed in advance with the paediatric cardiac team. Images from standard textbook were obtained [15,16] and inverted using standard Microsoft software. For the patient with congenitally corrected transposition of great arteries, the illustrations were obtained and inverted from an article by Barron et al [17]. These images and en face views were studied thoroughly.
Operative technique
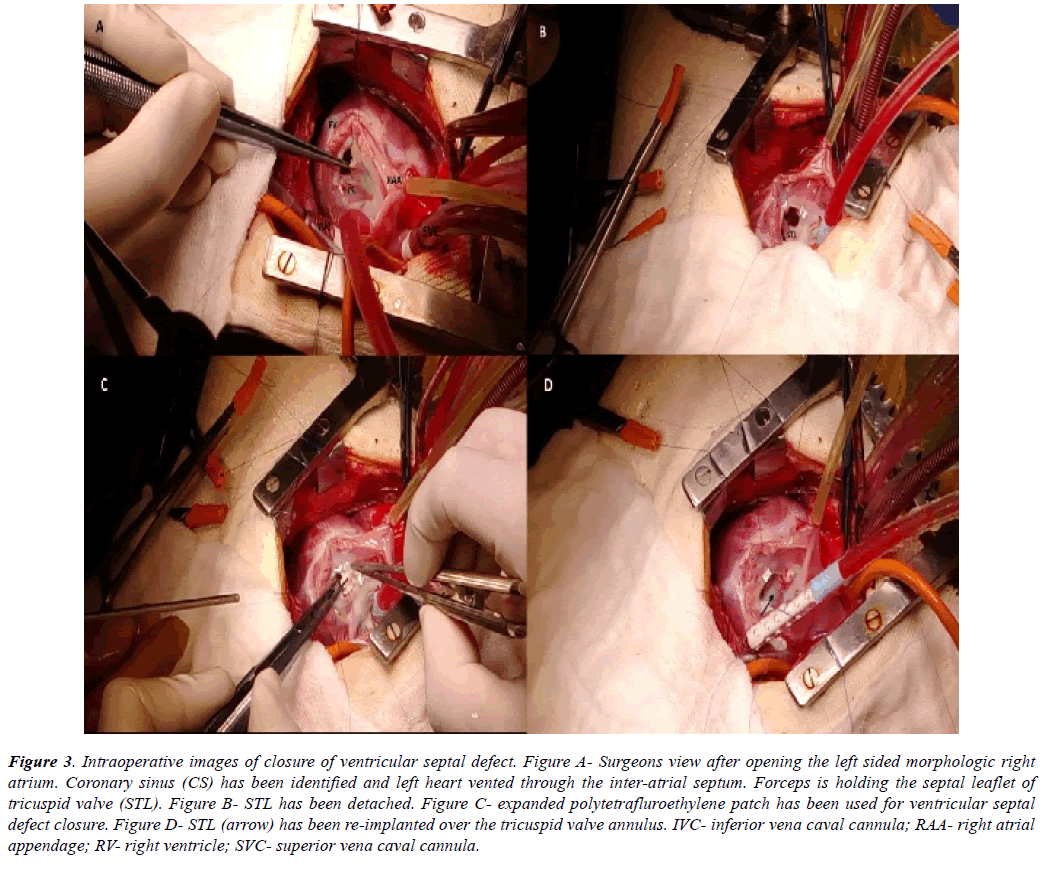
All the discussed illustrations and en face views were displayed in the operating room. Regular sternotomy was done from right side of the patient and pericardium was harvested whenever indicated. Cardiopulmonary bypass was established by standard aorto-bicaval cannulation. Under appropriate hypothermia, aorta was cross clamped and cardioplegic arrest achieved. The operating surgeon then came on the left side of the patient. The left sided right atrial appendage was retracted towards the right shoulder of the patient. Right atriotomy was performed from the tip of the appendage towards the left sided inferior vena cava (IVC), parallel to the atrio-ventricular groove. Left ventricle (LV) was vented through the inter-atrial septum (Figure 3A). Tricuspid valve was retracted at the midpoint of each leaflet to inspect the right ventricular (RV) interiors. Septal and anterior leaflets of tricuspid valve were frequently detached (n=7) [18] to demarcate the anatomy in detail (Figure 3B).
Figure 3: Intraoperative images of closure of ventricular septal defect. Figure A- Surgeons view after opening the left sided morphologic right atrium. Coronary sinus (CS) has been identified and left heart vented through the inter-atrial septum. Forceps is holding the septal leaflet of tricuspid valve (STL). Figure B- STL has been detached. Figure C- expanded polytetrafluroethylene patch has been used for ventricular septal defect closure. Figure D- STL (arrow) has been re-implanted over the tricuspid valve annulus. IVC- inferior vena caval cannula; RAA- right atrial appendage; RV- right ventricle; SVC- superior vena caval cannula.
Ventricular septal defect
From left side of the patient, an adequately tailored expanded polytetrafluroethylene patch was used to close the ventricular septal defect in all the cases (Figure 3C). Continuous fine polypropylene suture was used to fix the patch taking care of the conduction bundle at the postero-inferior margin of the defect. The patch was always reinforced with few interrupted pledgetted sutures. Saline was injected into the LV through the inter-atrial septum to check for any residual leaks. They were all managed by additional pledgetted sutures. Whenever detached (n=2), septal leaflet of tricuspid valve was sutured back to the annulus with a double layer of fine polypropylene suture (Figure 3D). Tricuspid valve was always checked for competency by saline injection test in these cases.
Tetralogy of fallot
In addition to the above mentioned steps, right ventricular outflow tract resection was done from left side of the patient.Then, various procedures were performed for right ventricular outflow tract enlargement like trans-annular patch, right ventricular outflow tract patch, right ventricle to pulmonary artery conduit and double barrel technique, all from the left side of the patient. RV and LV pressures were always checked after discontinuing cardiopulmonary bypass as a routine protocol, to confirm the adequacy of right ventricular outflow tract enlargement. Trans esophageal echocardiography was performed after bypass to ensure precise and complete repair with no residual defects.
Double outlet right ventricle
For all double outlet right ventricle cases (n=3), septal leaflet and part of anterior leaflet of tricuspid valve were detached to accomplish precise tunneling of LV to aorta. One case had inlet ventricular septal defect while another inlet defect with outlet extension resulting in large ventricular septal defect.
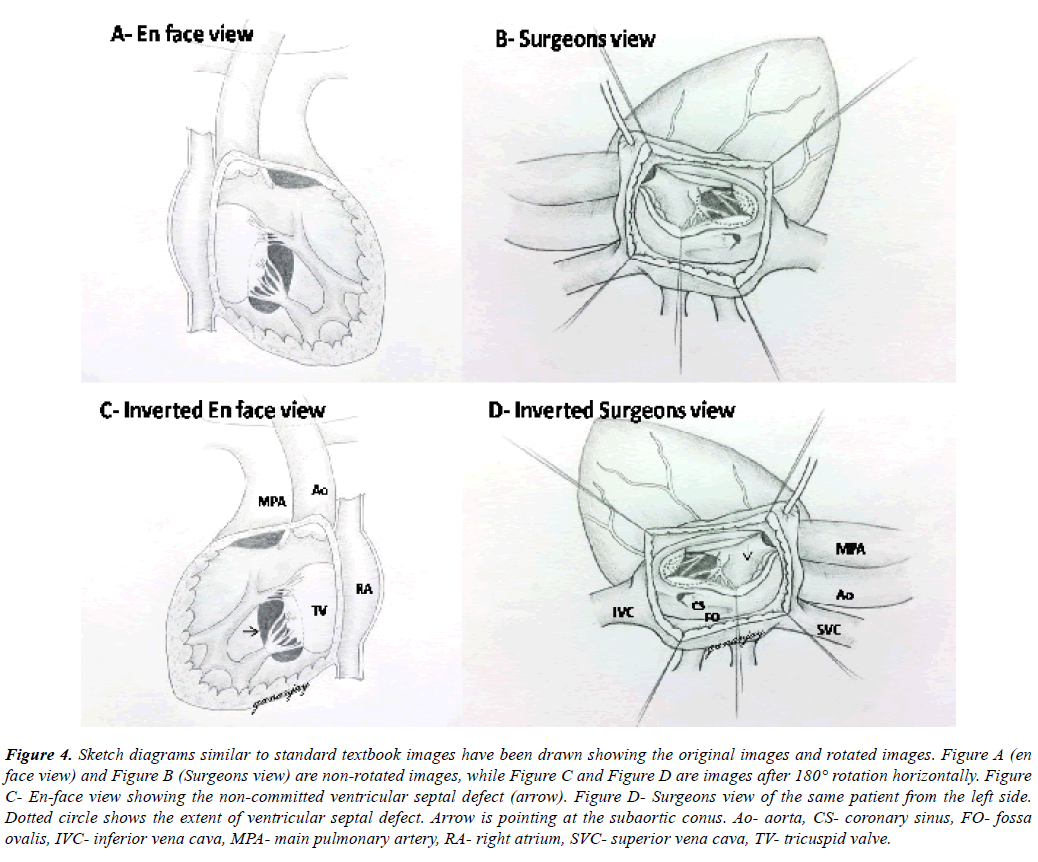
Both ventricular septal defects were non-committed and appeared difficult to tunnel as per the discussion in the preoperative meet (Figure 4A, Figure 4B, Figure 4C, Figure 4D). But on table, tunneling was attempted successfully in both the cases following adequate resection of subaortic conus and mobilization of tricuspid antero-septal commissure as well. One patient required disconnection of a conal papillary muscle chorda, which was reimplanted later onto the tunnel patch. Ventriculotomy was avoided in both the cases. One patient had an additional tiny apical muscular ventricular septal defect which was left untreated. Its clinical non-significance was confirmed by intraoperative epicardial echocardiography after the cardiopulmonary bypass was discontinued. We also confirmed no significant step-up in right atrial and pulmonary artery saturations.
Figure 4: Sketch diagrams similar to standard textbook images have been drawn showing the original images and rotated images. Figure A (en face view) and Figure B (Surgeons view) are non-rotated images, while Figure C and Figure D are images after 180° rotation horizontally. Figure C- En-face view showing the non-committed ventricular septal defect (arrow). Figure D- Surgeons view of the same patient from the left side. Dotted circle shows the extent of ventricular septal defect. Arrow is pointing at the subaortic conus. Ao- aorta, CS- coronary sinus, FO- fossa ovalis, IVC- inferior vena cava, MPA- main pulmonary artery, RA- right atrium, SVC- superior vena cava, TV- tricuspid valve.
Congenitally corrected transposition of great arteries
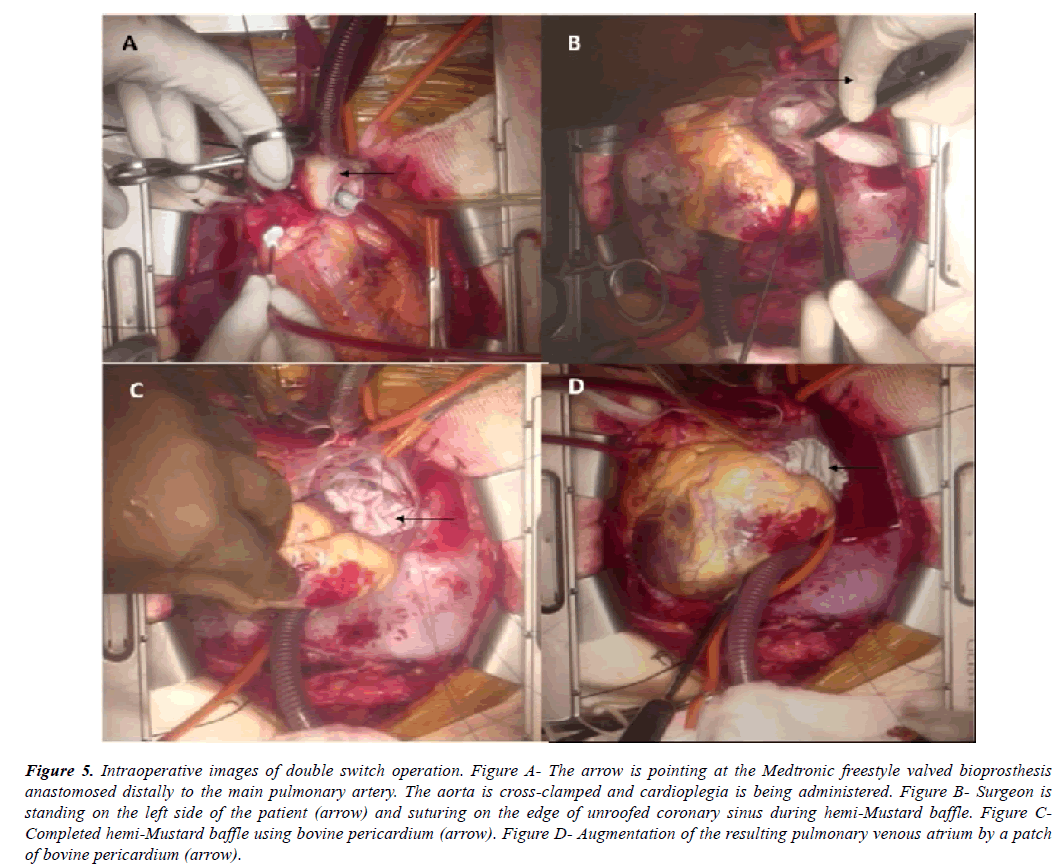
Once cardiopulmonary bypass was established, cooling was initiated towards profound hypothermia. From right side of the patient, main pulmonary artery (situated to the left and posterior to the ascending aorta) (Figure 2A, Figure 2B, Figure 2C) was transected and its proximal end was closed. Its distal open end was vented and a Medtronic freestyle valved bioprosthesis no. 21 (Medtronic, Minneapolis, MN) (Figure 5A) was sutured to it. Aorta was then cross clamped and cardioplegia was administered at regular intervals (Figure 5A). Right ventricular outflow tract was opened from the right side of the patient and ventricular septal defect was tunneled to the aorta using autologous pretreated pericardium.
Figure 5: Intraoperative images of double switch operation. Figure A- The arrow is pointing at the Medtronic freestyle valved bioprosthesis anastomosed distally to the main pulmonary artery. The aorta is cross-clamped and cardioplegia is being administered. Figure B- Surgeon is standing on the left side of the patient (arrow) and suturing on the edge of unroofed coronary sinus during hemi-Mustard baffle. Figure CCompleted hemi-Mustard baffle using bovine pericardium (arrow). Figure D- Augmentation of the resulting pulmonary venous atrium by a patch of bovine pericardium (arrow).
Then from left side of the patient, right atrium was opened as mentioned above. Coronary sinus was identified and unroofed. Inter-atrial septum was completely excised. Hemi-Mustard was performed by diverting left sided IVC return to the right sided tricuspid valve using an adequately tailored patch of bovine pericardium. Conduction system was avoided by staying onto the cut edge of unroofed coronary sinus (Figure 5B, Figure 5C). The resultant pulmonary venous atrium was closed with an augmentation patch of bovine pericardium (Figure 5D).
Patient was gradually rewarmed. Then the left sided bidirectional Glenn shunt was performed from right side of the patient by anastomosing left sided superior vena cava to the ipsilateral branch pulmonary artery in end-to-side fashion. Azygous vein was left patent. A bovine pericardial tube of adequate size was created and interposed between the right ventriculotomy and the proximal end of the bioprosthesis to establish RV to main pulmonary artery continuity.
Results
Summary of the results has been stated in Table 2. All patients underwent biventricular repair successfully through mirror image techniques. The approach was exclusively trans right atrial except for the patient with congenitally corrected transposition of great arteries, wherein right ventriculotomy was done for ventricular septal defect tunneling and for RV to main pulmonary artery conduit placement. None developed conduction related abnormalities. No patients with detachment of septal and anterior leaflets of tricuspid valve (n=7) developed valve related issues (stenosis/regurgitation).
There was no in-hospital mortality. All patients had a satisfactory postoperative recovery. Immediate postoperative echocardiography showed good repair with good biventricular function in all patients (Figure 1C, Figure 1D) except for the patient with congenitally corrected transposition of great arteries. She developed RV dysfunction which recovered gradually over a period of 7 days in intensive care unit. All patients were followed up at 10th day after discharge and then at one month. Follow-up was then extended at 3 months interval for one year, and then once in every 6 months for the next 5 years.
Two-dimensional echocardiography was repeated at one month on follow-up, and then whenever indicated; at least once in every 6 months. There was no late mortality in this study group (mean follow-up of 2.72 ± 1.84 years). One patient developed small residual ventricular septal defect in relation to the posterior aspect of the ventricular septal defect patch. On follow-up, the ventricular septal defect has progressively decreased to less than 3 mm in size. Two patients required readmission. One underwent right pulmonary artery balloon plasty after total correction for tetralogy of Fallot. Another was re-admitted for supra ventricular tachycardia (the patient with congenitally corrected transposition of great arteries) who was managed successfully with beta blockers. She remains in sinus rhythm till date and so do the rest of the patients in this study group. Another patient with the repair of tetralogy of Fallot with right ventricular outflow tract patch developed subpulmonic gradient. His contrast enhanced computerized tomography scan showed significant right ventricular outflow tract narrowing (Figure 6A, Figure 6B). He is awaiting further intervention for the same.
Discussion
Repair of congenital cardiac anomalies in situs inversus with dextrocardia is a challenging task because of the mirror image anatomy. These subsets usually undergo univentricular repair due to the associated anatomical complexity [5,6]. Surgical closure of isolated ventricular septal defects in these subsets remains technically demanding owing to the difficulty in locating the atrio-ventricular node and the bundle. Inverting the illustrations of normal hearts from any standard book would display the exact anatomy of the heart with dextrocardia and inversus morphology. This immensely facilitated us in identifying the precise location of the conduction system. Moreover, leaflet detachment whenever necessary, made the precision much easier. Perhaps that’s the dominant reason for successful closure of the ventricular septal defects while avoiding heart blocks in our series.
Operating from left side of the patient gives the familiar orientation of conventional trans-atrial approaches though in inverted fashion. Operating from left side of the patient, though results in mild discomfort for a right handed surgeon, still provides the orientation of RV interiors if we invert illustrations of any standard books. It simplifies a potentially challenging situation into not-so-formidable subsets. We change sides after cardioplegic arrest and we have not faced any obstacles in doing so and it will take less than 30 seconds most of the time. The fact that we avoid/minimize ventriculotomy while delivering the superior outcomes with this decision motivates us to pursue this approach.
Biventricular repair for congenital cardiac anomalies in situs inversus and dextrocardia (apart from congenitally corrected transposition of great arteries), though performed by few experienced centres, remains unreported till date. We applied our mirror image techniques to a variety of lesions including ventricular septal defect, tetralogy of Fallot and double outlet right ventricle (committed/ non-committed). Li et al. have reported biventricular repair for double outlet right ventricle with non-committed ventricular septal defects in situs solitus and levocardia. They employed various concomitant procedures for intracardiac tunneling viz. resection of subaortic conus, selective enlargement of ventricular septal defect anteriorly and superiorly, disconnection of conal papillary muscle or chordae and then reattachment on the surface of the tunnel patch, and excision of the tricuspid anteroseptal commissure and then reimplantation onto the surface of the baffle [4].
We followed the similar methodology in situs inversus, dextrocardia, double outlet right ventricle cases (n=3). Septal and part of anterior leaflet of tricuspid valve were detached in few cases of ventricular septal defect (n=2) and tetralogy of Fallot (n=2) as well. None of these patients developed tricuspid valve related problems.
Patients with tetralogy of Fallot needed special consideration during management of right ventricular outflow tract. We performed a wide spectrum of procedures for the same from left side of the patient which included trans-annular patch, right ventricular outflow tract patch, right ventricle to pulmonary artery conduit and double barrel technique. The ratio of pressures in RV and pressures in LV of less than 0.8 was considered acceptable.
Most of the literature available for repair of inverted dextrocardia revolves around corrected transposition [5-8]. Prasanna et al. did the biventricular repair in corrected transposition keeping morphologic RV as the systemic ventricle [5]. Sharma et al. opined that anatomic repair of congenitally corrected transposition of great arteries is better than the classic repair. However, it may not be applicable to all ventricular septal defect locations or in patients with marked imbalance of the ventricular sizes [7]. Di Donato et al. performed biventricular repair in situs inversus, dextrocardia, congenitally corrected transposition of great arteries, pulmonary stenosis with generous ventriculotomies (either right or left). The pulmonary stenosis was bypassed by LV to main pulmonary artery conduit and thus morphologic RV always remained as the systemic ventricle [8]. Suboptimal function of the systemic RV either at rest or on exercise, and its early failure has been well established in these lesions [19,20].
We therefore attempted anatomic correction in our patient with congenitally corrected transposition of great arteries, in spite of being inverted with dextrocardia, with an intention to provide her with LV as the systemic ventricle. We included bidirectional Glenn shunt in the circuit as it prolongs the conduit life, unloads the RV, reduces the complexity of complete Mustard, reduces the chances of sinus node related complications and decreases cross clamp time [12,13,17]. We also frequently augment the pulmonary venous atrium with an appropriate patch of pericardium whenever intra-atrial tunnels are constructed. This would result in a spacious atrial cavity while giving enough room for the intra-atrial tunnel.
This is one unique kind of study wherein a variety of congenital cardiac anomalies with situs inversus and dextrocardia have been clubbed. We herewith report superior outcomes of trans-atrial biventricular repair using mirror image techniques in this subset of patients. We emphasize on thorough pre-operative preparation which starts with production of appropriate illustrations for orientation of the anatomy and also includes proper planning of surgical steps to improve co-ordination and to reduce the pump time. Complete heart block is something we consider as a surgically induced disease to the patient, which not only imposes financial stress on the family in this part of the world, but also mandates reoperations /interventions to the child at regular intervals for change of impulse-generator. We could safely avoid the conduction bundle in all these cases, thanks to the detailed preoperative planning. Short and midterm follow up till date has been satisfying. Few cases have developed predictable complications on follow-up (Table 2).
| Mean ACC time | 101 ± 38 mins | |
| Mean CPB time | 153 ± 75 mins | |
| TCA time (for DSO) | 39 mins | |
| Mean hospital stay | 12.4 ± 2.6 days | |
| Mean follow-up | 2.72 ± 1.84 years | |
| Hospital/ Late mortality | 0 | |
| On follow-up | Residual VSD | 1 |
| RPA narrowing | 1 | |
| SVT | 1 | |
| RVOT re-stenosis | 1 | |
| ACC- aortic cross clamp, CPB- cardiopulmonary bypass, DSO- double switch operation, RPA- right pulmonary artery, RVOT- right ventricular outflow tract, SVT- supra ventricular tachycardia, TCA- total circulatory arrest, VSD- ventricular septal defect | ||
Table 2. Summary of results.
To conclude, trans-atrial biventricular repair for congenital cardiac anomalies in situs inversus and dextrocardia is possible using mirror image techniques. The aim of pre-operative planning should be to acquire thorough anatomical orientation of the complex anomaly/ anomalies in toto. Additionally this would avoid the injury to conduction system without prolonging surgical time significantly. The benefits of displaying of inverted standard illustrations help enormously for the same. Detachment of septal leaflet and part of anterior leaflet of tricuspid valve is one key step which we recommend for a full-proof correction, whenever indicated. We believe that we have improved the surgical outcomes of this subset of patients by avoiding ventriculotomies and univentricular repairs, without compromising the surgical safety.
References
- Bopp P, Bussat P, Lemonnier J. Rheumatic heart disease and dextrocardia. Arch Intern Med 1964;113:19-22.
- Jain VV, Gupta OP, Jain J. A rare case of situs inversus with dextrocardia, Lumtembacher Syndrome, and pericardial effusion. Heart Views 2011;12:107-11.
- Ma N, Jiang SL, Huang LJ, et al. Diagnosis of isolated dextrocardia using angiocardiography or surgery. Chin Med J (Engl) 2004;117:1655-8.
- Li S, Ma K, Hu S, et al. Biventricular repair of double outlet right ventricle with non-committed ventricular septal defect. Eur J Cardiothorac Surg 2015;48:580-7.
- Prasanna KGJ, Sharma R, Iyer KS, et al. Single versus biventricular repair for discordant hearts. Asian Cardiovasc Thorac Ann 1996;4:161-3.
- Hirooka K, Yagihara T, Kishimoto H, et al. Biventricular repair in cardiac isomerism. J Thorac Cardiovasc Surg 1995;109:530-5.
- Sharma R, Bhan A, Juneja R, et al. Eur J Cardiothorac Surg 1999;15:276-82.
- Di Donato RM, Wernovsky G, Jonas RA, et al. Corrected transposition in situs inversus. Biventricular repair of associated cardiac anomalies. Circulation 1991;84(Suppl):III193-9.
- Sapire DW, Ho SY, Anderson RH, et al. Diagnosis and significance of atrial isomerism. Am J Cardiol 1986;58:342-6.
- Marcelletti C, Di Donato R, Nijveld A, et al. Right and left isomerism: the cardiac surgeon’s view. Ann Thorac Surg 1983;35:400-5.
- Shivaprakasha K. Simplified double barrel repair with autologous pericardium for tetralogy of fallot with hypoplastic pulmonary annulus and anomalous coronary crossing right ventricular outflow. Ann Pediatr Cardiol 2008;1:34-7.
- Malhotra SP, Reddy VM, Qiu M, et al. The hemi-Mustard/bidirectional Glenn atrial switch procedure in the double-switch operation for congenitally corrected transposition of the great arteries: rationale and midterm results. J Thorac Cardiovasc Surg 2011;141:162-70.
- Sojak V, Kuipers I, Koolbergen D, et al. Mid-term results of bidirectional cavopulmonary anastomosis and hemi-Mustard procedure in anatomical correction of congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg 2012;42:680-4.
- Sivakumar S, Anil SR, Rao SG, et al. Closure of Muscular Ventricular Septal Defects Guided by En Face Reconstruction and Pictorial Representation. Ann Thorac Surg 2003;76:158–66.
- Kouchoukos NT, Blackstone EH, Hanley FL, et al. Ventricular Septal Defect. Kirklin/ Barratt-Boyes Cardiac Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2013:1295-6.
- Kouchoukos NT, Blackstone EH, Hanley FL, et al. Ventricular Septal Defect with Pulmonary Stenosis or Atresia. Kirklin/ Barratt-Boyes Cardiac Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2013:1386-9.
- Barron DJ, Davies B. The atrial switch component of the double-switch procedure: Management of venous anomalies and role of the superior cavopulmonary connection. Operative Techniques in Thoracic and Cardiovascular Surgery 2013;18:190-203.
- Hudspeth AS, Cordell AR, Meredith JH, et al. An improved transatrial approach to the closure of ventricular septal defects. J Thorac Cardiovasc Surg 1962;43:157-65.
- Peterson RJ, Franch RH, Fajman WA, et al. Comparison of cardiac function in surgically corrected and congenitally corrected transposition of the great arteries. J Thorac Cardiovasc Surg 1988;96:227–36.
- Parrish MD, Graham TP, Bender HW, et al. Radionuclide angiographic evaluation of right and left ventricular function during exercise after repair of transposition of the great arteries. Comparison with normal subjects and patients with congenitally corrected transposition. Circulation 1983;67:178–83.