Research Article - Current Pediatric Research (2025) Volume 29, Issue 2
Incidence and predictors of lost to follow-up among women under option B+ PMTCT program at Debre Berhan town, Amhara, Ethiopia 2024: A retrospective follow-up study.
Degefaw Denekew Hunegnaw1*, Awraris Hailu Bilchut2, Addisalem Workie Demsash3, Abebe Nigussie Ayele4, Esubalew Guday Mitkie4
1Department of Health Information Technology, Debre Berhan Health Science College, Debre Berhan, Ethiopia
2Department of Epidemiology, Debre Berhan University, Debre Berhan, Ethiopia
3Department of Health Informatics, Debre Berhan University, Debre Berhan, Ethiopia
4Department of Nursing, Debre Berhan Health Science College, Debre Berhan, Ethiopia
*Corresponding Author:
- Degefaw Denekew Hunegnaw
- Department of Health Information Technology
- Debre Berhan Health Science College
- Debre Berhan, Ethiopia
- E-mail:denekewdegefaw@gmail.com
Received: 31-Aug-2024, Manuscript No. AAJCP-24-147067; Editor assigned: 03-Sep-2024, AAJCP-24-147067 (PQ); Reviewed: 18-Sep-2024, QC No. AAJCP-24-147067; Revised: 16-Apr-2025, Manuscript No. AAJCP-24-147067 (R); Published: 23-Apr-2025, DOI: 10.35841/0971-9032.29.02.1-11
Abstract
Background: Option B+ is an advanced strategy in the Prevention of Mother-to-Child Transmission (PMTCT) recommended by the World Health Organization to enhance the care for HIV positive pregnant and lactating women. Lost to follow-up in the prevention of mother-to-child transmission service poses a significant public health challenge. Yet, there is limited understanding of the incidence and predictors influencing LTFU among HIV infected women under the option B+ PMTCT program.
Methods: A retrospective follow-up study was conducted at various health institutions in Debre Birehan town from March 11 to April 11, 2024. Simple random sampling was used to select 280 HIV infected pregnant and lactating women under option B+ PMTCT service. Data were extracted from the PMTCT registers and individual medical records using a checklist, and linked together for analysis. The patients' characteristics were analyzed in terms of frequency and percentage. The cumulative survival probability of loss to follow-up was determined using the Kaplan-Meier survivor estimator and predictors influencing LTFU were identified using the Cox regression model. All potential predictors with a p-value of <0.05 at a 95% confidence interval were declared statistically significant and presented as adjusted hazard ratios.
Result: The cumulative incidence rate of Loss to Follow-Up (LTFU) was found to be 8.04 (95% CI 5.6-11.3) cases per 1000 person-months with restricted mean survival time of 25.14 (95% CI: 24.25-26.04) months. The median follow-up time of the follow-up period was 16 (IQR 10-19) months. Lower level of formal education (AHR 9.8, 95% CI 1.2-21), non-disclosure of HIV status (AHR 3.0, 95% CI 1.6-12.4), and fair antiretroviral drug adherence (AHR 3.0, 95% CI 1.6-12.4) were significantly associated with lost to follow-up among women.
Conclusions and recommendations: The overall incidence rate of lost to follow-up is higher than the national and World Health Organization target. Lost to follow-up was found to be influenced by educational status, disclosure of HIV status, and level of medication adherence.
Keywords
Lost to follow-up, Option B+, PMTCT, Ethiopia
Introduction
Option B+ Prevention of Mother-to-Child Transmission (PMTCT) is the advanced approach of provision of universal lifelong Antiretroviral Therapy (ART) for all Human Immunodeficiency Virus (HIV) positive pregnant and lactating women, without eligibility criteria [1,2]. It is one of the four global strategies for eliminating HIV by enhancing the appropriate use of ART [3]. The other three strategies are the avoidance of HIV infection among childbearing women, prevention of unwanted pregnancy among HIV infected women, and providing appropriate management, and support to women living with HIV and their families [4].
Globally in 2022, about 36.9 million people are living with HIV AIDS, from these, 1.3 million pregnant women and girls and 1.8 million children under 15 years are newly HIV infected every year [5-7], of which 90% was recorded in Africa [8]. In sub-Saharan Africa, the rate of HIV transmission from mother to child without any interventions is 15–45%, and half of them may die before their 2nd birthday [9].
Lifelong ART has become significantly accessible and affordable due to the option B+ PMTCT program, and the women must be able to follow the prescribed course of action and remain under care for the effectiveness of the program. This strategy brought significant improvement in the engagement of lifelong ART among pregnant and lactating women living with HIV increasing from 45% in 2010 to 85% in 2019.
Prevention from Mother-to-Child Transmission (PMTCT) Guidelines have been revised since 2013 and made two major revisions in 2017 and 2021 for more integration and Ethiopia has implemented this strategy since 2013. The major reason for this revision is the revision of the national strategic plan including the Health Sector Transformation Plan (HSTP II), eliminating Mother-to-Child Transmission (eMTCT) of HIV, and congenital syphilis. Furthermore, the revision was done to meet the 95-95-95 targets for HIV testing, ART, viral suppression, and the four-pronged PMTCT approaches.
Lost to Follow-Up (LTFU) from option B+ PMTCT program can significantly affect the control of HIV by increasing mother-to-child HIV transmission and rising rates of maternal and infant mortality due to HIV infection. Thus, LTFU leads to the complicated stage of HIV, as well as contributes to the vertical transmission of HIV from mother to new born.
Evidence from African countries confirmed that the incidence of LTFU is higher than the WHO target, for instance, Uganda, Gondar Specialized Hospital, Nekemt Specialized Hospital, and northwest Ethiopia was 17, 9, 9.4, and 12 cases per 1000- person month observation respectively.
A study conducted in Malawi revealed that younger women and rural residents have a strong relationship with LTFU from the option B+ program. Another study conducted in Uganda, Ethiopia reported that the educational status of women was another predictor of LTFU. The study reported in Kenya discovered, that the level of WHO stages I and II increased the probability of LTFU, and a study from Ethiopia show that disclosure of HIV serostatus can strongly affect the retention of PMTCT. According to studies done in Uganda and Nekemt, Ethiopia, ART initiation on the same day strongly correlates with the LTFU from option B+ PMTCT program. Similar studies in Ethiopia showed that the level of drug adherence played a great role in the LTFU from the option B+ program.
Previous studies define LTFU different from the 2021 national guideline. Some of them are defined as “patients who miss their appointment for more than 3 months” and others are defined as “patients who miss their appointment more than 1 month”. These contrary ideas and research outputs could not provide insight for research and may not support for policy making. Thus, investigations are curtail based on the new guideline and standards to track LTFU and to identify associated factors. In addition to this, there are some potential variables not addressed by the previous study like the More at Risk Population (MARPs) group and the nutritional status of HIV infected pregnant and lactating women. Therefore, this study aimed to determine the incidence rate and identify potential predictors of LTFU among HIV infected pregnant and lactating women incorporating the missed variables in previous studies. The findings of this study can provide a potential information source for future researchers in the area, decisionmakers, program evaluators, and clinicians for enhancement and better care in the PMTCT program.
Materials and Methods
Study design, setting, and period
An institution-based retrospective follow-up study was conducted at Debre Berhan town, North Shoa, Amhara, Ethiopia from March 11 to April 11, 2024. The town has two public hospitals and eight health centers. Of those one hospital and seven health centres provide PMTCT services for HIV infected pregnant and lactating women within the catchment area. The PMTCT program began on June 23, 2013, and since then 1,039 women with HIV have been enrolled for PMTCT services and treatment through December 31, 2024.
Population
Source population: All HIV infected pregnant and lactating women who enrolled under option B+ PMTCT program in Debre Berhan town.
Study population: All HIV infected pregnant and lactating women who enrolled under option B+ program Debre Berhan town from January 1, 2021, to December 31, 2024.
Inclusion and exclusion criteria
All HIV infected pregnant and lactating women enrolled under option B+ PMTCT program and whose last appointment was two months before the date of data extraction were eligible to be included in the study and incomplete charts of HIV infected pregnant and lactating women with clinical data on the records concerning the variables of interest including date of enrolment to PMTCT, date outcome occurred, or women whose outcome/ event was not recorded were excluded from the study.
Sample size determination
The required sample size (n) for this study was calculated via the survival sample size calculation power approach using STATA 16 software with Cox proportional assumptions.
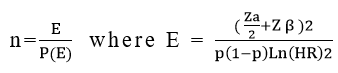
Where,
n=required number of sample size, E=total number of events required, p(E)=probability of event, p=proportion of subjects under exposure variable, HR=hazard ratio, zα/2 (critical value at 95% confidence level)=1.96, Zβ=0.8, standard deviation=0.5, proportion of withdrawals=0.1 and correlation of covariate=0 (Table 1).
| Variables from previous studies | Probability of event | CHR | Sample size |
| Adherence level | 0.2 | 2.2 | 280 (max) |
| Disclosure of HIV | 0.34 | 4.1 | 52 |
| Educational status | 0.25 | 3.9 | 76 |
| Residence | 0.57 | 7 | 17 |
| Pregnancy status | 0.33 | 3.2 | 79 |
| Enrolment type | 0.4 | 6.5 | 25 |
| Religion | 0.25 | 2.5 | 149 |
| Drug side effect | 0.5 | 6.5 | 20 |
Table 1. Sample size calculation using STATA V16 software from a similar study
Therefore, from the calculation, the largest sample taken as a final sample size was 280 charts.
Sampling techniques
Debre Berhan town has 10 public health facilities. Of these, nine health facilities are PMTCT sites and five of them don’t have cases during the study time, whereas one health facility is not a PMTCT site. Therefore, those four health facilities that provide PMTCT service were included in the study. Lists of 350 HIV infected pregnant and lactation women enrolled in option B +PMTCT program from January 1, 2021, to December 31, 2024, were obtained from those four health facilities that provide PMTCT service. Namely, Debre Berhan Compressive Specialized Hospital (DBCSH), Debre Berhan Health Center (DBHC), Ayertena Health Center (AHC), Chacha Health Center (CHC), each containing 220, 132, 36, and 22 HIV infected pregnant and lactating women respectively. Therefore, a simple random sampling technique was used to select 280 HIV infected lactation and pregnant women enrolled in PMTCT from those four health facilities that provide PMTCT service.
Data collection methods procedures
Data was extracted from patients’ charts like ART intake forms, HIV care follow-up cards, and the PMTCT register using a pretested checklist in the English language which is adapted from previous similar studies and the national guideline and face validity was performed by at least five experts.
Two BSc holder midwiferies and one MSc midwifery supervisor were recruited for the data collection process and training was provided for one day on the objective, and the relevance of the study.
Study variables
Dependent variables: Lost to follow-up from option B+ PMTCT program.
Independent variables: Socio-demographic, health facility related, clinical related and treatment related variables.
Operational definitions
Lost to follow-up: a patient missing 60 days after the last documented visit from option B+ PMTCT and not recorded as “dead, retained,” or “transferred out” on the patient’s PMTCT logbook or medical cards is considered as lost.
Censored: A patient who did not develop an event or LTFU that could be dead, transferred out, or on active treatment at the end of the study period is defined as censored.
Data quality control
Data collectors were trained in handling ethical issues and maintaining confidentiality and privacy. The data extraction tool (checklist) was pretested on 5% of the sample size in one of the health facilities other than the study facility before use to check for consistency and reliability. It was checked by the chrombach-alpha test and the reliability coefficient was 0.70. Close supervision was made daily to ensure the completeness and consistency of each checklist.
Data process and analysis
The data entry was done by using the Kobo toolbox data collection tool and was exported to STATA Version 16 for cleaning, coding, and further analysis. To calculate the descriptive analysis, the Kaplan–Meier estimator was used. On the other hand, the Cox proportional hazard regression model was employed to determine LTFU predictors. All potential predictors in the bi-variable with a P-value less than 0.25 were reserved as candidates for the multivariable Cox regression mode. All predictors from the multivariable Cox proportional hazard regression model with a p-value of less than 0.05 at 95% CI were used to declare statistical significance with Adjusted Hazard ratios (AHR). Multi-collinearity between covariates was checked by using the Variance Inflation Factor (VIF), which resulted in a mean VIF of 1.21. The proportional hazard Assumption was satisfied and checked by the Sheffield residual test and the Goodness-of-fit test by the Cox Snell residual test (distribution of residual follows 45-degree line. Finally, the results of quantitative data were presented using text, tables, and figures.
Results
Description of study units
A total of 280 women’s charts were evolved during the study period. Of those, 265 of them were included in the study with inclusion criteria. Unfortunately, 15 women’s charts were excluded from the study exclusion criteria. Therefore, the response rate was 94.6%.
Socio-demographic characteristics
The mean age of the women who enrolled in the PMTCT was 26 ± 5 SD years. About 168 (63.40%) of the women were married and 59 (22%) of the women had no formal education. About one-fourth 59 (22.3%) of the women had no formal education (Table 2).
| Variable | Category | Frequency (n) | Percent (%) |
| Age | 15-25 | 132 | 49.81 |
| 26-49 | 133 | 50.19 | |
| Residence | Urban | 162 | 61.13 |
| Rural | 103 | 38.87 | |
| Occupation (n=206) | Housewife | 103 | 50 |
| Gov’t and NGo | 85 | 41.2 | |
| Other | 18 | 8.7 | |
| Marital status | Never married | 48 | 18.11 |
| Married | 168 | 63.4 | |
| Divorced | 49 | 18.49 | |
| Educational status | No education | 59 | 22.26 |
| Primary education | 108 | 40.75 | |
| Secondary above | 98 | 36.98 | |
| Occupation | Housewife | 103 | 38.87 |
| Gov’t. and NGO | 85 | 32.08 | |
| Other1 | 21 | 7.92 | |
| Religion | Orthodox | 208 | 78.49 |
| Muslim | 53 | 20 | |
| Others2 | 4 | 1.51 | |
| Population category (n=159) | MAPS3 | 11 | 4.15 |
| General population | 148 | 55.85 | |
| Note: 1Merchant, daily laborer, has no occupation, 2Catholic, Protestant, and the like, 3Female commercial sex workers, long-distance drivers, daily laborers, and prisoners | |||
Table 2. Socio-demographic and facility type characteristics of HIV infected women in Debre Berhan town Amhara, Ethiopia 2024.
Clinical related characteristics
Of the total women enrolled in the study, 224 (84.5) had WHO clinical stage I, while the remaining 41 (15.5) had WHO clinical stage II and above. During the follow-up, most of the women 218 (82.3%) were enrolled during their pregnancy period while 47 (17.7%) of them joined the PMTCT program after giving birth. Moreover, more than one-fourth 72 (27.2%) of the women did not disclose their sero status to their families (Table 3).
| Variable | Category | Frequency n | (%) |
| WHO clinical stage | WHO stage 1 | 224 | 84.5 |
| WHO stage 2-4 | 41 | 15.5 | |
| Recent CD4 count | Less than 200 | 29 | 10.9 |
| 200-350 | 56 | 21.1 | |
| Greater than 350 | 180 | 67.9 | |
| Disclosure status | Yes | 193 | 72.8 |
| No | 72 | 27.2 | |
| Functional status | Working | 250 | 94.3 |
| Ambulatory | 15 | 5.7 | |
| Partner serostatus | Negative | 41 | 15.5 |
| Positive | 135 | 50.9 | |
| Unknown | 89 | 33.6 | |
| Status at enrolment | Lactating | 47 | 17.7 |
| Pregnant | 218 | 82.3 | |
| Opportunistic infection | Yes | 16 | 6 |
| No | 249 | 94 |
Table 3. Clinical related characteristics of HIV infected women in Debre Berhan town Amhara, Ethiopia 2024.
Treatment related characteristics
During the follow-up time, just more than half of the HIV infected women 147 (55.4%) were newly diagnosed HIV Positive pregnant and lactating women. Among women’s chart review, almost all HIV-infected women 237 (89.4%) had no side effects and more than half of the women 137 (51.7%) started the ART drug the same day they were diagnosed.
About 147 (55.5%) of HIV-infected women had a history of nutritional status of Moderate Acute Malnutrition (MAM) while 3 (1.13%) of the women had a history of Severe Acute Malnutrition (SAM) nutritional status (Table 4).
| Variable | Category | Frequency | % |
| Type of enrolment to PMTCT | Known HIV positive | 118 | 44.5 |
| New HIV positive | 147 | 55.4 | |
| Drug adherence level | Good | 207 | 78.1 |
| Fair | 58 | 21.9 | |
| Drug side effect | Yes | 28 | 10.6 |
| No | 237 | 89.4 | |
| Date of ART initiation | Later day | 128 | 48.3 |
| Same day | 137 | 51.7 | |
| Change of ART regimen | Yes | 23 | 8.68 |
| No | 242 | 91.3 | |
| BMI | Under weight | 3 | 1.13 |
| Normal weight | 262 | 98.9 |
Table 4. Treatment related characteristics among HIV infected women in Debre Berhan town Amhara, Ethiopia 2024.
Incidence rate LTFU and survival status of HIV infected women
A total of 265 women were followed with a median follow-up time of 16 (IQR 10-19) months. During the follow-up time, a total of 3977 person-month time risk was observed. The incidence density was calculated by taking the total person month as a denominator because the follow-up was a dynamic cohort.
The overall incidence rate of LTFU was 8.04 (95% CI 5.69-11.37) cases per 1000 person months’ observation time with restricted mean survival time of 25.14 (95% CI: 24.25-26.04) months. The incidence rate of LTFU among HIV infected women in hospitals and health centers were 10.5 and 5.46 cases per 1000 person-months of observation time respectively. The highest incidence of LTFU was observed at the 8th month of follow-up 63 (95% CI 20.15-193) cases per 1000 person-months observation time and shows a gradual decrement to the end of the follow-up. At the end of the follow-up, almost 50% of the women are on treatment (Table 5).
| Variable | Category | Person time in Month | LTFU n (%) | IR per 10,000 (95% CI) |
| Age | 15-25 | 1936 | 27 (84.4) | 13.9 (9.5-20.3) |
| 26-49 | 2041 | 5 (15.6) | 2.4 (1.0-6.0) | |
| Residence | Urban | 2411 | 12 (37.5) | 4.9 (2.8-8.7) |
| Rural | 1566 | 20 (62.5) | 12.7 (8-19) | |
| Marital status | Never married | 680 | 5 (15.6) | 7.3 (3-17.6) |
| Married | 2555 | 22 (68.8) | 8.6 (5.6-13) | |
| Divorced | 742 | 5 (15.6) | 6.7 (2.8-16.7) | |
| Education | No formal education | 864 | 18 (56.3) | 20.8 (13-33) |
| Primary education | 1699 | 9 (28.1) | 5.2 (2.7-10) | |
| Secondary and above | 1414 | 5 (15.6) | 3.5 (1.4-8.4) | |
| Occupation (n=206) | Housewife | 1613 | 15 (46.9) | 9.2 (5.6-15.4) |
| Gov’t and NGO | 1225 | 6 (18.8) | 4.8 (2.2-10.9) | |
| Other | 243 | 2 (6.3) | 8.2 (2.0-32.9) | |
| Religion | Orthodox | 3133 | 27 (84.4) | 8.6 (5.9-12.5) |
| Muslim | 809 | 5 (15.6) | 6.1 (2.5-14.8) | |
| Other | 35 | 0 (0%) | 0 (?.) | |
| Population category (n=159) | MARPs4 | 155 | 4 (12.5) | 25.8 (9.6-68.7) |
| General population | 2027 | 28 (87.5) | 13.8 (9.5-20) | |
| Type of health facility | Hospital | 2199 | 22 (68.8) | 10 (6.5-15.1) |
| Health center | 1778 | 10 (31.3) | 5.6 (3-10) | |
| WHO stage | WHO stage 1 | 3399 | 25 (78.1) | 7.3 (4.9-10.8) |
| WHO stage 2-4 | 578 | 7 (21.9) | 12.1 (5.7-25.4) | |
| CD4 count | Less than 200 | 409 | 2 (6.3) | 4.8 (1.2-19.5) |
| 200-350 | 866 | 6 (18.8) | 6.9 (3.3-15.7) | |
| Greater than 350 | 2702 | 24 (75.0) | 8.8 (5.9-13.2) | |
| Disclosure status | Yes | 2885 | 13 (40.6) | 4.5 (2.6-7.7) |
| No | 1092 | 19 (59.4) | 17.3 (11-27.2) | |
| Partner serostatus | Negative | 661 | 8 (25.0) | 12.1 (6.3-24.5) |
| Positive | 1966 | 3 (9.4) | 1.5 (0.44-0.48) | |
| Unknown | 1350 | 21 (65.6) | 16.7 (10.9-25.7) | |
| Status at enrolment | Lactating | 718 | 14 (43.8) | 19.4 (11.5-32.9) |
| Pregnant | 3096 | 18 (56.3) | 5.8 (3.6-9.2) | |
| Type of enrolment to PMTCT | Known HIV positive | 1716 | 5 (15.6) | 2.9 (1.2-7.0) |
| New HIV positive | 2098 | 27 (84.4) | 12.8 (8.8-18.7) | |
| Drug adherence | Good | 3184 | 17 (53.1) | 5.3 (3.3-8.5) |
| Fair | 630 | 15 (46.9) | 23.8 (14.3-39.4) | |
| Time of PMTCT initiation | Later day | 2074 | 8 (25.0) | 3.8 (1.9-7.7) |
| Same day | 1903 | 24 (75.0) | 12.6 (8.4-18.8) | |
| Note:4Female commercial sex workers, long-distance drivers, daily laborers, and prisoners | ||||
Table 5. Incidence rate of LTFU among HIV infected women in Debre Berhan town Amhara, Ethiopia 2024.
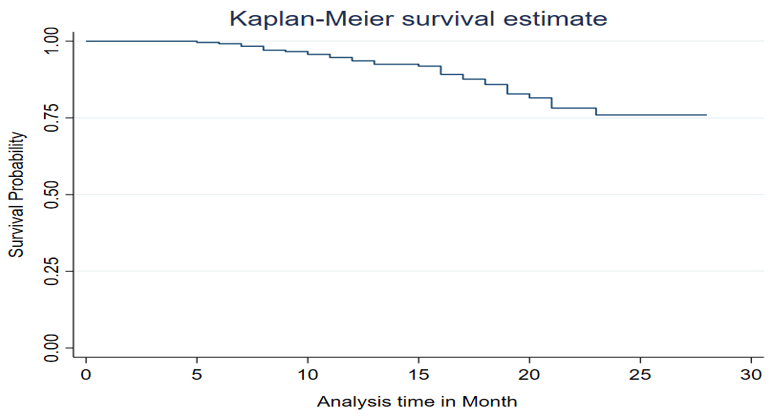
The overall Kaplan-Meir survival curve estimate
The Kaplan–Meir survival curve shows the survival risk of LTFU from the PMTCT program decreased over time (Figure 1). The first case was detected during the 5th month of followup. The highest number of LTFU was detected during 15 months and The Cumulative survival probability was 0.74 at the end follow-up (Figure 2).
Figure 1. Life table of LTFU among HIV infected women in Debre Berhan town Amhara, Ethiopia 2024.
Kaplan-Meier survival graphs for selected predictors
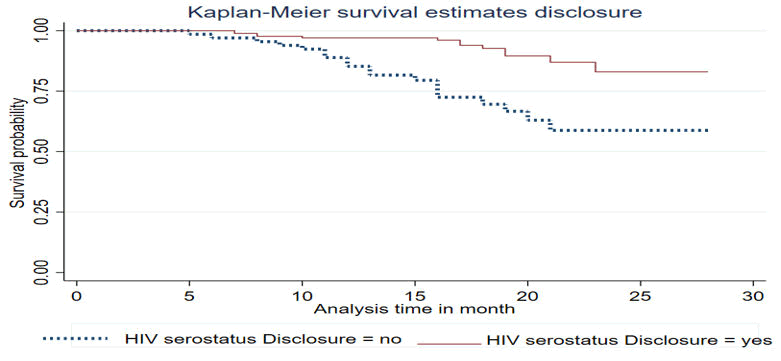
The result of this study shows women who didn’t disclose HIV serostatus decreased the survival probability of the follow-up time as compared to women who disclosed HIV serostatus (Figure 2).
Figure 2. KM curve of HIV serostatus of disclosure for LTFU among HIV infected women in Debre Berhan town Amhara, Ethiopia 2024.
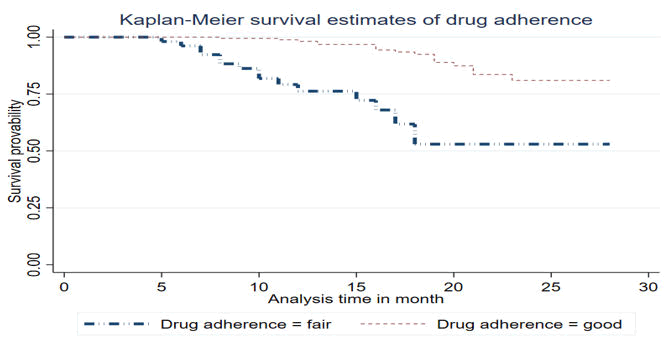
Women with fair drug adherence during the follow-up time have a higher risk of losing from the follow-up as compared to women who had good drug adherence and a woman whose drug adherence level is fair has a risk of losing from the follow-up within 17 months, while those whose adherence level is good have a risk of lost from the follow-up for more than 24 months (Figure 3).
Figure 3. KM curve of drug adherence level for LTFU among HIV infected women in Debre Berhan town Amhara, Ethiopia 2024.
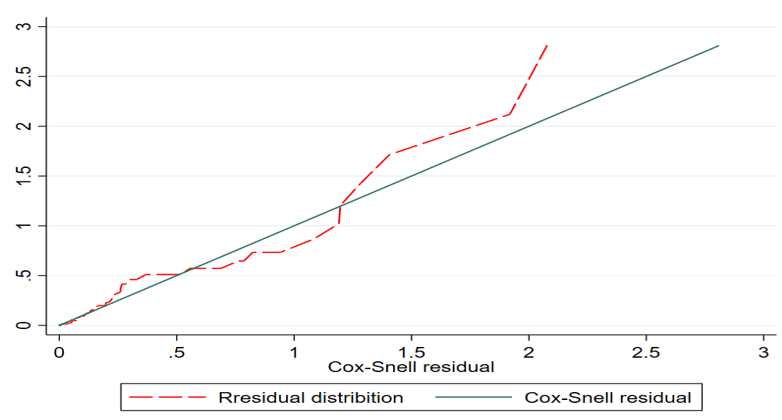
Goodness-of-Fit (GOF)
The Goodness-of-Fit (GOF) was checked by graphically using the Cox Snell residuals plot to assess the assumption of the Cox proportional hazard model. Cox Snell residual plot is used to assess the overall goodness of fit in survival models. This is done graphically using the usual Cox-Snell plots and it is observed that residuals from a properly fitted model follow an exponential unity distribution along the 45-degree slope (baseline). So, the assumption was satisfied (Figure 4).
Figure 4. Cox Snell residual test assumption using graphically.
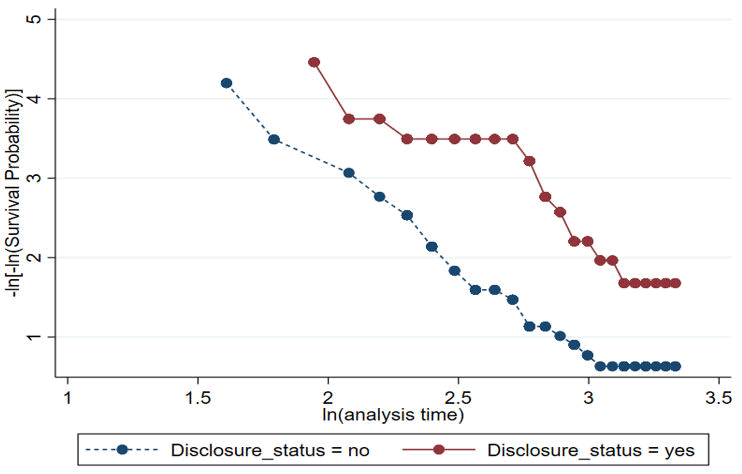
PH assumption checking using a graphical method
Graphical assumption checking for those covariates significantly associated with LTFU using the–ln(-ln(survival)) against –ln (analysis time) graphs are roughly parallel (not crossed each other) which indicates the assumption is roughly fulfilled (Figure 5).
Figure 5. PH assumption checking using a graphical method.
Predictors of Lostto Follow-Up (LTFU) among HIV infected women
After running a bi-variable Cox proportional regression analysis age, residence, educational status, type of health facility, WHO clinical stage, disclosure status, status at enrolment, date of ART initiation, type of enrolment, and drug adherence level were associated with LTFU at p-value ≤ 0.25.
Variables with a p ≤ 0.25 on bi-variable analysis and passing for the assumption of proportionality test were sent for multivariable analysis. Moreover, educational status, disclosure status, and Drug adherence level showed a significant association with LTFU in the multivariable model at a significance level of (p-value) <0.05. From the final model analysis, those HIV-infected women who had no formal education increased the hazard of LTFU by almost 10 times compared to women who had secondary and above education (AHR 9.8 CI 95% 1.2-21) keeping other variables constant. Moreover, those women who had not disclosed their HIV status increased the hazard of LTFU by 3 times compared to those who had disclosed their HIV status to their families (AHR 3.0 CI 95% 1.6-12.4) keeping other variables constant.
Another significant predictor of LTFU was the drug adherence level of the women which played a great role in LTFU. Women with fair drug adherence increase the hazard of LTFU by 5 times compared to women with a good level of drug adherence (AHR 5.0 CI 95% 2.1-11.6) keeping other variables constant (Table 6).
| Variable | Category | Survival status | CHR (95% CI) | AHR (95% CI) | P-value | |
| LTFU no (%) | Censored no (%) | |||||
| Age of the mother | 15-24 | 27 (10) | 105 (39.6) | 6.2 (2.3-16.2) | 2.9 (0.9-8.5) | 0.051 |
| 25-49 | 5 (1.8) | 128 (48.3) | 1 | 1 | ||
| Residence | Urban | 12 (4.5) | 150 (56.6) | 0.39 (0.19-0.81) | 1.6 (0.45-5.5) | 0.31 |
| Rural | 20 (7.5) | 83 (31.3) | 1 | 1 | ||
| Educational status | No formal education | 18 (6.8) | 41 (15.5) | 5.7 (2.1-15.6) | 9.8 (1.2-21) | 0.01* |
| Primary | 9 (3.4) | 99 (37.4) | 1.3 (0.44-3.95) | 1.1 (.2- 4.3) | 0.85 | |
| Secondary and above | 5 (1.9) | 93 (35.1) | 1 | 1 | ||
| Type of health facility | Health centre | 10 (3.8) | 95 (35.8) | 1 | 1 | 0.42 |
| Hospital | 22 (8.3) | 135 (50.9) | 2 (0.95-4.2) | 1.5 (0.5-4.7) | ||
| WHO clinical stage | Stage 1 | 25 (9.4) | 199 (75.1) | 1 | 1 | 0.56 |
| Stage 2-4 | 7 (2.6) | 34 (12.8) | 1.7 (0.7-4.1) | 1.3 (0.4-4.0) | ||
| Disclosure status | Yes | 13 (4.9) | 180 (67.9) | 1 | 1 | 0.00* |
| No | 19 (7.2) | 53 (20.0) | 3.8 (1.9-7.8) | 3.06 (1.6-12.4) | ||
| Status at enrolment | Pregnant | 14 (5.3) | 33 (12.5) | 0.6 (0.3-1.2) | 1.1 (0.41-3.1) | 0.8 |
| Lactating | 18 (6.8) | 200 (75.5) | 1 | 1 | ||
| Date of ART initiation | Later day | 8 (3.0) | 120 (45.3) | 1 | 1 | 0.06 |
| Same day | 24 (9.1) | 113 (42.6) | 3.7 (1.6-8.2) | 2.29 (0.9-5.5) | ||
| Type of enrolment | Known positive | 5 (1.9) | 113 (42.6) | 1 | 1 | 0.05 |
| New HIV positive | 27 (10.2) | 120 (45.3) | 4.6 (1.7-11.9) | 2.7 (0.9-7.7.) | ||
| Drug adherence level | Good | 17 (6.4) | 190 (71.7) | 1 | 1 | 0.00* |
| Fair | 15 (5.7) | 43 (16.2) | 5.9 (2.9-12.2) | 5.0 (2.1-11.6) | ||
| Note: AHR: Adjusted Hazard Ratio; CHR: Crude Hazard Ratio; CI: Confidence Interval *significant at p-value<0.05 for AHR | ||||||
Table 6. Bi-variable and multilevel survival model analysis for predictors of LTFU among HIV infected women in Debre Berhan town Amhara, Ethiopia 2024.
Discussion
The overall incidence rate of LTFU in this study is 8.04 (95% CI 5.6-11.3) cases per 1000 person month’s observation time. This evidence is in line with the previous study done in Gondar University Hospital 9 cases per 1000 person months’ observation time and Nekemt Specialized Hospital, Western Ethiopia 9.4 cases per 1000 person months’ observation time.
This might be due to the study times in which different strategies were implemented to decrease HIV infection nationally as well as globally including integrating ART related services to maternal healthcare services. In addition to this, case managers and social workers offer adherence and psychosocial services at these ART health facilities to decrease interruption from services.
However, it is lower than the previous study done in Uganda 17 cases per 1000-person month’s observation time, and in Pawi General Hospital 12 cases per 1000 people’s month’s observation time. This difference might be due to the difference in the study time that the study was initiated. For instance, the previous study report in Pawi General Hospital was done from June 2013 and March 2021 before the 2021 national guideline was introduced. Again, this difference may as a result of study time in which this study was employed after the recent national guideline introduced in which most strategies at a country level were implemented to decrease LTFU, such as bringing services closer to communities by expanding PMTCT sites that improve the accessibility of the service. Source populations of a study might be one cause of variation. For instance, the source population of a study done in Uganda was only lactating HIV-infected women whereas the source of the current study was both pregnant and lactating HIV-infected women. In addition to this, the deference might be due to the difference in the operational definition of LTFU as described earlier.
The current study revealed that the restricted mean survival time is 25.14 (95% CI: 24.25-26.04) months. This finding is lower than the study conducted in Pawi General Hospital with 29.4 months mean time survival time. This discrepancy might be due to the difference in the length of follow-up time. For instance, the previous study's follow-up time was seven years whereas the current study's follow-up time was three years. However, the result of this study is higher than previous studies conducted in Gondar University Hospital with 10.4 months mean time survival time, and Nekemt Specialized Hospital with 16.9 months mean time survival time. This disagreement might be due to the sample size variation between the studies. The sample size in the previous study was relatively higher than in the current study. Again, the difference might be due to the difference in the definition or measurement of LTFU.
Based on the findings of this study, the educational status of the women was significantly associated with LTFU. Women who had no formal education increased the hazards of LTFU by almost 10 times compared to women who had secondary and above education (AHR 9.8 CI 95% 1.2-21). This evidence is supported by other studies done in Kenya with AHR 2.3. This might be due to lower-educated women being less empowered to actively engage with the healthcare system and adhere to PMTCT protocols. In addition to this, both Kenya and Ethiopia are categorized as developed nations in which educational coverage is lower than in developed nations. However, Women with higher levels of education often have more independence and decision-making authority in their homes and communities, which may make it easier for them to begin and maintain PMTCT programs. To overcome these obstacles, enhancing the educational status of women can help to empower them in better decision-making about their healthrelated activities.
Disclosure of HIV serostatus was another significant predictor of LTFU. Those HIV-infected Pregnant or lactating women who didn’t disclose their HIV serostatus increased the hazards of LTFU by 3 times compared to those who had disclosed their HIV status to their families (AHR 3.0 CI 95% 1.6-12.4). The finding of this study is supported by studies done in Nekemt Specialized Hospital and Pawi General Hospital with AHR of 2.80 and 2.59 respectively. This might be due to discrimination and stigma by their partners, family, and community. Additionally, it could be partner discordant and confidentialityrelated issues. Hence partner, parent, and community support may play a great role in enhancing the retention of lifelong treatment of HIV-infected women.
According to the evidence of this finding, the risk of LTFU in most studies, poor ARV drug utilization level had a strong association with the LTFU and this study also revealed the same result with those studies. Women with fair drug adherence levels raise the hazard of LTFU by 5 times compared to women with fair or poor levels of drug adherence (AHR 5.0 CI 95% 2.1-11.6). The finding of this study is supported by studies done in Pawi General Hospital, Nekemt Specialized Hospital, and Gondar University Hospital with AHR of 3.96, 2.80, and 2.59 respectively. This might be due to both the current and the previous study population being similar (pregnant and lactating). Poor drug adherence may result from an inadequate understanding of benefits of ARV drugs. Cultural and Traditional Beliefs might discourage women from the continuity of PMTCT follow-up.
Conclusion
The overall incidence rate of Lost to Follow-Up (LTFU) exceeds the national and WHO target. Particularly, LTFU was found to be influenced by factors such as educational status, disclosure of HIV status, and level of medication adherence. Moreover, age, date of ART initiation, residence, and residence of the respondent were significantly associated factors in most previous studies. However, they are not associated with the LTFU in the current study.
Strengths and Limitations
Because of retrospect follow-up study used secondary data, it might be overestimate or underestimate the outcomes due to incomplete records. Therefore, future researchers can conduct a prospective approach to determine the effect of important predictors of LTFU like behavioural, cultural, and socioeconomic factors.
Declaration
The study was conducted after ethical approval was obtained from the Institutional Review Board (IRB) of Debre Berhan University, Asrat Woldeyes Health Science Campus, and a permission letter from each health facility. The data were extracted after ethical approval and permission letters were gained. During the data collection, confidentiality was assured by maintaining anonymity and the use of codes instead of the patient’s name.
Consent for Participant
In this manuscript, the consent for participant is not applicable as well as have not minor case.
Consent for Publication
Not applicable.
Availability of Data and Materials
All the data generated, and analyzed during the study are included in this article.
Authors' Contributions
DDH made a major contribution to conceptualization, proposal development, data analysis, and manuscript writing.
AHB and AWD played important roles in the work's conceptualization, supervision, and reviewing of the work.
ANA and EGM participated in in review he final report and manuscript writing. Allauthors read and approved the final manuscript for submission.
Competing Interests
The authors declare that they have no competing interests
Funding
No funding.
Acknowledgments
We would like to thank data collectors and supervisors for their active involvement in the data collection process.
References
- Tolossa T, Mulisa D, Fetensa G, et al. Magnitude and factors associated with lost to follow-up among women under option B+ PMTCT program at East Wollega public health facilities, Western Ethiopia. Int J Afr Nurs Sci. 2020; 13:100212.
- Chopra NK, Ni H, Lim V. Past present and future status of HIV-AIDS pandemic problem in world. Microbiol Infect Dis. 2019; 3(1):1-6.
- Kyaw KW, OO MM, Kyaw NT, et al. Low mother-to-child HIV transmission rate but high loss-to-follow-up among mothers and babies in Mandalay, Myanmar; a cohort study. PloS One. 2017; 12(9):e0184426.
[Crossref] [Google Scholar] [PubMed]
- Kiirya Y, Musoke P, Obeng-Amoako GA, et al. Loss to follow up after pregnancy among mothers enrolled on the option B+ program in Uganda. Public Health Pract. 2021; 2:100085.
[Crossref] [Google Scholar] [PubMed]
- Azanaw MM, Baraki AG, Yenit MK. Incidence and predictors of loss to follow-up among pregnant and lactating women in the Option B+ PMTCT program in Northwestern Ethiopia: a seven-year retrospective cohort study. Front Glob Womens Health. 2023; 4:1128988.
[Crossref] [Google Scholar] [PubMed]
- Tolossa T, Kassa GM, Chanie H, et al. Incidence and predictors of lost to follow-up among women under Option B+ PMTCT program in western Ethiopia: a retrospective follow-up study. BMC Res Notes. 2020; 13:1-7.
[Crossref] [Google Scholar] [PubMed]
- Geremew H, Wolde A, Kassa GM. Incidence and predictors of loss to follow-up among women on option B+ PMTCT program in northwest Ethiopia. A retrospective follow-up study. PLoS One. 2023; 18(1):e0280546.
[Crossref] [Google Scholar] [PubMed]
- Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow?up among women in O ption B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014; 19(11):1360-6.
[Crossref] [Google Scholar] [PubMed]
- Kweyamba M, Buregyeya E, Kusiima J, et al. Loss to follow?up among HIV positive pregnant and lactating mothers on lifelong antiretroviral therapy for PMTCT in rural Uganda. Adv Public Health. 2018; 2018(1):7540587.