- Journal of RNA and Genomics (2011) Research Article
In vivo knock-down of multidrug resistance transporters ABCC1 and ABCC2 by AAV-delivered shRNAs and by artificial miRNAs
| Florie Borel1,2, Richard van Logtenstein1, Annemart Koornneef1, Piotr Maczuga1,3, Tita Ritsema1, Harald Petry1, Sander JH van Deventer1,3, Peter LM Jansen2, Pavlina Konstantinova1,* 1Department of Research and Development, Amsterdam Molecular Therapeutics, Amsterdam, The Netherlands, 2Department of Gastroenterology and Hepatology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, 3Department of Gastroenterology and Hepatology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands |
| *Correspondence to: Pavlina Konstantinova, Email: p.konstantinova@amtbiopharma.com, Tel: +31 205662214. Fax: +31 205669272 |
| Received; 05 May 2011, Revised; 03 June; 2011, Accepted; 06 June 2011, Published online; 17 June 2011 |
| © Copyright The Authors: This is an open access article, published under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/). This license permits noncommercial use, distribution and reproduction of the article, provided the original work is appropriately acknowledged with correct citation details. |
Abstract
ABC transporters export clinically-relevant drugs and their over-expression causes multidrug resistance. In order to knock-down ABC transporters, ABCC1 and ABCC2, 13 shRNAs were developed. Four shRNA candidates were tested in vivo using self-complementary adeno-associated virus serotype 8. A strong, specific knock-down of Abbc2 was observed in mice liver, but at the cost of toxicity caused by oversaturation of the RNAi machinery due to high shRNA expression. Subsequent generation of artificial miRNAs showed better efficacy profile. These results demonstrate the feasibility of knocking down Abbc2 via AAV-delivered shRNAs to the liver, and encourage the use of miRNA in further therapeutics development.
Keywords |
| shRNA, miRNA, AAV, Abbc1, Abbc2, multidrug resistance, hepatocellular carcinoma |
Introducation |
| Hepatocellular carcinoma (HCC) is the fifth most common cause of cancer worldwide, with about 750,000 patients reported globally each year (International Agency for Research on Cancer, website: http://globocan.iarc.fr). Poor survival of HCC patients has several causes, frequently including resistance to chemotherapy. Many ATP-binding cassette (ABC) transporter family members can decrease the intracellular concentration of toxic compounds (Cole et al, 1994; Hopper-Borge et al, 2004; Tian et al, 2006; Hopper-Borge et al, 2009; Lagas et al, 2009). These transmembrane pumps are over-expressed in tumor cells, hence causing the multidrug resistance phenotype. ABCB1 and ABCC3 have been shown to be up-regulated in HCC (Grude et al, 2002; Mizukoshi et al, 2008) and the upregulation of ABCC1 has been associated with a more aggressive HCC phenotype (Vander et al, 2008). Thus far ABCB1 inhibitors have failed to show benefit during clinical trials, but more are being tested (Kuppens et al, 2007). Decreasing the expression of other ABC transporters is a desirable alternative as it could potentially reverse the multidrug resistance phenotype. |
| RNA interference (RNAi) is an attractive approach to achieve this goal, as it would allow combinatorial, possibly patient-tailored targeting of ABC transporters. RNAi is a naturally-occurring post-transcriptional gene silencing mechanism, which can induce sequencespecific degradation of a messenger RNA (mRNA), thus reducing gene expression. RNAi can be induced by synthetic small interfering RNAs (siRNAs), or by intracellular expression of short hairpin RNAs (shRNAs) and artificial microRNAs (miRNAs). shRNAs and miRNAs are processed by Dicer into siRNAs, which are loaded onto the RNA-induced silencing complex (RISC), where they mediate sequence-specific mRNA recognition, ultimately causing its degradation. In vivo siRNA-mediated knock-down of Abbc1 has been demonstrated after intratumoral siRNA injection followed by in situ electroporation of the tumor; this led to a reduction in tumor weight in response to epirubicin (Wu et al, 2011). In addition, Abbc2 was inhibited in vitro and in vivo by plasmid-, adenovirus- and lentivirusdelivered shRNAs, which respectively resulted in reversed cisplatin and paclitaxel resistance (Materna et al, 2006), decreased bilirubin transport (Narvaiza et al, 2006), and reduced growth of cisplatin-treated tumors (Xie et al, 2008). Despite these findings, the major problem of RNAi applications still lies in sustained and tissue-specific delivery of the effector molecules in vivo. Recombinant adeno-associated virus (AAV) has emerged as the vector of choice for gene therapy and for RNAimediated therapy, as it yields long-term, tissue-specific expression without any apparent pathogenicity (Daya et al, 2008). In the current study we assessed the feasibility of in vivo AAV-mediated knock-down of two murine endogenous ABC transporters: ABCC1 and ABCC2. Initially, we verified the knock-down activity of 13 shRNA constructs and selected two candidates targeting Abbc2 for further in vivo testing. A single injection of self-complementary AAV8 (scAAV8) carrying shAbcc2 resulted in efficient Abbc2 knock-down in murine liver. Concomitant signs of toxicity including elevated transaminases were attributed to high levels of shRNAs being processed into siRNAs, causing oversaturation of the RNAi machinery. Nevertheless, subsequent translocation of the validated shRNA sequences into a miRNA scaffold offers the perspective of safe AAVmediated in vivo knock-down. |
| Materials and Methods |
| DNA constructs |
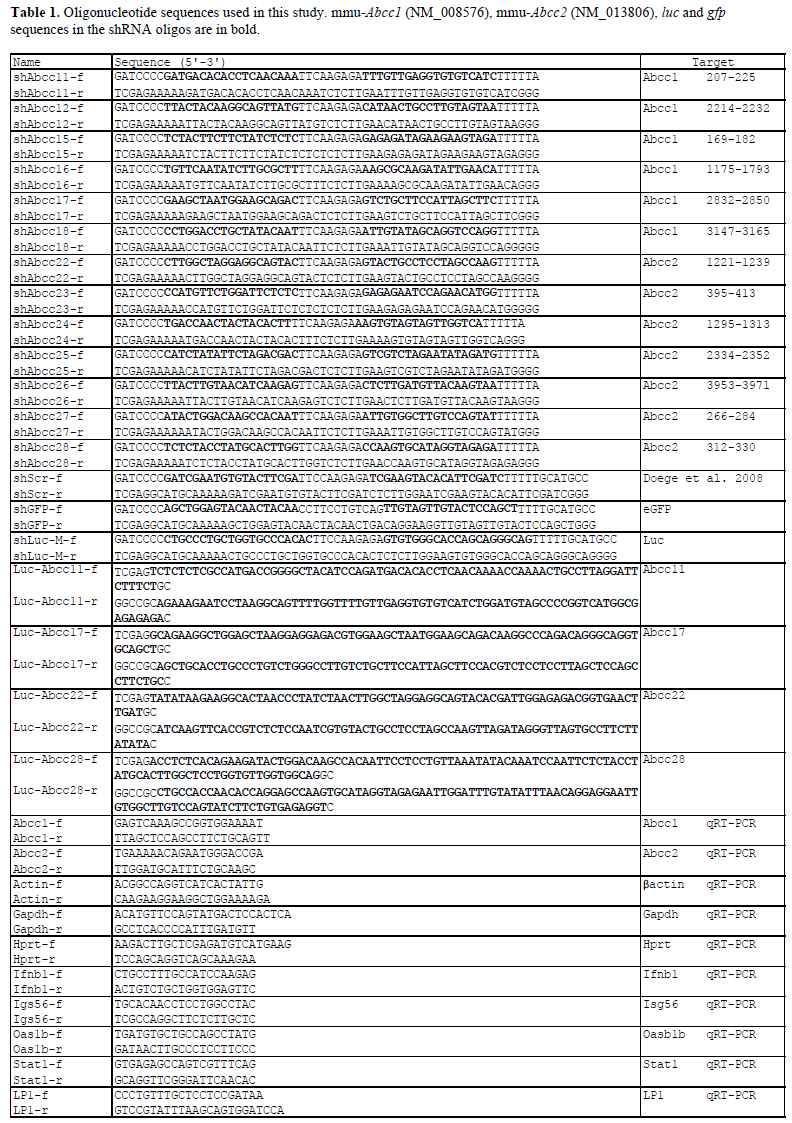
| Six shRNA constructs targeting murine Abbc1 and seven targeting murine Abbc2, and control shRNAs targeting eGFP (shGFP) and Luciferase (shLuc) and a scramble sequence (shScr) were made by annealing of complementary oligonucleotides and ligating them into the pSuper vector containing the H1 Pol III promoter (OligoEngine, Seattle, WA). The sequence for constructing the negative control hairpin shScr has been described previously (Doege et al, 2008). The sequences of oligonucleotides used in this study are listed in Table 1. Luciferase reporters Luc-Abbc11/17 and Abbc22/28 were made by cloning of Abbc1/2 sequences behind renilla luciferase in the siCheck vector (Promega, Madison, WI). |
| For cloning in the AAV backbone, the H1-shRNA expression cassettes were subcloned in pCR-Blunt IITOPO vector (Invitrogen, Carlsbad, CA), and then ligated in the pVD287 vector. pVD287 contains the egfp gene under the control of the liver-specific LP-1 promoter and generates scAAV due to a mutation in one terminal repeat (McCarty et al, 2003). |
| Cell culture and transfections |
| Human embryonic kidney (HEK) 293T and murine hepatoma (Hepa1-6) cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 10% (v/v) fetal calf serum, 100U/ml penicillin and 100U/ml streptomycin, at 37oC and 5% (v/v) CO2. Cells were plated in 6-, 24- or 96-well plates one day prior to transfection. Transfections were performed with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. For the interferon response experiment we transfected 2μg of a synthetic analog of dsRNA, polyinosinic:polycytidylic acid (poly I:C), as a positive control. |
| Luciferase assays |
| For luciferase assays, cells were co-transfected with 10ng Luc-Abbc reporter that contains both firefly and renilla luciferase genes, and 0, 0.5, 2.5, 10 or 50ng of the corresponding shAbcc expression construct. Transfected cells were assayed at 48hr post-transfection and firefly and renilla luciferase were measured with the Dual-Luciferase Reporter Assay System (Promega). Relative luciferase activity was calculated as the ratio between the renilla and firefly luciferase activities, and transfection with shScr was set at 100%. Data are represented as mean values ±SD from a representative experiment conducted with three technical replicates. |
| RNA isolation, quantitative RT-PCR (RT-qPCR), siRNA and miRNA Taqman assays |
| For in vitro experiments, total RNA was isolated from cells 18hr or 72hr post-transfection using the Nucleospin kit (Clontech, Mountain View, CA). For in vivo experiments, total RNA was isolated from frozen liver sections at two weeks post-injection (p.i.) using Trizol (Invitrogen) according to the manufacturer’s protocol. DNAse-treatment and RT-qPCR were performed as described previously (Koornneef et al, 2011) and data are represented as mean values ±SD from a representative experiment conducted with three technical replicates. siRNA and miRNA expression was quantified with custom-made siRNA assays or miRNA-specific Taqman assays (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions. |
| AAV vector production and in vivo experiments |
| Self-complementary AAV8 vectors were produced and purified as previously described (Zolotukhin et al, 1999; Gao et al, 2002; Koornneef et al, 2011) with an added fractionation step yielding higher virus concentration. Final concentration was determined by qPCR with LP1 primers. All animal experiments were conducted according to the guidelines of the local animal welfare committee. Six-to-eight-week-old male C57BL/6 mice received 2.2x1011gc AAV-shScr, 2.6-3x1012gc AAVshAbcc22 or -shAbcc28 per animal intravenously via the tail vein. Heparinized blood samples were taken by retroorbital bleeding at 1 and 2 weeks p.i. for plasma analysis. Mice were sacrificed on day 15 p.i. and livers were examined for Abbc1 and Abbc2 knock-down. Plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin were analyzed on Modular Analytics P800 analyzer (Roche Diagnostics, Basel, Switzerland). Data are represented as mean values ±SEM (n = 4-5). |
 |
Results and Discussion |
| Design and validation of shRNAs targeting murine Abcc1 and Abcc2 |
| Six shRNAs were designed against murine Abcc1 mRNA (shAbcc11-shAbcc17) (Figure 1A and 1C), and seven against murine Abcc2 mRNA (shAbcc22-shAbcc28) (Figure 1B and 1D). The ability of these constructs to knock-down endogenous Abcc1 and Abcc2 was assessed in vitro in Hepa1-6 cells using shGFP and shLuc as negative controls. shAbcc11, shAbcc17, and all 7 shAbcc2 constructs, respectively inhibited endogenous Abcc1 and Abcc2 expression by more than 50% (Figure 1E and 1F). For each target we selected the 2 most efficient shRNAs: shAbcc11 and shAbcc17, shAbcc22 and shAbcc28. |
| The specificity of Abcc1 and Abcc2 knock-down by these 4 constructs was tested on luciferase reporters containing the Abcc1 or Abcc2 target sequences. For all 4 constructs increasing concentrations of the shRNA construct induced dose-dependent inhibition of the specific luciferase reporter (Figure 1G). These results support the sequencespecificity of endogenous Abcc1 and Abcc2 knock-down by these shRNAs. |
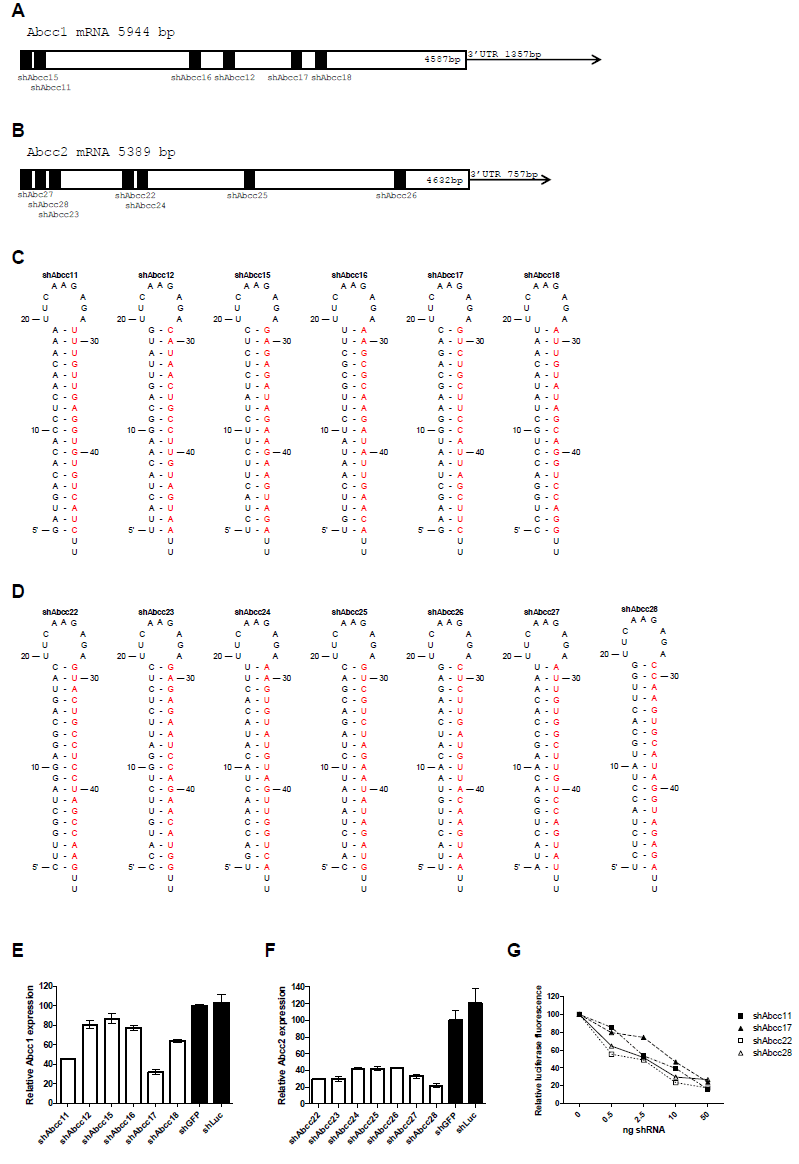
| siRNA processing was examined in vitro in two cell lines and was compared with the siRNA processing of a validated active shRNA, shApoB (Koornneef et al, 2011). Efficient processing of the shRNA would indicate efficacy of the shRNA in vivo. HEK293T and Hepa1-6 cells were transfected with increasing amounts of plasmids encoding shAbcc22, shAbcc28 and shApoB. All shRNAs expressed similar amounts of siRNAs in HEK293T (Figure 1H) and Hepa1-6 cells (Figure 1I), indicating that they do not suffer from any misprocessing that could impair the anticipated in vivo knock-down. |
| Double-stranded RNA (dsRNA), including siRNAs, can induce the interferon response (Sledz et al, 2003). We determined that none of the 4 selected shRNAs induced expression of marker genes of the interferon response in vitro following transfection of Hepa1-6 cells (Figure 2). |
| In vivo knock-down of Abcc1 and Abcc2 via scAAVdelivered shRNAs |
| C57BL/6 mice were injected with 4x1012gc/kg scAAV8 encoding shAbcc11, shAbcc17, shAbcc22, shAbcc28 and the shScr and PBS as controls. Animals were sacrificed at two weeks p.i. and Abcc1, Abcc2 knock-down and siRNA expression in liver was determined, as well as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in plasma and interferon pathway genes expression in white blood cells. Transduction efficiency by scAAV8-shAbcc was greater than 90% as determined by fluorescence microscopy (data not shown). However, Abcc1 and Abcc2 mRNA expression was not affected significantly, and we could determine only very low levels of siRNA (data not shown). We concluded that at this viral dose, these shRNAs were not expressed at a level sufficient to show a detectable effect. We subsequently narrowed our focus on the highly-expressed Abcc2 gene and injected C57 BL/6 mice with a 10-30 fold higher dose of 1.2-1.3x1014gc/kg AAV-shAbcc22, AAV-shAbcc28, and AAV-shScr and PBS as controls. Mice injected with AAV-shAbcc22 and AAVshAbcc28 were therefore sacrificed on day-13p.i. in agreement with the guidelines of the local animal welfare committee as the animals presented some signs of physiological stress and one AAV-shAbcc22-injected mouse died. Animals from AAV-shScr and PBS groups were sacrificed on day-15p.i. Gene expression analysis by RT-qPCR in the livers revealed a profound knock-down of Abcc2 mRNA up to 83% by shAbcc22 and shAbcc28 (Figure 3A). Since there were signs of toxicity, we determined whether the Abcc2 knock-down was sequencespecific rather than due to general toxicity. Therefore, the expression of the housekeeping genes, β-Actin, glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and hypoxanthine guanine phosphoribosyl transferase (Hprt), was determined in RNA isolated from mouse livers. Our results indicated no detectable down-regulation of expression of the housekeeping genes between the 3 AAVinjected groups (data not shown), thus indicating the sequence-specificity of Abcc2 knock-down by AAVshAbcc22 and AAV-Abcc28 in vivo. Further quantification of the amount of siAbcc22 and siAbcc28 by Taqman revealed a higher number of siRNA in the AAV-shAbcc22- injected mice than in the AAV-shAbcc28-injected mice (Figure 3B). |
| Interestingly, the mouse injected with AAV-shAbcc22, which first showed signs of toxicity and died was indeed the one presenting the highest levels of siRNA. While in vitro the amount siRNA produced by shAbcc22 and shAbcc28 was similar, in vivo there were differences between mice injected with the same dose of AAVshAbcc22 and AAV-shAbcc28. We conclude that our in vitro studies were insufficient predictors of the shRNAs processing in vivo. Nevertheless, in vitro efficacy correlated well with in vivo results, which, following highly-efficient transduction of hepatocytes with AAV8, achieved 83% knock-down of the target mRNA. |
| During the first week p.i. the mice from the AAVshAbcc22 and AAV-shAbcc28 groups did not gain any weight, while the AAV-shScr and the PBS groups did (data not shown). It appears that the over-expression of shAbcc22 and shAbcc28 in vivo resulted in severe toxicity. Mice presented several indications of physiological stress. Analysis of plasma sampled on day- 8p.i. revealed elevated levels of AST, ALT and total bilirubin indicating liver toxicity in the AAV-shAbcc groups (Figure 4A). On day-13p.i. one of the AAVshAbcc22- injected mice died and subsequently all mice from both AAV-shAbcc22 and AAV-shAbcc28 groups were sacrificed on the same day. Plasma analysis at two weeks p.i. revealed highly-elevated AST and ALT levels in the AAV-shScr group (data not shown), and weight loss was also observed during the second week p.i., indicating a slower onset of the toxicity, most likely due to the lower viral dose that was administered to this group. |
 |
 |
 |
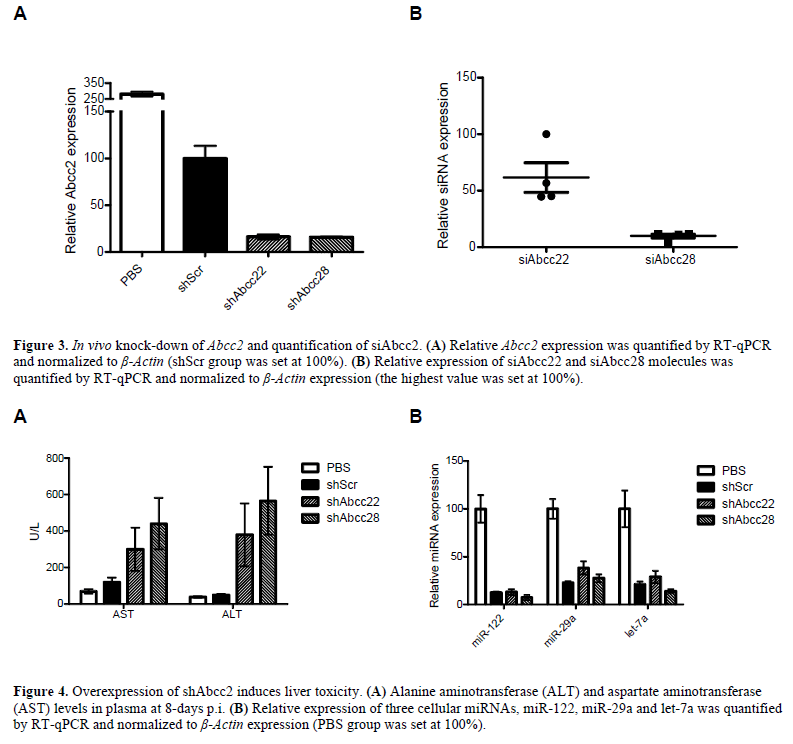 |
| It has been reported that high siRNA expression from shRNA cassettes containing a strong promoter can lead to lethal toxicity by over-saturating the RNAi machinery and affecting endogenous miRNA processing (Grimm et al, 2006). We determined the expression levels of 3 cellular miRNAs, namely miR-122, miR-29a and let-7a, in the livers of the injected animals. If saturation of the RNAi machinery occurred to the point where endogenous metabolic processes are expected to be significantly affected, expression of these liver miRNAs should be significantly decreased. Taqman assays for the 3 cellular miRNAs revealed down-regulation in the mouse livers injected with AAV-shScr, AAV-shAbcc22 and AAVshAbcc28 compared to the PBS group (Figure 4B), indicating oversaturation of the RNAi machinery. |
| Circumventing shRNA toxicity |
| Expression of shRNA from a weaker promoter and from a miRNA scaffold can circumvent toxicity problems (McBride et al, 2008). Therefore, shAbcc11 and shAbcc28 sequences were expressed from a miRNA backbone (Figure 5A and 5B) and their ability to knock-down Luc- Abcc11 and Luc-Abcc28 luciferase reporters was assessed in vitro in HEK293T cells. Comparison of knock-down induced by shAbcc11 and miAbcc11, and by shAbcc28 and miAbcc28 showed that both constructs had a similar efficiency – the miRNA being slightly better (Figure 5C and 5D). Furthermore, quantification of the amount of siRNA molecules necessary to achieve this knock-down effect by Taqman assay showed that miAbcc28 produced 55%-75% less siAbcc28 molecules than shAbcc28 (Figure 5E). Since these miRNAs were expressed from a significantly weaker Pol II promoter, less siRNA molecules were apparently being produced while retaining equal inhibitory properties compared to the shRNA. This indicates that miRNAs may be more potent molecules for induction of RNA silencing than shRNAs. Considering the toxicity issues raised in this study the possibility of expressing siRNA sequences from a miRNA backbone, |
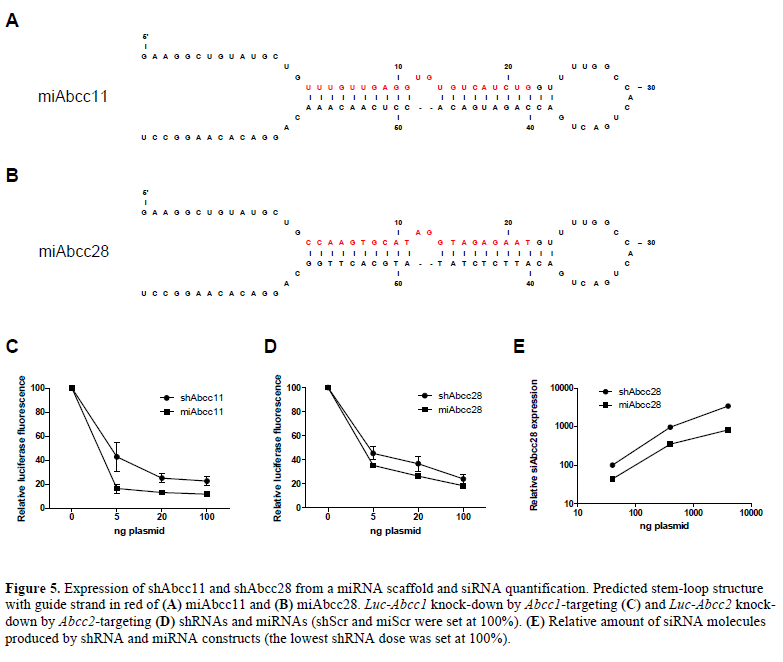 |
| would present reduced toxicity risks in in vivo studies is of considerable interest. We are currently comparing the long-term efficacy and toxicity profile of siRNAs expressed from AAV-shRNA and AAV-miRNA backbone in vivo. Future research will therefore focus on expressing siRNA from a miRNA backbone to achieve efficient and safe in vivo target knock-down. |
| To our knowledge, this is the first report to show AAVmediated knock-down of Abcc2 in vivo. Concomitant toxicity was observed and was attributed to a previously described mechanism of oversaturation of the RNAi machinery (Grimm et al, 2006). Subsequent generation of miRNAs showed a better efficacy profile, i.e., the same effect mediated by significantly less siRNA molecules. We therefore expect that these constructs will yield a strong and safe knock-down following liver transduction with AAV. |
Conclusions |
| • Expression of multiple anti-Abcc1 and anti-Abcc2 shRNAs |
| • In vitro studies are insufficient predictors of the shRNAs processing in vivo |
| • Strong knock-down of Abcc2 in vivo following AAVshAbcc delivery |
| • Concomitant toxicity due to oversaturation of the RNAi machinery |
| • Toxicity can be circumvented by siRNA expression from a natural miRNA scaffold |
| Acknowledgements |
| Amsterdam Molecular Therapeutics funded the research presented in this article. The authors thank Bas Blits for his help with the animal experiments committee, Angelina Huseinovic, Niccolò Bacchi and Ruiqi Han for technical assistance, and Rachid Benchaouir for the iodixanol fractions protocol. The selfcomplementary AAV vector containing the LP-1 promoter was kindly provided by Amit C Nathwani (St Jude Children’s Research Hospital, Memphis, TN). |
Completing Ineterests |
| The authors declared no conflict of interest. Amsterdam Molecular Therapeutics declared no commercial interest in the conclusions. |
List of Abbrevations |
| gc; Genomic copies |
| p.i.; Post injection |
| PBS; Phosphate-buffered saline |
References
|