Research Article - Biomedical Research (2017) Volume 28, Issue 19
Impact of different concentrations of 1,25(OH)2D3 on expressions of AdipoR2, p38MAPK, LPL, and triglyceride in HepG2 cells
Yan Yang, Beibei Liu, Ling Gao*, Qi Li, He Wang and Liandi Wang
Department of Endocrinology, Affiliated Hospital of Zunyi Medical College, Zunyi, PR China
- *Corresponding Author:
- Ling Gao
Department of Endocrinology
Affiliated Hospital of Zunyi Medical College, PR China
Accepted date: September 23, 2017
Abstract
The aims of this study were to observe the effects of different concentrations of 1, 25 dihydroxyvitamin D3 (1,25(OH)2D3) on the content of Triglyceride (TG), as well as the gene and protein expressions of Adiponectin receptor 2 (AdipoR2), p38 Mitogen-Activated Protein Kinase (P38MAPK), and Lipoprotein Lipase (LPL) in TG’s metabolic pathway, in the HepG2 cells so as to provide theoretical basis for further studying the mechanism by which Vitamin D reduces TG. The HepG2 cells were treated with different concentrations of 1,25(OH)2D3 0 ng/ml (CON), 12.5 ng/ml (L-dose), 25 ng/ml (M-dose), and 50 ng/ml (H-dose)) for 24 h, respectively, and then detected the gene and protein expressions of AdipoR2, p38MAPK, and LPL by Real-Time Polymerase Chain Reaction (RT-PCR) and Western blotting, as well as the content of TG by the enzyme method. Compared with Group CON, the gene and protein expressions of AdipoR2, p38MAPK, and LPL in Group L, M, and H-dose were all increased while the contents of TG were decreased (P<0.05). The levels of 1,25(OH)2D3 decreases the TG content in the HepG2 cells may be achieved by upregulating the expressions of AdipoR2, p38MAPK, and LPL.
Keywords
1,25(OH)2D3, Adiponectin receptor 2, p38 mitogen-activated protein kinase, Lipoprotein lipase.
Introduction
Vitamin D (VD) is a multi-functional vitamin, and in addition to participating in regulating the in vivo metabolism of Ca and P and osteogenesis, it can also inhibit cell growth and regulate immune function [1]. Recent studies have found that the level of VD exists certain relationships with the level of blood lipids [2,3]. Wang found a significant negative correlation between serum VD and Triglyceride (TG) in a study targeting Chinese populations [4]. It has also been confirmed that VD has certain protective effects against the livers in diabetic rats [5]. Active VD, namely 1, 25 dihydroxyvitamin D3 (1,25(OH)2D3), can reduce the liver fat content in mice [6]. However, the mechanism by which 1,25(OH)2D3 regulates the lipid metabolism has not been fully elucidated. Adiponectin receptor 2 (AdipoR2), p38 Mitogen-Activated Protein Kinase (p38MAPK), and Lipoprotein Lipase (LPL) are the key genes in the metabolic pathway of glycolipid, which play important roles in lipid metabolism, Insulin Resistance (IR), etc., and can lead to the occurrence of IR via direct or indirect channels. Therefore, in-depth studies in this field can be helpful to elucidate the molecular mechanism of IR. The impact of 1,25(OH)2D3 on the expressions of AdipoR2, p38MAPK, and LPL in in vitro hepatocytes has not been reported in China or abroad. Therefore, this study investigated the impact of 1,25(OH)2D3 on the expressions of AdipoR2, p38MAPK, and LPL, as well as the content of TG, in the HepG2 cells, aiming to preliminarily investigate the dose-dependent relationships of 1,25(OH)2D3 with AdipoR2, p38MAPK, LPL, and TG and to provide theoretical basis for further studying the mechanism by which vitamin D regulates the metabolism of TG in the HepG2 cells.
Materials and Methods
Experimental grouping
The HepG2 cells were divided into 4 groups according to the concentrations of 1,25(OH)2D3 (3 repetitive wells for each group, and the result was the mean of the 3 wells): 1. Group CON: no VD; 2. Group L-dose: 12.5 ng/ml; 3. Group M-dose: 25 ng/ml; 4. Group H-dose: 50 ng/ml.
Cell culture
HepG2 cells were purchased from the Chinese Academy of Medical Sciences Concord Cell Center. 1,25(OH)2D3 was purchased from American R & D Company. Dulbecco Minimum Essential Medium (DMEM) and fetal bovine serum were purchased from American Life Technologies. The HepG2 cells were incubated in 10% FBS-containing DMEM medium at 37°C and 5% CO2, with the culture medium changed once every 2-3 d. When the cells fused to about 90% of the area, the cells were passaged and then incubated with different concentrations of VD (0 ng/ml, 12.5 ng/ml, 25 ng/ml, 50 ng/ml) for 24 h. The HepG2 cells (grew well, 80%) were then sampled for trypsin digestion, and kept subculture in 6-well culture plates with the cell density as 2 × 105 cells/ml until the cells adhered to the plate wall. The cell density was then microscopically adjusted to 5000 cells/well, followed by PBSrinsing twice, and cultivation in serum-free DMEM.
Detection of TG
TG Assay Kit (purchased from Zhong Sheng Bei Kong Biotechnology Co., Ltd. China), dimethyl sulfoxide (DMSO) was purchased from Shanghai Boyao Biotechnology Co., Ltd., China. Taq enzyme was purchased from American Life Technologies. After absorbed the medium in each well, 2 ml of pre-cooled PBS buffer was added and shaken gently several times so as to remove the culture medium as much as possible (twice). After rinsing, the appropriate amount of PBS buffer was added so as to scrap the wall-adherent cells and transfer them into new pre-cooled centrifuge tubes for 10 min centrifugation (1000 r/min at 4°C); after discarded the supernatant, 200 μL of isopropanol was then added to extract the cell lipid. After several repetitive freeze-thaw at -70°C/ 37°C, the totally broken cells (microscopically confirmed) were centrifuged at 9000 r/min for 10 min. The supernatant was then transferred to another clean 1.5 ml centrifuge tube, added 20 μL of 1 mol/L NaOH to dissolve the protein precipitate in the tube for at least 12 h, and determined the protein content by the BCA method. The TG detection agent was then added and incubated at 37°C for 30 min. The TG concentration was then calculated by comparing the absorbance (A) value with those of the standards; the TG concentration was divided by the protein concentration so as to calculate the intracellular TG content (unit: mg/g).
Real-time polymerase chain reaction (RT-PCR)
PCR primers were synthesized by TaKaRa Bioengineering (Economic and Technological Development Zone, Dalian City, Liaoning Province, China); Reverse transcription kit and Trizol regent were purchased from American Life Technologies. After 24 h 1,25(OH)2D3 treatment, the HepG2 cells were rinsed three times by PBS, and then determined the mRNA by RT-PCR.
20 μL reaction system to extract the total RNA (the primer sequences are shown in Table 1). Procedures: reaction conditions: pre-denaturalization at 95°C for 5 min, denaturalization at 95°C for 30 s, annealing at 58°C~75°C for 30 s, extension at 72°C for 1 min, for 28~33 cycles, extension at 72°C for 5 min. In order to correct the errors, this study used the housekeeping gene β-actin as the internal reference, and the relative content of the target gene was obtained by dividing the average copy number of the target gene of the sample by the average copy number of the internal reference gene of this sample. The copy number of the template in samples can be calculated according to its related SDS-generated Ct value from the standard curve.
| Primer | Sequence (5’-3’) |
|---|---|
| β-actin | upstream: 5’-TAAAGACCTCTATGCCAACACAGT-3’ |
| downstream: 5’-CACGATGGAGGGGCCGGACTCATC-3’ | |
| AdipoR2 | upstream: 5’-TGCGCACACGTTTCAGTCTCCT-3’ |
| downstream: 5’-TTCTATGATCCCCAAAAGTGTGC-3’ | |
| P38MAPK | upstream: 5’-CCGTTTCAGTCCATCATTCA-3’ |
| downstream: 5’-TCATTTCGTCATCAGTGTGC-3’ | |
| LPL | upstream: 5’-ATGGAGAGCAAAGCCCTGCT-3’ |
| downstream: 5’-CACGCCAGCAGCATGGGCTC-3’ |
Table 1. Specific primers used for real-time PCR.
Western blotting
AdipoR2, p38MAPK, LPL were purchased from Santacruz (Shanghai Golden Bank Biological Technology Co., Ltd, China). The second antibody and cell protein extraction kit were purchased from Takara (Economic and Technological Development Zone, Dalian City, Liaoning Province, China). Impact of different concentrations of 1,25(OH)2D3 on the expressions of AdipoR2, p38MAPK, and LPL in the HepG2 cells by Western blotting. The above-treated cells were collected, rinsed three times with cold PBS, and then extracted 30 μg of proteins by protein lysate for 12% Polyacrylamide Gel Electropheresis (PAGE). After the electrophoresis, the products were transferred onto one Polyvinylidene Fluoride (PVDF) membrane, followed by overnight incubation with the primary antibodies of β-actin (1:1000), AdipoR2 (1:1000), p38MAPK (1:1000), and LPL (1:300), film rinsing, 50 min incubation with the HRP-labeled secondary antibody (1:1000), alkaline phosphatase staining, and exposure. The relative expressions of AdipoR2, p38MAPK, and LPL were expressed by the ratios of their respective gray values to the gray value of β-actin.
Statistical analysis
SPSS19.0 was used for the statistical analysis. The mRNA and protein levels of AdipoR2, p38MAPK, and LPL and the TG content were expressed as (͞x ± s). The normally distributed data were then applied ANOVA and pairwise comparison for analyzing the differences among them.
Results
Gene and protein expressions of AdipoR2, p38MAPK, and LPL
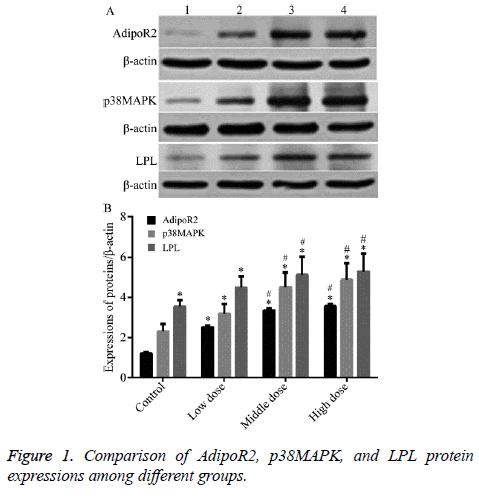
1. Compared with Group CON, the gene and protein expressions of AdipoR2, p38MAPK, and LPL in different 1,25(OH)2D3-treatment groups were significantly increased (T=29.256, 34.187, 35.274; 29.614, 35.162, 36.371; 30.081, 35.725, 36.932, P<0.05); 2. Compared with group L-dose, the gene and protein expressions of AdipoR2, p38MAPK, and LPL in group M- and H-dose were significantly increased (T=25.156, 29.187, 30.673; 25.772, 29.657, 31.134, P<0.05); 3. Compared with group M-dose, the gene and protein expressions of AdipoR2, p38MAPK, and LPL in Group H-dose were increased, but no significant difference can be found (P>0.05) (Tables 2 and 3 and Figure 1).
| Group | AdipoR2 | p38MAPK | LPL | TG |
|---|---|---|---|---|
| CON | 2.20 ± 0.10 | 2.30 ± 0.57 | 3.54 ± 0.32 | 532.17 ± 57.23 |
| L-dose | 3.51 ± 0.29* | 4.17 ± 0.49* | 4.48 ± 0.38* | 427.18 ± 50.85* |
| M-dose | 5.35 ± 0.38*# | 5.59 ± 0.62*# | 6.12 ± 0.49*# | 341.26 ± 43.13*# |
| H-dose | 5.75 ± 0.40*# | 5.87 ± 0.72*# | 6.51 ± 0.53*# | 331.72 ± 40.83*# |
Table 2. Comparison of AdipoR2, p38MAPK, LPL gene expression and TG levels among different groups (͞x ± s).
| Group | AdipoR2 | p38MAPK | LPL |
|---|---|---|---|
| CON | 1.20 ± 0.06 | 2.78 ± 0.67 | 3.68 ± 0.33 |
| L-dose | 2.51 ± 0.10* | 3.17 ± 0.91* | 4.35 ± 0.58* |
| M-dose | 3.35 ± 0.15*# | 4.50 ± 1.22*# | 5.02 ± 0.89*# |
| H-dose | 3.55 ± 0.17*# | 4.87 ± 1.33*# | 5.27 ± 0.92*# |
Table 3. Comparison of AdipoR2, p38MAPK, LPL protein expression among different groups (͞x ± s).
Contents of TG
1. Compared with Group CON, the TG contents in different 1,25(OH)2D3-treatment groups were significantly decreased (T=21.249, 24.631, 25.285, P<0.05); 2. Compared with Group L-dose, the TG contents in Group M- and H-dose were significantly decreased (T=21.242, 22.174, P<0.05); 3. Compared with group M-dose, the TG content in group H-dose was decreased, but no significant difference can be found (P>0.05) (Table 2).
Discussion
In addition to regulating the in vivo Ca and bone mineral balance, VD can also participate in the processes of body inflammation, autoimmune response, or synthesis and secretion of insulin [1]. Studies have shown that patients with type 2 diabetes exhibit significantly increased sensitivity of insulin and significantly improved blood lipid metabolism with the elevation of in vivo VD [7-9]. Furthermore, VD can effectively prevent the occurrence and development of type 2 diabetes [10]. The liver is the target organ of in vivo insulin, and plays important roles in maintaining stable blood glucose and IR formation. IR promotes a large number of free fatty acids to enter the liver, in which they are activated to acyl CoA and form TG through combining with glucosylated phosphonate glycerol; however, there has been no report about the specific mechanism of VD’s regulatory roles against TG currently. The results of this study show that 1,25(OH)2D3 has no significant effect on cholesterol in the HepG2 cells, but the TG content is significantly decreased. The mechanism may be explained as that active VD promotes the lipid transfer and reduces the accumulation of TG in liver tissues, consistent with related foreign research [11]. However, whether VD affects the level of TG in the liver through regulating the expressions of such lipid metabolism related genes as AdipoR2, p38MAPK, or LPL has not been reported in China or abroad yet, so this study observed the impact of different concentrations of 1,25(OH)2D3 on the levels of AdipoR2, p38MAPK, LPL, and TG in the HepG2 cells so as to explore the regulatory mechanism of 1,25(OH)2D3 toward TG in the HepG2 cells.
A large number of studies have shown that as an important member of the MAPK family, the p38MAPK signaling pathway not only plays important roles in inflammation and stress response but also is involved in cell survival, differentiation, and apoptosis, so it’s considered as the transduction x station of a large number of intracellular pathways. IR, high levels of free fatty acids, and inflammatory cytokines can all activate the p38MAPK signaling pathway [12]. The activation of the p38MAPK signaling pathway can increase the level of Adiponectin (APN), which then plays the role of reducing liver TG content by binding to AdipoR2 [13]. Studies have shown that high-fat high-energy diet can promote the expression of AdipoR2 in rat liver tissue [14], thus leading to dyslipidemia [15]. The changes of AdipoR2 may be involved in liver fat deposition and IR formation [16], and the AdipoR2 polymorphic receptors are significantly associated with the accumulation of liver TG [17,18]. On the other hand, the p38MAPK signaling pathway can activate the Peroxisome Proliferator-Activated Receptor-α (PPAR-α), and the latter plays an important role in the regulation of lipid metabolism. LPL, involved in the transportation and oxidation of fatty acids, is a target gene of PPAR-α. PPAR-α regulates the expression of LPL [19], and as one hydrolase of TG, LPL is one of the key enzymes toward lipid metabolism and mainly hydrolyzes TG in Chylomicrons (CM) and Very Low Density Lipoprotein (VLDL). The structure and activity abnormalities of LPL can lead to TG-based lipid metabolism disorders, followed by subsequent glucose metabolism disorders and IR [20,21]. At the same time, animal experiments using LPLknocked heterozygous mice have shown that: with age increase, glucose and lipid metabolism disorders in mice increase, and IR increases significantly [22]. Therefore, TG metabolism may be related to the expressions of AdipoR2, p38MAPK, and LPL in the liver.
The results of this study have shown that 1,25(OH)2D3 can increase the expressions of AdipoR2, p38MAPK, and LPL and decrease the TG content in the HepG2 cells in a dosedependent manner. When the 1,25(OH)2D3 concentration was increased from low to middle dose, the expressions of AdipoR2, p38MAPK, and LPL are increased gradually, while the TG content was decreased gradually. However, there was no significant difference between the high dose (50 ng•ml-1) and the middle dose, suggesting that 1,25(OH)2D3 can reduce the TG content in the hepatocytes in a dose-dependent manner. The mechanism of 1,25(OH)2D3 reducing the liver TG content may be achieved by upregulating the expressions of AdipoR2, p38MAPK, and LPL, but the specific mechanism still needs further investigation.
Acknowledgements
This study were supported by the National Natural Science Foundation of China (81460168), Projects of Guizhou Science and Technology Foundation (Qiankehezi J LKZ (2013) No. 19) and PhD Starting Foundation of Zunyi Medical College ((2012) F-574).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol 2014; 5: 151.
- Sun X, Cao ZB, Tanisawa K, Ito T, Oshima S, Ishimi Y, Tabata I, Higuchi M. Associations between the serum 25(OH)D concentration and lipid profiles in Japanese men. J Atheroscler Thromb 2015; 22: 355-362.
- Jungert A, Roth HJ, Neuhäuser-Berthold M. Associations of serum 25-hydroxycholecalciferol and parathyroid hormone with serum lipids differ by sex and VD status. Public Health Nutr 2015; 18: 1684-1691.
- Wang Y, Si S, Liu J, Wang Z, Jia H, Feng K, Sun L, Song SJ. The associations of serum lipids with vitamin D status. PLoS One 2016; 11: 0165157.
- Su D, Nie Y, Zhu A, Chen Z, Wu P, Zhang L, Luo M, Sun Q, Cai L, Lai Y, Xiao Z, Duan Z, Zheng S, Wu G, Hu R, Tsukamoto H, Lugea A, Liu Z, Pandol SJ, Han YP. VD signaling through induction of paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models. Front Physiol 2016; 7: 498.
- Ning C, Liu L, Lv G, Yang Y, Zhang Y, Yu R, Wang Y, Zhu J. Lipid metabolism and inflammation modulated by VD in liver of diabetic rats. Lipids Health Dis 2015; 14: 31.
- Gandhe MB, Jain K, Gandhe SM. Evaluation of 25(OH) Vitamin D3 with Reference to Magnesium Status and Insulin Resistance in T2DM. J Clin Diagn Res 2013; 7: 2438-2441.
- Shaat N, Iqnell C, Katsarou A, Berntorp K. Glucose homeostasis, beta cell function, and insulin resistance in relation to vitamin D after gestational diabetes mellitus. Acta Obstet Gynecol Scand 2017; 96: 821-827.
- Mohamad MI, EI-Sherbeny EE, Bekhet MM. The effect of VD supplementation on glycemic control and lipid profile in patients with type 2 diabetes mellitus. J Am Coll Nutr 2016; 35: 399-404.
- Xuan Y, Zhao HY, Liu JM. Vitamin D and type 2 diabetes mellitus (D2). J Diabetes 2013; 5: 261-267.
- Kasapoglu B, Turkay C, Yalcin KS, Carlioglu A, Sozen M, Koktener A. Low VD levels are associated with increased risk for fatty liver disease among non-obese adults. Clin Med (Lond) 2013; 13: 576-579.
- Ng RC, Cheng OY, Jian M, Kwan JS, Ho PW, Cheng KK, Yeung PK, Zhou LL, Hoo RL, Chung SK, Xu A, Lam KS, Chan KH. Chronic adiponectin deficiency leads to Alzheimers disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol Neurodegener 2016; 11: 71.
- Santamarina AB, Oliveira JL, Silva FP, Carnier J, Mennitti LV, Santana AA, de Souza GH, Ribeiro EB, Oller do Nascimento CM, Lira FS, Oyama LM. Green tea extract rich in epigallocatechin-3-gallate prevents fatty liver by AMPK activation via LKB1 in mice fed a high-fat diet. PLoS One 2015; 10: 0141227.
- Park PH, Sanz-Garcia C, Nagy LE. Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in the liver. Curr Pathobiol Rep 2015; 3: 243-252.
- Holland WL, Xia JY, Johnson JA, Sun K, Pearson MJ, Sharma AX, Quittner-Strom E, Tippetts TS, Gordillo R, Scherer PE. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol Metab 2017; 6: 267-275.
- Ma Y, Liu D. Hydrodynamic delivery of adiponectin and adiponectin receptor 2 gene blocks high-fat diet-induced obesity and insulin resistance. Gene Ther 2013; 20: 846-852.
- Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc Diabetol 2014; 13: 103.
- Ma H, You GP, Zhang XP, Yang XJ, Lu HD, Huang YL, Zhang WQ. A novel role of globular adiponectin in treatment with HFD/STZ induced T2DM combined with NAFLD rats. Sci World J 2014; 2014: 230835.
- Deehan R, Maerz-Weiss P, Catlett NL, Steiner G, Wong B, Wright MB, Blander G, Elliston KO, Ladd W, Bobadilla M, Mizrahi J, Haefliger C, Edgar A. Comparative transcriptional network modeling of three PPAR-α/γ co-agonists reveals distinct metabolic gene signatures in primary human hepatocytes. PLoS One 2012; 7: 35012.
- Blaak EE. Characterisation of fatty acid metabolism in different insulin-resistant phenotypes by means of stable isotopes. Proc Nutr Soc 2017; 1-7.
- Olivecrona G. Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol 2016; 27: 233-241.
- Li DD, Su DY, Xue L, Gao W, Pang WY. Relationship between a lipoprotein lipase gene polymorphism in placental tissue and insulin resistance in patients with gestational diabetes mellitus. Genet Mol Res 2015; 14: 7751-7758.