- Biomedical Research (2005) Volume 16, Issue 1
Increased levels of Interleukin-4, 5 and 10 and decreased levels of Interleukin-2 and Interferon-gamma in lymphatic filariasis
Alpana Sharma1*, Medha Rajappa2, P.L.Mehndiratta3 and A. Saxena21Department of Biochemistry All India Institute of Medical Sciences, New Delhi-110 029, India
2Department of Biochemistry, Maulana Azad Medical College, New Delhi-110 002
3Department of Microbiology, Maulana Azad Medical College, New Delhi-110 002
- *Corresponding Author:
- Dr. Alpana Sharma
Department of Biochemistry, All India Institute of Medical
Sciences Ansari Nagar New Delhi 110 029 India
Phone: 0091-98990-61974
E-mail: dralpanasharma ( at ) hotmail.com
Accepted date: December 14, 2004
Abstract
Lymphatic filariasis is a global problem, with about 120 million people infected worldwide in 80 endemic countries. Filarial infections have been associated with the development of strongly polarized Type 2 host immune response and a severe impairment of type 1 cytokine production in humans. The aim of this study was to assess the immune responsiveness of patients with lymphatic filariasis, by studying their cytokine secretion profile. Samples were collected from 45 patients of different clinical manifestations such as chronic with lymphatic pathology (n=15), microfilaraemics (n=15) and endemic controls (n=15). Fifteen samples from non-endemic healthy controls were also included in the study. The levels of interleukin-2 (IL-2) and interferon-γ (IFNγ) for type 1 and interleukin-4, interleukin-5 and interleukin-10 (IL-4, IL-5 and IL-10) for type 2 responses were estimated by ELISA in peripheral blood mononuclear cell (PBMC) culture supernatants. The cells were cultured and sensitised with purified W. bancrofti extracted micro filarial antigens. The results showed the induction of type 2 immune responses with down regulation of type 1 responses. Levels of IL-4, IL-5 and IL-10 were increased in all chronic, microfilaraemic and endemic control cases. The analysis of data showed significant decrease in IL-2 (p<0.01) and IFNγ (p<0.02) levels in microfilaraemic patients as compared to chronic and endemic control cases. Our findings suggested an attenuated T-helper 1 response in patients with filariasis. The data presented here suggest a complex relationship between the host immune response and parasite establishment and survival that cannot be simply ascribed to the Th1/Th2 paradigm. There is an up regulation of IL-4, IL-5 and IL-10 expression in patients with lymphatic filariasis and a simultaneous decrease in levels of the interferon-gamma and IL-2. The profile of different cytokines in this study may prove useful in further stage-specific diagnosis of filarial patients of different clinical manifestations.
Keywords
Interleukin-2, 4, 5 and 10, Interferon-gamma, Lymphatic filariasis, Wuchereria bancrofti, Th1 and Th2 responses.
Introduction
Lymphatic filariasis is caused by the nematode parasite Wuchereria bancrofti in tropical regions of the world including India. It is a global problem, with about 120 million people infected worldwide in 80 endemic countries. It is thought to be the second leading cause of permanent and long-term disability. In India, the population at risk of developing filariasis was 454 million in the year 2001 [1]. All the filarial parasites are vector borne, transmitted by mosquitoes, biting midges, tabands and black flies. These parasites elicit a broad spectrum of clinical manifestations like asymptomatic microfilaraemia, amicrofilaraemic chronic infection with lymphatic pathology, endemic controls resisting infection, etc [2].
Parasite antigens stimulate CD4+ cells preferentially to produce elevated levels of IL-4 and IL-5, whereas antigens from intracellular organisms (mycobacteria or viruses) are more likely to induce increased production of cytokines such as IL-2 and IFNγ [3-7]. In filariasis, antigen specific cellular hyporesponsiveness has been observed among microfilaraemic (Mf) persons. Recent studies have suggested that this hyporesponsiveness is restricted to the Th-1 subset of CD4+ helper cells as Th-2 type responses to parasite antigens, specifically IL-4 and IL-5 production, appear not to differ between W. bancrofti-infected persons with Mf and chronic patients [6-11]. Clonal deletion has been suggested as one possible mechanism by which parasite-reactive lymphocytes, that may be capable of mediating resistance and/or immunopathology, are silenced in the microfilaraemic subjects [12]. Helminth infections are usually associated with a strong Th-2 response, as shown by up regulation of IL-4, IL-5 and IL-10 levels in animal models especially during chronic stages of infection [10,13-15].
Varying degrees of immune responsiveness in different clinical manifestations make the immune status studies and stage-specific detection of filariasis much more difficult. This paper describes the cytokine production profile and hence, the immune responsiveness in filarial patients, with different clinical manifestations. The profile of different cytokines in this study may prove useful in further stage specific diagnosis of filarial tients of different clinical manifestations.
Materials and Methods
Study populations
A total of 45 sera samples were collected from patients with different clinical manifestations. Fifteen samples of clinically infected patients with signs of chronic filariasis such as elephantiasis, hydrocoele with lymphatic pathology, 15 samples of microfilaraemic patients showing microfilariae on blood examination and 15 endemic control samples (asymptomatic and amicrofilaraemic but who can resist infection) were collected from NICD, filarial Clinic and Institute of Medical Sciences, Varanasi, India. Fifteen non-endemic healthy controls (individuals who have never travelled to filariasis endemic areas) were also included in this study.
Antigen extraction
Antigen was extracted from W. bancrofti microfilariae, which were isolated from patients with high counts of Mfs (>500-1000 Mfs/ml) living in the outskirts of Varanasi. Blood was collected in heparin and diluted with PBS (1:10), layered on a percoll gradient (25-35%) and centrifuged at 400 g for 30 min. The layer containing Mfs was collected, diluted with PBS and pelleted at 10,000 g for 10 min. The pelleted material was suspended in 2 ml of PBS and then passed through 0.5 μm nucleopore membrane. The membrane filter was placed in a petridish containing PBS and Mfs were collected then homogenized and TRIS-EDTA extraction (10 mM Tris and 20 mM EDTA, pH 5.0) was done as described earlier [16]. Protein concentration was estimated by Lowry’s method [17].
The extracted antigen was purified by affinity purification on CNBr activated CL sepharose 4B beads. Chronic filarial antibodies (gamma globulins) were precipitated by ammonium sulphate and used as immunosorbent to purify filarial antigen as described earlier [18].
PBMC culture
Peripheral blood mononuclear cells (PBMC) were separated from heparinised blood on Histopaque-1077 (Sigma, USA) density gradient. Cells were counted by haemocytometer and viability assessed by 0.5% trypan blue dye test. These mononuclear cells were cultured at 5×106 cells/ml in 24 well tissue culture plates (Laxbro, India) in RPMI–1640 medium supplemented with 2 mM glutamate, 100 IU/ml penicillin, 100 μg/ml streptomycin and 5% fetal calf serum. Cells were sensitised with 10 μg/ml W. bancrofti filarial antigen. Cells were incubated at 37°C in 5% CO2 for 5 days.
Measurement of cytokine profiles
The PBMC culture supernatant was collected at 48 hour for IL-2, IL-5 and IL-10 and at 96 hours for IL-4 and IFNγ estimations. The levels of different cytokines were estimated by ELISA (Diaclone Research, France) using biotinylated antibody with the streptavidin peroxidase enzyme system.
Statistical analysis
The comparison of cytokine levels in all the groups (chronic microfilaraemic and endemic) with non-endemic controls was made by non-parametric Mann-Whitney ‘U’ test. Significance was calculated using Kruskal Wallis test.
Results
In patients with lymphatic filariasis, cytokine profiles were studied, with respect to non-endemic controls.
Type-1 Cytokines
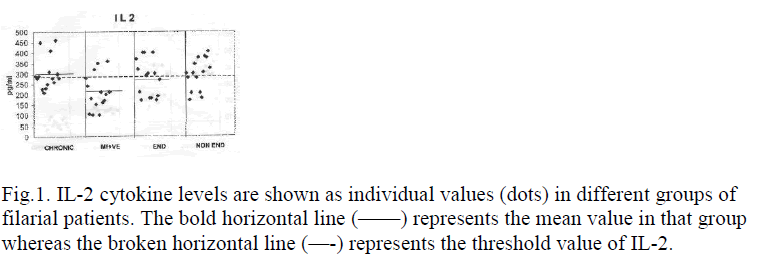
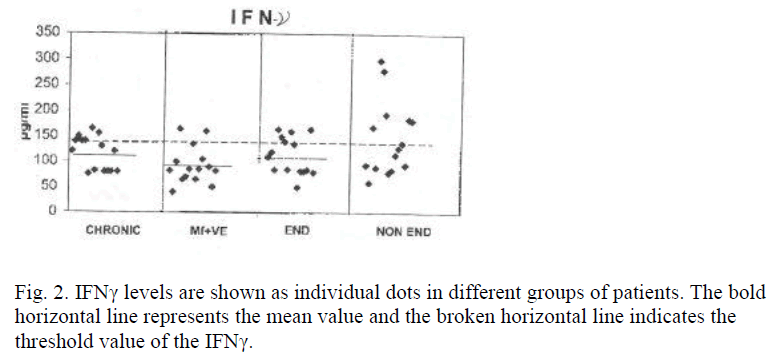
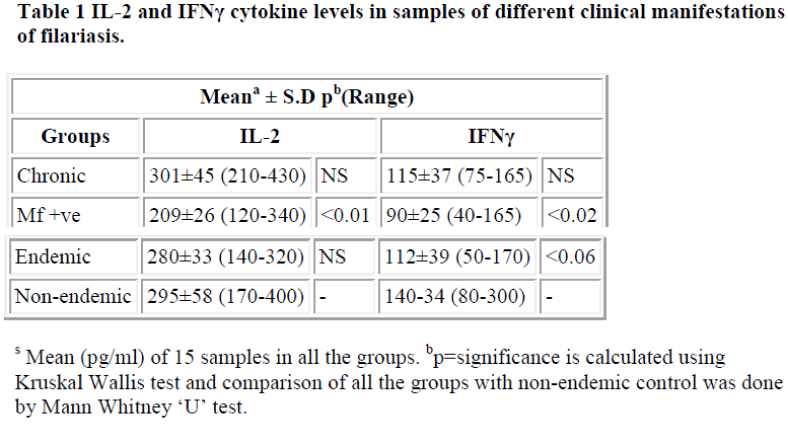
Levels of IL-2 and IFNγ in PBMC culture supernatant showed no increase in all the groups of filarial patients. Rather decreased levels of IL-2 and IFNγ were found in microfilaraemic patients (mean value of 15 samples for IL-2 = 209 pg/ml and IFNγ = 87 pg/ml). The down regulation of Th-1 responses mainly in microfilaraemic cases showed a significant decrease in IL-2 (p<0.01) and a less significant decrease in IFNγ (p<0.02) levels. Table 1 shows mean values of cytokines IL-2 and IFNγ. Figures 1 and 2 show the individual values of IL-2 and IFNγ.
Type-2 Cytokines
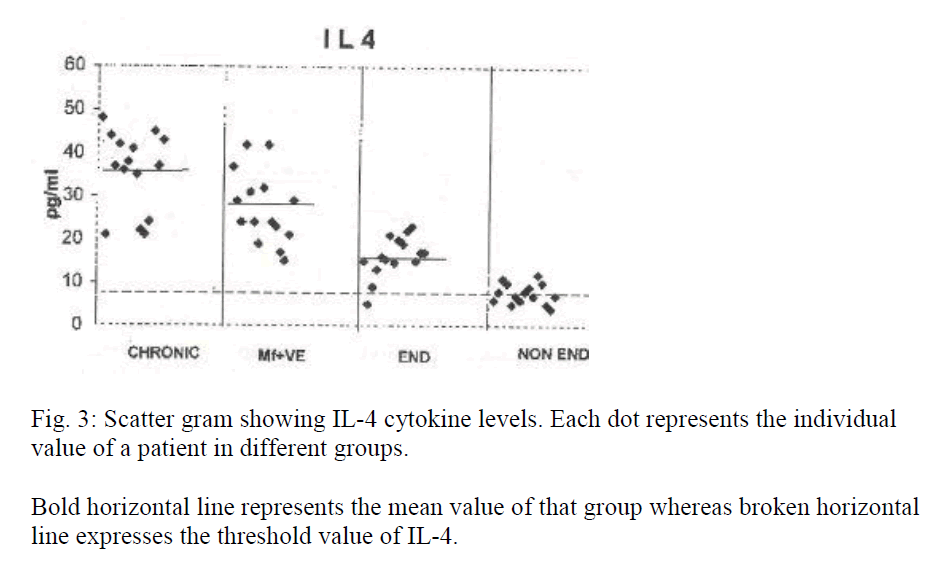
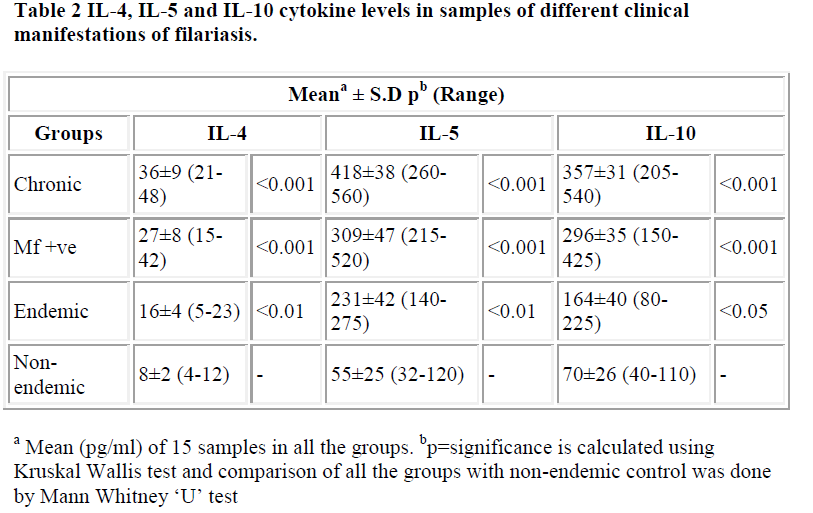
IL-4, IL-5 and IL-10 cytokine levels showed remarkable up regulation in all the patients. The maximum increase was observed in chronic cases (mean IL-4 = 36 pg/ml, IL-5 = 418 pg/ml and IL-10 = 357 pg/ml). The enhanced levels were highly significant (p<0.001) in both chronic and microfilaraemic cases.
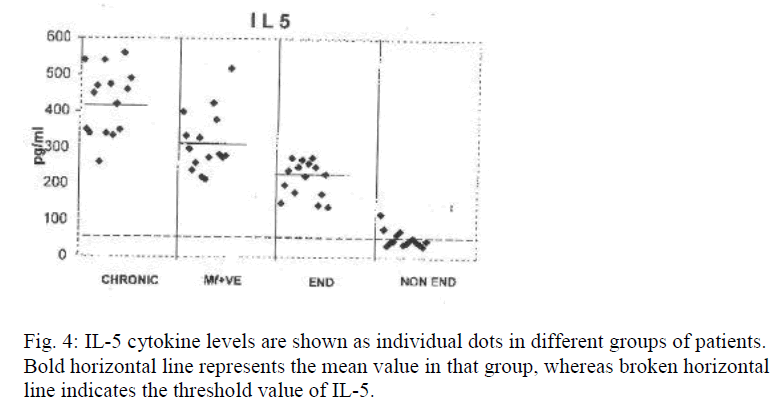
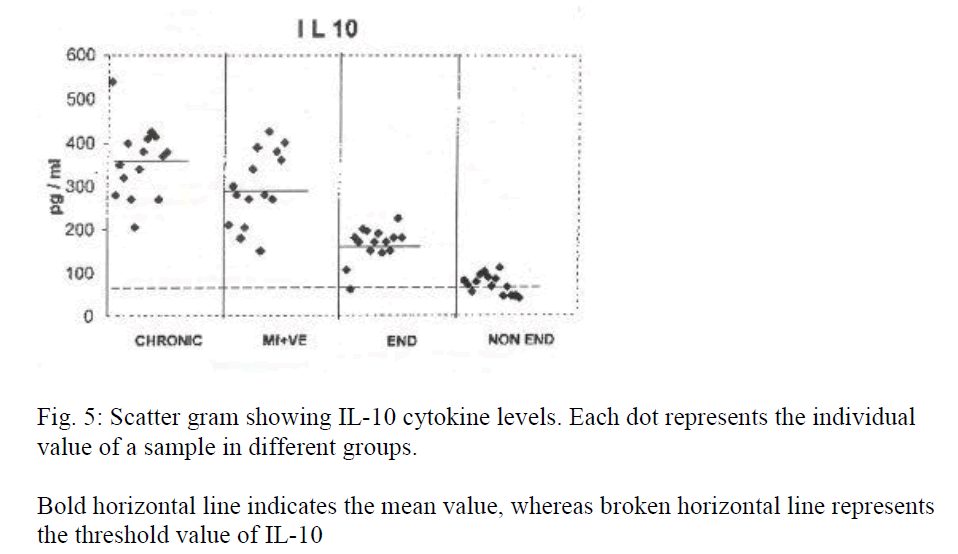
Table 2 shows levels of IL-4, 5 and 10 of different groups containing 15 samples each. Significance is represented by p value. Figures 3, 4 and 5 represent scatter grams of IL-4, IL-5 and IL-10 cytokine levels, respectively.
Bold horizontal line represents the mean value of that group whereas broken horizontal line expresses the threshold value of IL-4.
Bold horizontal line indicates the mean value, whereas broken horizontal line represents the threshold value of IL-10
Discussion
Under normal in-vivo conditions Th-1 and Th-2 patterns co-exist in a balance so that a protective immune response develops, while under pathologic conditions this balance may be shifted in either direction (13). It has been reported that Th-1 and Th-2 responses are conditioned by the antigen presenting cells [13]. Macrophages are better presenting cells for Th-1 responses, whereas B cells preferentially induce Th-2 activation [19]. Th-1 responses appear to mediate functions related to cytotoxicity and macrophage activation and therefore play an important role in combating intracellular micro organisms [19-22]. On the other hand, Th-2 responses are effective in helping B cells to produce antibodies and are important in combating extracellular bacteria or parasites in the induction of humoral immunity [22-27].
In parasitic helminth infections including filariasis more or less similar Th-1/Th-2 response pattern has been shown. Earlier reports have demonstrated an increased Th-2 response producing IL-4 in human lymphatic filariasis [8]. The high antibody titre in chronic cases could be associated with a strong Th-2 response. Although the mechanism involved in the shift in responses towards Th-2 predominance in helminth infection is not very clear, the parasite antigen load can cause maximum up regulation of Th-2 responses [5,28]. Yazdanbaksh et al [29] have compared the plasma levels of soluble CD25 and CD27 (sCD25 and sCD27) and IL-4 and IFNγ release by peripheral blood mononuclear cells of filarial patients. They observed that both chronic and microfilaraemic patients had significantly higher levels of sCD27 and IL-4 as compared to endemic and non-endemic controls. Moreover, plasma from chronic patients contained higher sCD27 levels than plasma from microfilaraemics. Similar findings were reported by Nielsen et al [7], who found an elevation in levels of IL-4 and IL-10 in chronic and microfilaraemics, as compared to endemic and non-endemic controls.
Our findings suggest an attenuated Th-1 response in patients with filariasis. Although the mechanisms are unclear, others have suggested that clonal deletion [12] or a block of CTLA-function [30] may be important.
In the present study, our results showed up regulation of Th-2, mainly producing IL-4, IL-5 and IL-10 in chronic filariasis cases, to a greater extent than in microfilaraemic and endemic controls. The higher degree of activation of Th-2 in chronic cases is in agreement with the observed hyper immune responsiveness in these patients [19,25,31]. Similarly, earlier reports of parallel regulation of IL-4 and IL-5 in human helminth infections and Th-2 biased lymphatic filarial patients are in good agreement with our results [8,31].
In this study, the down regulation of Th-1, as estimated by IL-2 and IFNγ cytokine levels confirmed the Th-1 type (cellular) hyporesponsiveness in filarial patients [32-34]. The microfilaraemic cases showed remarkable decrease in Th-1 response as compared to other groups of patients. These results are no different than our earlier reports of low levels of macrophage migration inhibition and leukocyte migration inhibition in filariasis [18,35]. The data presented here suggest a complex relationship between the host immune response and parasite establishment and survival that cannot be simply ascribed to the Th1/Th2 paradigm.
In the present study, although 3 cases showed slight discordance in the pattern and did not show a decrease in IL-2 and IFNγ levels, yet this might be due to heterogeneity in antigen preparation [18,35]. The cross-regulated mechanism of action of cytokines may explain the inhibition of IL-2 and IFNγ which could be due to IL-4 and IL-5 secreting T-cell expansion. Once the ratio of Th-1/Th-2 cells has shifted to Th-2 balance, the dominant population is likely to down regulate the induction or expansion of the other subset through a cross-regulating cytokine such as IL-10 [34,36]. The profile of different cytokines in this study may prove useful in further stage-specific diagnosis of filarial patients of different clinical manifestations.
Acknowledgements
The financial assistance provided by Council of Scientific and Industrial Research (CSIR) and Indian Council of Medical Res-earch (ICMR), New Delhi is gratefully acknowledged.
References
- WHO Global Programme to eliminate Lymphatic Filariasis-Annual Report on lymphatic filariasis 2002; 28: 7-61.
- Ottesen EA, Immmunopathology of lymphatic filariasis in man. Springer Semin. Immunopathol 1980; 2: 373-385.
- Gazzinelli RT, Makino M, Chattopadhyay SK, Snapper CM, Sher A, Hugin A W, and More H, CD4+ subset regulation in viral infection. Preferential activation of Th-2 cells during progression of retrovirus – induced immunodeficiency in man. J Immunol. 1992; 148: 182-185.
- Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A, Down regulation of Th-1 cytokine production accompanies induction of Th-2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med 1991; 173: 159-166.
- Urban JFJ, Madden KB Svetic A, Cheever A, Trotta PP, Gause WC, Katona IM, Finkelman FD, The importance of Th-2 cytokines in protective immunity to nematodes. Immunol Rev 1992; 127: 205-209.
- Mahanty S, King CL, Kumaraswami V, Regunathan J, Maya A, Jayaraman K, Abrams JS, Ottesen EA, Nutman TB, IL-4 and IL-5 secreting lymphocyte populations are preferentially stimulated by parasitic derived antigens in human tissue invasive nematode infections. J Immunol 1993; 151: 3704-3711.
- Nielsen NO, Bloch P, Simonsen PE. Lymphatic filariasis- specific immune responses in relation to lymph oedema grade and infection status. I. Cellular responses. Trans R Soc Trop Med Hyg. 2002; 96: 446-452.
- Mohanty S, Abrams JS, King CL, Limaye AP, Nutman TB, Parallel regulation of IL-4 and IL-5 in human helminth infections. J Immunol 1992; 148: 3567-3571.
- King CL, Connelly M, Alpers MP, Bockarie M, Kazura JW. Transmission intensity determines lymphocyte responsiveness and cytokine bias in human lymphatic filariasis. J Immunol 2001; 166: 7427-7436.
- Steel C, Ottenson EA. Evolution of immunologic responsiveness of persons living in an area of endemic bancroftarian filariasis a 17-year follow-up. J Infect Dis 2001; 184: 73-79.
- Raman U, Eswaran D, Narayanan RB, Jayaraman K, Kaliraj P. Proinflammatory Cytokines secreted by monocytes of filarial patients. Microbiol Immunol. 1999; 43: 279-283, 1999.
- Jenson JS, O’Connor K, Osborne J, Devaney E. Infection with microfilariae induces apoptosis of CD4 (+) T- lymphocytes: A mechanism of immune unresponsiveness in filariasis. Eur J Immunol 2002; 32: 858-867.
- Romagnani S, Human Th-1 and Th-2 subsets doubt no more. Immunology Today. 1991; 12: 256-257.
- Malhotra I, Ouma JH, Wamachi A, Kioko J, Mungai P, Njzovu M, Kazura JW, King CL Influence of maternal filariasis on childhood infection and immunity to Wuchereria bancrofti in Kenya. Infect Immun 2003; 71: 5231-5237.
- Cox FEG, and Liew FY. Cell subsets and cytokines in parasitic infections. Immunology Today 1992; 13: 445.
- Sharma A, Saxena A, and Talukdar B, Detection of W. bancrofti circulating antigen in patients with S. digitata glucan combination directed filarial antibodies. Biomed Res 1999; 10: 227-233.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ, Protein measurement with the Folin-Phenol reagent. J Biol Chem 1951; 193: 265-275.
- Sharma A, Upadhyay SN. Cellular Immunoresponsiveness in rabbits with Setaria digitata filarial antigen and TDM adjuvant. Int J Immunopharmacol 1993; 15: 395-400.
- Gajewski TF, Pinnas M, Wong T, Fitch FW. Murine Th-1 & Th-2 clones proliferate optimally in response to distinct antigen presenting cell populations. J Immunol 1991; 146: 1750-1758.
- Sanchez FO, Rodriguez JI, Agndelo G Garcia LF, Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infec Immun 1994; 62: 5673-5678.
- Yamamura M, Wang XH, Ohmen JD, Uyemura K, Rea TH, Bloom BR, Modlin RL Cytokine patterns of immunologically mediated tissue damage. J Immunol 1992; 149: 1470-1475.
- Chirgwin SR, Elzer PH, Coleman SU, Nowling JM, Hagius SD, Edmonds MD, Klei T R. Infection outcome and cytokine gene expression in Brugia pahangi infected gerbils (Meriones unguiculatus) sensitized with Brucella abortus. Infect Immun 2002; 70: 5938-5945.
- Nutman TB, Kumaraswami V. Regulation of the immune response in lymphatic filariasis perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol 2001; 23: 389-399.
- King, CL, Ottesen EA, Nutman TB. Cytokine regulation of antigen driven immunoglobulin production in filarial parasite infections in human. J Clin Invest 1990; 85: 1810-1815.
- Limaye AP, Abrams JS, Silver JE, Ottesen EA, Nutman TB. Regulation of parasite-induced eosinophilia selectively increased IL-5 production in helminth- infected patients. J Exp Med 1990; 172: 399-404.
- King CL, Mahanty S, Kumaraswami V, Abrams JS, Regunathan J, Jayaraman K, Ottesen EA, Nutman TB. Cytokine control of parasite-specific anergy in human lymphatic filariasis preferential induction of a regulatory T helper type 2- lymphocyte subset J Clin Invest 1993; 92: 1667-1673.
- Lawrence RA, Devaney E. Lymphatic filariasis parallels bet-ween the immunology of infection in humans and mice. Parasite Immunol 2001; 23: 353- 261.
- King CL, Nutman TB. Biological role of helper T-cell subsets in helminth infections. Chem Immunol 1992; 54: 136-165.
- Yazdanbaksh M, Sartono E, Kruize YCM, Kurniawan A., et al, Elevated levels of T- cell activation antigen CD27 and increased IL-4 production in human lymphatic filariasis. Eur J Immunol 1993; 23: 3312-3317.
- Steel C, Nutman TBCTLA-4 in Filarial Infections Implications for a Role in Diminished T Cell reactivity. J Immunol 2003; 170: 1930-1938.
- Sartono E, Kruize YCM, Kurniawan A, Maizels RM, Yazdanbaksh M. In Th-2 biased lymphatic filarial patients, responses to purified protein derivative of Mycobacterium tuberculosis remain Th1. Eur J Immunol 1996; 26: 501-504.
- Ottesen EA, Weller PF, Heck L. Specific cellular unresponsiveness in human filariasis. Immunol 1997; 33: 413-421.
- Piessens WF, Partono F, Hoffman SL, Tatiwayanto S, Piessens PW, Palmeieri JR, Koiman J, Dennis DT, Carney WP. Antigen-specific suppressor T lymphocytes in human lymphatic filariasis. N Eng J Med 1982; 307: 144-148.
- Ravichandran M, Mahanty S, Kumaraswami V, Nutman TB, Jayaraman K. Elevated IL-10 mRNA expression and downregulation of Th1-type cytokines in microfilaraemic individuals with Wuchereria bancrofti infection. Parasite Immunol 1997; 19: 69-77.
- Sharma A, Upadhyay SN. Antibody detection of filarial patients with S. digitata antigen using thin layer immunoassay and ELISA tests. Med Sci Res 1995; 23: 337-338.
- Mossman, TR, Moore KW, The role of IL-10 in cross-regu-lation of Th-1 and Th- 2 responses. Immunol. Today 1991; 12: A49-53.