Research Article - Biomedical Research (2022) Volume 33, Issue 4
Identifying potential adverse effects in development and maintenance of masticatory system due to tooth loss.
Mohammed Enamur Rashid1,2*, Khaleda Akhter3,4
1Department of Oral Basic and Clinical Sciences, College of Dentistry, Taibah University, Al Madinah Al Munawara, Kingdom of Saudi Arabia
2Department of Oral Growth and Developmental Biology, Hiroshima University, School of Dentistry, Hiroshima, Japan
3Department of Periodontology and Oral Pathology, Pioneer Dental College and Hospital, Dhaka University, Dhaka, Bangladesh
4Department of Oral and Maxillofacial Surgery, Division of Cervico-Gnathostomatology, Hiroshima University, School of Dentistry, Hiroshima, Japan
- Corresponding Author:
- Mohammed Enamur Rashid
Department of Oral Growth and Developmental Biology
Hiroshima University
School of Dentistry
Hiroshima, Japan
E-mail: mrashid@taibahu.edu.sa
Accepted on April 20, 2022
Abstract
Many investigations have been conducted previously on the association of different factors with low mastication ability. In the similar context, the current study aims to examine the adverse impact in developing and maintaining masticatory system in individuals with tooth loss. Pregnant female mice were included in the study and experiments were conducted on both the tooth extracted mice (experimental) and mice with intact teeth (control) on 30th, 60th, 90th, 120th, 180th, and 360th following their birth. There was no substantial difference in the mean body weights of the mice in both classes, according to the findings. Furthermore, in the test group of mice, there was no substantial difference in the number of named Me5 and TG neurons on the right and left sides. There was a substantial decrease in the number of labelled Me5 and TG neurons in the tooth extracted mice from the 60th to the 360th postnatal day. On the 40th day, there were no degenerative fibres on the intact side of the tooth extracted mouse, but normal degenerative changes were present in the axons and myelin sheaths on the tooth extracted side. The study concluded masticatory system’s development and maintenance involves sensory input from periodontal mechanoreceptive afferents.
Keywords
Mastication, Neurons, Tooth loss.
Introduction
Optimum cognition status is essential for quality of life regardless of age factor. Cognitive changes occur as the age increases and are often associated with certain extrinsic and intrinsic risk factors [1,2]. Considering the neurocognitive disorders, the cognitive changes are associated with memory, reasoning, learning, thinking, and judgmental abilities [3]. Severe cognitive impairment in an individual causes a serious negative impact on his professional and social life [4]. It is believed that proper cognitive level can be maintained through social engagements, education and physical exercises [5].
Optimal mastication is known as one of the factors responsible for preserving the cognitive function besides its main role in the intake and swallowing of food [6]. The impaired masticatory function triggered by either functional factors (poor masticatory performance or weak biting force) or structural factor (tooth loss) is termed as masticatory dysfunction [7,8]. This may result in the loss of periodontal support that leads to loss of tooth movement [9]. It has been shown that in older individuals (with less than 20 teeth), the masticatory ability scores and biting force decrease significantly [10]. In this context, Ikebe et al. [7] stated that tooth loss and inappropriate mastication are significantly associated with each other.
It is believed that periodontal disease is caused by the relationship between number of teeth and presence of a serious inflammatory disease. Consequently, periodontal disease may lead to multiple tooth loss. There is a negative impact of inflammatory cytokines and periodontal disease on the blood vessels of the heart and brain. A study has reported prevalence of carotid plaque in individuals who have lost 10 or more teeth [11]. It is obvious that individuals suffer from insufficient nutrient intake due to their low masticatory ability. Hajimohammadi et al. [12] showed that edentulous individuals suffer from the deficiency of plasma vitamin C that has antioxidant activity. This clarifies that it is reasonable to expect that elderly people suffering from low masticatory ability due to multiple tsooth loss are undernourished.
Researchers have conducted systematic reviews that focused on the association of different factors with low mastication ability on the individuals [13,14]. However, these studies failed to focus on the adverse effects of low mastication ability among the individuals. Moreover, none of the recent researches have emphasized on the negative impact of masticatory system development on individuals with lost teeth. Therefore, the current study tries to reduce the heterogeneity by focusing on the adverse effects of developing and maintaining masticatory system on the individuals with lost teeth.
Materials and Methods
Study subjects and experiment
The study included pregnant female mice, which were alternatively kept in darkness and light for 12 hours. On the day 5 after the birth, the litter was reduced to 10 male pups. Consequently, on day 20, lower and upper molars were extracted unilaterally. Afterwards, experiments were conducted on both the tooth extracted mice (experimental) and mice with intact teeth (control) on 30th, 60th, 90th, 120th, 180th, and 360th day following their birth to assess the impact of loss of tooth on the masticatory neurons.
Ethical consideration
Both laboratory operations, including the treatment and use of animals for experimental procedures, were carried out in compliance with Hiroshima University’s Laboratory Animal and Testing Facilities’ guidelines.
Horseradish peroxidase tracing methods
The method of Horseradish Peroxidase-Wheat Germ Agglutinin (HRP-WGA) tracing was used for explaining the role of periodontal afferent in developing and maintaining the masticatory system in mice between 30th and 360th day following their birth. For this purpose, mice of both the control and the experimental groups (each group comprised 6 mice) were selected and HRP-WGA method was applied on the 30th, 60th, 90th, 120th, 180th and 360th postnatal day. Each animal was given anaesthesia through an intra-peritoneal injection of sodium pentobarbital after measuring their body weight (0.06 mg/g). The bilateral Inferior Alveolar Nerves (IAN) were exposed in the anterior side of the mandibular foramen through the surgical procedure.
A solution of 5% HRP-WGA (Toyobo, Osaka, Japan) was injected with the help of glass. The solution, into each nerve bundle, was entrapped in a 1% hypoallergenic polyacrylamide gel. 200 ml of heparinized saline was perfused after 24 hours in deeply anesthetized animals through the ascending aorta. This was followed by immediate perfusion of 300 ml of fixative (1% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer) having pH of 7.4. Later, the fixative was flushed out with the help of 200 ml of 10% sucrose in 0.1 M phosphate buffer having pH of 7.4. The trigeminal ganglion was stored in solution of 30% sucrose in phosphate buffer with pH of 7.4 at 4ºC.
50 μm thick transverse frozen sections were cut serially and it was later processed based on the Tetramethylbenzidine (TMB) protocol [15]. Light microscope (AX70; Olympus, Tokyo, Japan) was used for the examination of these sections and count the total number of labelled neurons in Mesencephalic trigeminal nucleus (Me5) and Trigeminal Ganglion (TG). Camera lucida was used for shaping each labelled cell with clear nucleus in order to avoid double counting. The obtained data was analyzed to show the differences through t-test analysis with significance value <0.05
Electron microscope observations
Following the birth of mice, they were being perfused with solution of 0.25% glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer with pH 7.4 on the 40th day. The inferior alveolar nerves were removed bilaterally as they got exposed to mandibular foramen posteriorly. Later, the nerves were being immersed for 24 hours in the same fixative. 1% of Osmium tetroxide was used for post fixing the blocks of tissues. The staining of these tissues was done using 1% aqueous uranyl acetate that was embedded in Epon 812 and dehydrated with propylene oxide and ethanol. The ultra-thin sections were stained with the help of lead citrate and uranyl acetate, and were being examined under electron microscope (JEM- 1010; JEOL, Japan).
Results
Post-natal changes in mean body weight
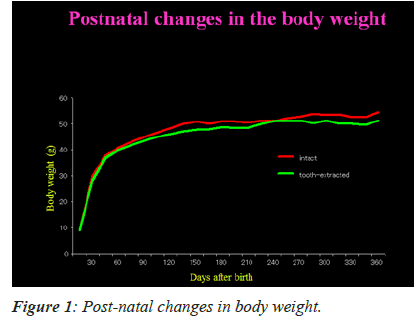
Figure 1 shows post-natal changes in the mean body weights of mice with intact and extracted tooth. The figure depicts that there is gradual increase in body weight between 15th and 360th day. During this time, however, there was no substantial change in the mean body weights of mice in both the groups. This specifically indicates that unilateral tooth extraction has no dietary effects in the mice tested.
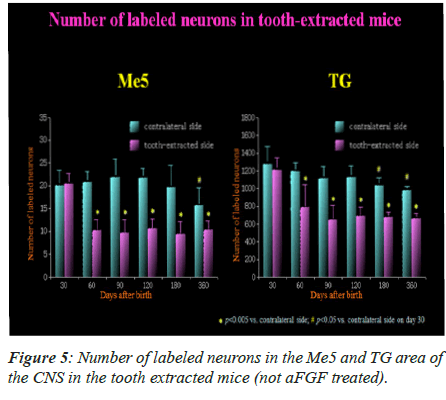
Labeled Me5 and TG Neurons
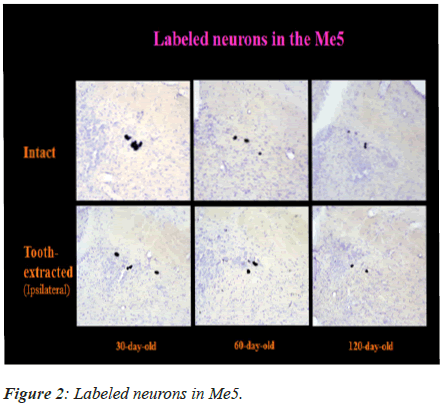
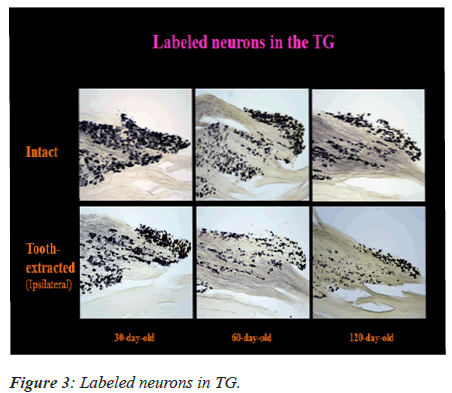
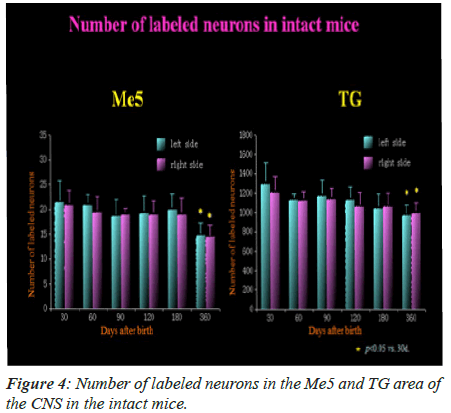
The labeled neurons in Me5 and TG are shown in Figures 2 and 3 in both groups of mice, respectively. There was no significant difference in the numbers of labeled Me5 and TG neurons throughout the time periods between right and left sides (Figures 4A and 4B). However, significant reduction in the number of Me5 and TG neurons were observed in the aged mice on the 360th day in comparison to 30-day old mice. Again, significant decreases were observed in total of the labelled Me5 and TG neurons as shown in Figures 5A and 5B respectively in tooth- extracted mice while comparing the tooth extracted side with the intact side. Figures 4 and 5 show the total number of neurons in Me5 and TG in mouse on the contralateral side, which was not different from the mice with intact teeth. After the 60th day, significant differences were noted in the total number of labeled Me5 and TG neurons in CNS of the tooth extracted mice while comparing the tooth extracted side with the intact side in the same mouse (Figures 5A and 5B).
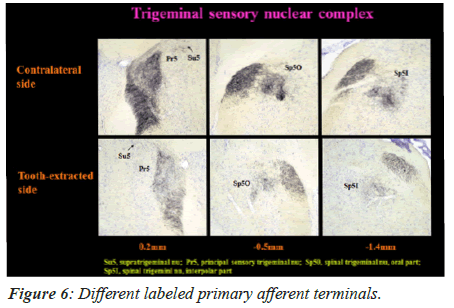
Labeled primary afferent terminals
On 60th day, the primary afferent terminals that were labeled in Spinal trigeminal nucleus interpolar part (Sp5I), Spinal trigeminal nucleus oral part (Sp50), Principal sensory trigeminal nucleus (Pr5), and Supra-trigeminal nucleus (Su5) are shown in Figure 6.
In the experimental mice group, the primary afferent terminals disappeared on the tooth extracted side on the 60th day, unlike the intact side. No significant difference was noted in the labeled terminals between the contralateral side in tooth-extracted mice and both sides (ipsilateral side and contralateral side) in mice with intact tooth.
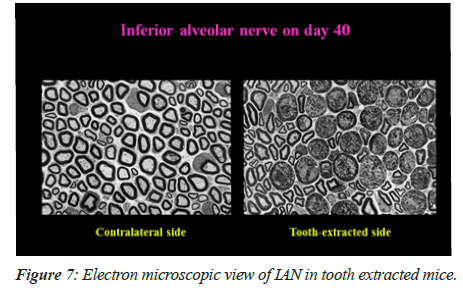
Electron microscopy of IAN
On 40th day, there was observance of majority of the degenerated fibers in the tooth extracted mice. Figure 7A shows that there was no degenerative fiber on the contralateral side (i.e. intact tooth side) and degenerative changes are being shown in Figure 7B in the axons and myelin sheaths on the tooth extracted side.
Discussion
During some stages of animal development, sensory feedback from oro-facial sensory receptors plays a significant role in regulating the formation of the masticatory system. In mice with unilateral molar extraction and Osteopetrotic (op/op) mice with no teeth extraction, periodontal ligaments are likely to be less developed. The sensory feedback from the PMRs also controls the formation and preservation of the jaw closing muscle in animals. Suemune et al. [16] found that the number of jaws closing motoneurons innervating the masseter muscle decreased dramatically, along with a substantial decline in masticatory sensory neurons. As a result, sensory feedback from the PMRs is thought to play a key role in the postnatal growth of the masticatory system and the maintenance of homeostatic conditions for jaw movement control.
The current research shows severe IAN degeneration in tooth-extracted experimental mice before a substantial decrease in the number of neurons in Me5 and TG, as well as primary afferents in the TSNC. However, the number of primary afferents and neurons was significantly reduced in aged mice. A similar study by Byers and Dongs [17] reported that Me5 and TG neurons innervate the mechanoreceptors in periodontal ligaments. Another study conducted by Shigenaga et al. [18] showed that main projection of Me5 neurons’ central axons towards Su5. Su5 holds excitatory neurons and inhibitory neurons, considering the jaw closing motoneurons. This clearly suggests that both tooth extracted and aged mice suffer from disorder of jaw closing activity.s
The terminals to TSNC are formed from the primary afferents originating from TG, which further project to Mo5 through Sp50 [19]. The study further showed that primary afferents of intra-oral structure are received by dorsomedial part of Sp50; while, their neurons have widely spread axons and dendrites that project towards Mo5 [18]. The formation of di-or multi-synaptic segmental reflex pathway relates to Sp50 neurons that receive periodontal Me5 input. The presence of interneurons in rostrodorsomedial part of the Sp50 that corresponds to jaw opening and closing of motor neurons in Mo5 was investigated by Yoshida et al. [20]. The significant decrease in TG neurons was reported by Suemune et al. [16] after injecting HRP into the IAN. According to the findings, the decline in Sp50 terminals in the osteopetrotic mouse arises as a consequence of the normal decrease in sensory feedback to Sp50 through TG from periodontal sensory endings. Similar results have been deduced by the experimental model in the current study for labeled primary afferents in TSNC. These results clearly show that normal individuals may also suffer from severe disorder of mastication induced by tooth loss. Kobayashi et al. [21] stated that formation of masticatory rhythm is not inhibited in osteopetrotic mice, as normal masticatory rhythm can develop in normal animal as early as the weaning period.
Conclusion
The current study has provided a stabilized model to examine the survival and death of sensory neurons with progressing age and injured periodontal mechanoreceptive afferents after tooth loss. The sensory feedback from periodontal mechanoreceptive afferents is specifically suggested in developing and maintaining the masticatory system. Many investigations have been conducted previously on the association of different factors with low mastication ability.
Declarations
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interest
The author declares no competing interest.
Funding
This research is not funded by any resource.
Acknowledgement
The author is very thankful to all the associated personnel in any reference that contributed in/for the purpose of this research.
References
- Harada CN, Love N, Triebel K. Normal cognitive aging. Clin Geriatr Med 2013; 29: 737-752.
[Crossref] [Google Scholar] [PubMed]
- Marchesi VT. Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative‐induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. FASEB J 2011; 25: 5-13.
[Crossref] [Google Scholar] [PubMed]
- Murman DL. The impact of age on cognition. Semin Hear 2015; 36: 111-121.
[Crossref] [Google Scholar] [PubMed]
- Hugo J, Ganguli M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin Geriatr Med 2014; 30: 421-442.
[Crossref] [Google Scholar] [PubMed]
- Campbell NL, Unverzagt F, LaMantia MA, Khan BA, Boustani MA. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med 2013; 29: 873-893.
[Crossref] [Google Scholar] [PubMed]
- Teixeira FB, Fernandes LD, Noronha PA, dos Santos MA, Gomes-Leal W, Maia CD, Lima RR. Masticatory deficiency as a risk factor for cognitive dysfunction. Int J Med Sci 2014; 11: 209.
[Crossref] [Google Scholar] [PubMed]
- Ikebe K, Matsuda KI, Kagawa R, Enoki K, Okada T, Yoshida M, Maeda Y. Masticatory performance in older subjects with varying degrees of tooth loss. J Dent 2012; 40: 71-76.
[Crossref] [Google Scholar] [PubMed]
- Lin CS. Revisiting the link between cognitive decline and masticatory dysfunction. BMC Geriatr 2018; 18: 1-4.
[Crossref] [Google Scholar] [PubMed]
- Kosaka T, Ono T, Yoshimuta Y, Kida M, Kikui M, Nokubi T, Maeda Y, Kokubo Y, Watanabe M, Miyamoto Y. The effect of periodontal status and occlusal support on masticatory performance: The suita study. J Clin Periodontol 2014; 41: 497-503.
[Crossref] [Google Scholar] [PubMed]
- Hashimoto M, Yamanaka K, Shimosato T, Ozawa A, Takigawa T, Hidaka S, Sakai T, Noguchi T. Oral condition and health status of elderly 8020 achievers in aichi prefecture. Bull Tokyo Dent Coll 2006; 47: 37-43.
[Crossref] [Google Scholar] [PubMed]
- Zeng XT, Leng WD, Lam YY, Yan BP, Wei XM, Weng H, Kwong JS. Periodontal disease and carotid atherosclerosis: A meta-analysis of 17,330 participants. Int J Cardiol 2016; 203: 1044-1051.
[Crossref] [Google Scholar] [PubMed]
- Hajimohammadi M, Mansouri S, Eimery S, Saadat SZ, Davallou P, Shab-Bidara S, Neyestani TR. A posteriori dietary patterns are related to C-reactive protein levels: Results from a systematic review and meta-analysis. J Nutr Sci Diet 2017; 14: 35-48.
- Chen X, Fu F. Social learning of prescribing behavior can promote population optimum of antibiotic use. Front Phys 2018; 6: 139.
- Tada A, Miura H. Association between mastication and cognitive status: A systematic review. Arch Gerontol Geriatr 2017; 70: 44-53.
[Crossref] [Google Scholar] [PubMed]
- Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 1978; 26: 106-117.
[Crossref] [Google Scholar] [PubMed]
- Suemune S, Maeda N. Reduction in the number of motor and sensory neurons that are involved in the jaw-opening reflex in the toothless (op/op) mouse. Biomed Res 1994; 15: 263-269.
- Byers MR, Dong WK. Comparison of trigeminal receptor location and structure in the periodontal ligament of different types of teeth from the rat, cat, and monkey. J Comp Neurol 1989; 279: 117-127.
[Crossref] [Google Scholar] [PubMed]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol 1988; 268: 489-507.
[Crossref] [Google Scholar] [PubMed]
- Tsuru K, Otani K, Kajiyama K, Suemune S, Shigenaga Y. Central terminations of periodontal mechanoreceptive and tooth pulp afferents in the trigeminal principal and oral nuclei of the cat. Brain Res 1989; 485: 29-61.
[Crossref] [Google Scholar] [PubMed]
- Yoshida A, Fukami H, Nagase Y, Appenteng K, Honma S, Zhang LF, Bae YC, Shigenaga Y. Quantitative analysis of synaptic contacts made between functionally identified oralis neurons and trigeminal motoneurons in cats. J Neurosci 2001; 21: 6298-6307.
[Crossref] [Google Scholar] [PubMed]
- Kobayashi M, Masuda Y, Kishino M, Ishida T, Maeda N, Morimoto T. Characteristics of mastication in the anodontic mouse. J Dent Res 2002; 81: 594-597.
[Crossref] [Google Scholar] [PubMed]