Research Article - Biomedical Research (2017) Volume 28, Issue 11
Hydrochloric acid treated polyurethanes for temporary blood contacting biomaterials applications
Saravana Kumar Jaganathan1,2,3* and Mohan Prasath M31Department for Management of Science and Technology Development, Ton Duc Thang University, Ho Chi Minh City, Vietnam
2Faculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, Vietnam
3Faculty of Biosciences and Medical Engineering, IJNUTM Cardiovascular Engineering Centre, Universiti Teknologi Malaysia, Johor Bahru, Malaysia
- *Corresponding Author:
- Saravana Kumar Jaganathan
Department for Management of Science and Technology
Development
Ton Duc Thang University,Vietnam
Email: saravana@tdt.edu.vn
Accepted on April 9, 2017
Abstract
This research paper investigated the surface characterization and blood compatibility of polyurethane surface modified with Hydrochloric Acid (HCl) treatment for 30 min and 60 min respectively. The surface modified polyurethane with HCl shows increase contact angle and resulted in well-defined structure with unique orientation evident by SEM study. To evaluate the effect of acid treatment on the coagulation cascade, Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) were measured. The HCl treated PU showed increasing Prothrombin Time (PT) and Activated Partial Thrombin Time (APTT) implying improved blood compatibility of the surfaces. The results of Hemolysis assay of the treated surface showed less number of damaged RBC’s compared to control. Compared to control the number of platelet adhesion on the surface of surface modified PU was found less and thereby reduce the chances of activation of blood coagulation cascade suitable for blood contacting biomaterial applications.
Keywords
Polyurethane, HCl treatment, Blood compatibility, Blood contacting biomaterials.
Introduction
Polyurethanes are an important class of biomaterials frequently used in various biomedical applications due to its excellent physical and mechanical properties, and promising blood compatibility [1]. Much effort has been focused on polyurethanes as blood-contacting materials, such as cardiovascular implants, Central Venous Catheters (CVC), hemodialysis blood line sets and IV bags [2,3]. Recently, Polyurethane materials are also used coatings in artificial heart and vascular grafts [4]. Segmented polyurethane synthesized from various polyols, diisocyanates and chain extenders are structurally manipulated to attain an extensive variety of properties for various biomedical applications [5] and its unique combination of physicomechanical properties and degradability makes it a great deal in cardiovascular applications [6].
The surface of the biomaterial that comes in contact with the blood plays a crucial role in the case of blood contacting applications, and it is therefore of interest to modify the surface of the biomaterial in order to enhance its blood compatibility. Events occurring within minutes of contact with blood are protein adsorption, cell adhesion and inflammation leading to thrombus formation and fibrinolysis. Longer exposure leads to embolism and change in biomaterial properties [7,8]. Past studies have shown that the small scale well defined orientation on the materials surface can induce a number of cellular reactions including orientation of cell growth, cell attachment, cell activation and influence of the growth rate [9]. Surface wettability and surface cell adhesion are other two important factors that enhance the biocompatibility and also interactions between material and surroundings [10,11].
A number of surface modification techniques are done in order to change the chemical nature and the morphology of the polymer surface. Some of the commonly used techniques are chemical and mechanical methods, grafting co-polymerization [12], plasma treatment [13], UV and laser irradiation [14], dielectric discharge [15] and microwave plasma irradiation [16]. These techniques require extensive investments and also occupy large spaces. Hence there is a need for cost effective and simple procedures. In this work, we have investigated the use of HCl as a tool for surface modification. HCl is a commonly available laboratory chemical with unique benefits like easy storage, versatile and portable compared to the above mentioned conventional methods.
Literature studies showed a number of researchers have worked on the acid induced reactions on polymer surfaces in order to improve the blood compatibility [17-20]. In this study, the surface of the polyurethane was treated with hydrochloric acid and its surface was carefully examined for any surface changes.
Materials and Methods
Materials
The Pellethane® 2102-90A polyurethane was purchased from LubriZol, USA. The obtained polyester polycaprolactone based polyurethane possess excellent resistance to fuels and oils with low compression set and good hydrolytic stability compared to other polyester based TPUs [21]. The commercially available HCl was obtained from Merck, Malaysia.
Sample preparation and acid treatment
Samples of polyurethane where cut in the measurements 1 cm × 1 cm and washed with 70% ethanol and distilled water thoroughly to clean the surface of the polymer from impurities. Hydrochloric acid of 5-6 ml is pipetted out into a petridish and the polymer is placed in the acid for 30 min to 120 min in a sterilized environment. Later, the samples were removed and incubated with physiological saline at 37ºC to remove the unwanted the acid attached to the surface of the polymer, before being used for surface characterization and blood compatibility tests.
Surface characterization
The prepared samples were subjected for surface characterization tests - Contact angle and SEM in order to define the change in surface wettability and microstructure that have taken place on the polymer.
Contact angle measurement: The contact angle measurements were carried out in a Dynamic contact angle Tensiometer-Dataphysics DCAT 11. The contact angles were calculated automatically when the sample is placed inside the Tensiometer. Measurements were carried out for three samples- Control, 30 min acid treated and 1 h acid treated. The acceding, receding and mean contact angles were recorded and tabulated in the system interfaced with the equipment.
Scanning electron microscope (SEM): The microstructure changes of the treated (30 min and 1 h acid treated) and control polymers were observed with JEOL JSM 5800 SEM with OXFORD ISI 300 EDS X-ray Microanalysis System (CRF). The samples were studied at 1500X magnification after under the gold sputtering treatment on the samples.
Coagulation assays
The blood compatibility of a polymer or a biomaterial is defined by the four major tests namely Prothrombin Time (PT), Activated Partial Thromblastin Time (APTT), hemolysis ratio and platelet adhesion which reveals intrinsic pathway, extrinsic pathway, RBC damage count and platelet-polymer surface interactions respectively.
Prothrombin time (PT): Platelet poor plasma (PPP) (100 ml at 37°C) was applied on the surface of the control and treated substrates along with NaCl-thromboplastin (Factor III; 100 ml Sigma) containing Ca2+ ions. The time taken for the onset of fibrin clot was estimated using stopwatch and a steel hook (n=3) [22].
Activated partial thromblastin test (APTT): APTT is widely used to assess the ability of the blood to coagulate through the intrinsic pathway and to assess the effect of biomaterial on possible delay of the process. A drop of blood is placed on the surface of the polymer to which Platelet Poor Plasma (PPP) is incubated at 37°C with phophospholipid (cephalin) and a contact activator (e.g. Kaolin) is added followed by the calcium (all pre-warmed to 37°C). Addition of calcium initiates clotting and the timer is started. The time taken from the addition of calcium till the clot is formed is recorded as the Activated Partial Thromblastin Time (APTT) (n=3) [23].
Hemolysis ratio: Initially, a physiological saline of 0.9%w/v (37°C, 30 min) was prepared. The control and treated samples (30 min and 1 h) were equilibrated with the physiological saline for 30 min to remove the acid droplets present on the surface of the polymer. Later, it was incubated with 3 ml aliquots of citrated blood diluted with saline in the ratio 4:5 by volume for 60 min at 37°C. These mixtures were then centrifuged and the absorbance was measured at 540 nm. A mixture of blood and distilled water was prepared in the ration 4:5 by volume which was taken as positive control as complete hemolysis take place in it. Physiological saline is taken as the negative control. The absorbance of positive control was normalized to 100% and the absorbance of different samples was expressed as a percentage of hemolysis compared with their positive control [22,23].
Platelet adhesion: Freshly prepared Platelet Rich Plasma (PRP) was obtained from the Dindigul blood bank. The samples were treated in HCl for 30 min and 1 h and were incubated in NaCl and placed on the rotatory shaker for an hour to cleanse from the acid residue on the surface of the polymer. The control and treated samples were immersed in each 1 ml fresh PRP and were incubated at 37°C for an hour. PRP was decanted off and the membranes were rinsed with NaCl and dried. Later, the samples were viewed through the microscope. The surface of the polymer was photographed and numbers of platelets were counted on a region at 40X magnification [24].
Results and Discussion
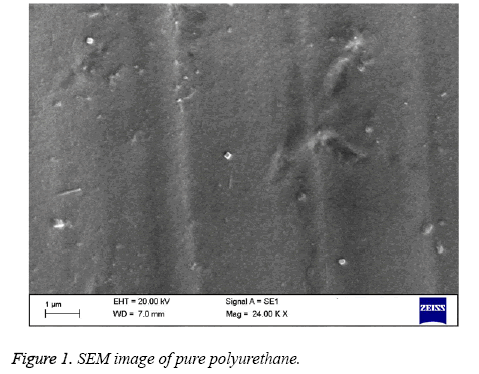
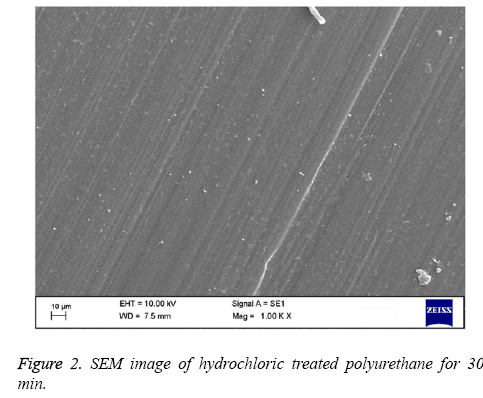
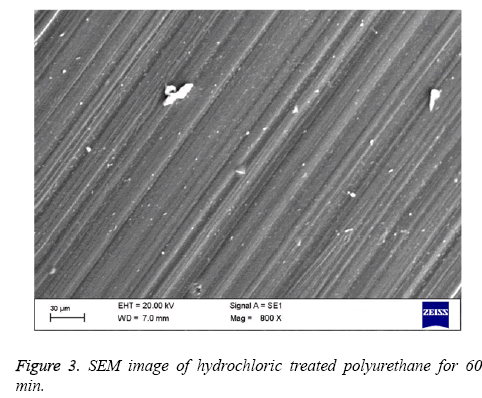
Polyurethane used for tissue engineering applications are the ones with polyester soft segment (e.g.: polycaprolactone) and others with polyether soft segment (e.g.: polyethylene glycol) [6,25]. Polyester soft segment polyurethane is hydrophobic in nature and shows slow degradation rates [6] but in case of mechanical properties, they show poor moduli and ultimate stress [26]. Ultimately, polyester soft segment polyurethanes have the right structural integrity for any tissue engineering scaffold formation. Hence in this work we have utilized polyester based polyurethane (Pellethane® 2102-90A). In order to enhance the blood compatibility feature, polymer surfaces should be modified with proper surface treatment methods. The polymer surface properties are mostly controlled by the chemical nature and morphology of the surface. The best approach for this major issue of interfacial blood compatibility between a synthetic prosthesis and blood is to modulate the blood/biomaterial interface [23]. The contact angle analysis shows that the hydrochloric treatment of polyurethane expressed the increase in contact angle indicating the treated polymer surface approaching to be hydrophobic. The Table 1 shows the hydrochloric treatment of polyurethane treated for 30 min and 60 min and compared with control. The treated polyurethane samples shows higher contact angle of 54.04 ± 1.95° for 30 min and angle of 61.19 ± 1.24° for 60 min while for control the contact angle found was 43.24 ± 1.71° respectively. On increasing the time of reaction we did not observe any significant improvement in the contact angle measurement. By increasing the HCl exposed times for 90 min and 120 min, contact angle measurement were performed. The contact angle was observed to be 58 ± 1.05° and 60 ± 1.91° respectively for 90 and 120 min exposed PU. Since there are no significant changes between the 60 min and the increasing times, we have utilized 30 min and 60 min treated polyurethane for blood compatibility and platelet adhesion studies. The hydrophobic nature of PU suggests that the polymer used is soft segment polyester which is used in various tissue engineering applications [25]. More over the past studies have proved that the PU used for tissue engineering applications is hydrophobic in nature as it contains polyester soft segment [6]. Change in contact angle proves that the treated sample has undergone morphological changes on its surface [27]. In case of surface wettability of polymeric materials, optimal cell adhesion has been reported to polymer surfaces having moderate wettability with WCAs 40°-70° [28-30]. The hydrophobic surface was found to improve the efficiency of Embryonic Stem (ES) cell differentiation [31]. All these studies show that the increase in the contact angle of polyurethane after surface treatment may result in improved biocompatibility of the samples and can be utilized for various blood contacting biomaterials. The Figures 1-3 indicates the SEM images of pure polyurethane and hydrochloric treated PU samples for 30 min and 60 min respectively. The microstructure analysis shows that the control sample has a rough surface with irregular orientations. While for hydrochloric acid treated samples have well-defined and proper structure with unique orientation pattern on its surface. The SEM study clearly proved that hydrochloric treatment seems to develop a smooth surface which implies the change in polymer surface with well-defined and unique orientation. Last works on polyurethane also show the similar kind of results i.e.) change of samples from rough surface with pores to smooth surface due to the improved compatibility of the hard and soft segments present in the polymer [32]. The surface characterization tests proved that a change has occurred to the polymer surface. In order to validate the surface is favorable for cardiovascular applications; various blood compatibility tests were continued.
| S. No | Sample | Average contact angle in degrees* |
|---|---|---|
| 1 | Pure Polyurethane | 43.24 ± 1.71 |
| 2 | Hydrochloric treatment of polyurethane for 30 min | 54.04 ± 1.95 |
| 3 | Hydrochloric treatment of polyurethane for 60 min | 61.19 ± 1.24 |
| 4 | Hydrochloric treatment of polyurethane for 90 min | 58 ± 1.05 |
| 5 | Hydrochloric treatment of polyurethane for 120 min | 60 ± 1.91 |
*Mean differences were significant compared with pure PU (p<0.05)
Table 1. Contact angle measurements of pure PU and hydrochloric treatment of PU for 30, 60, 90 and 120 min.
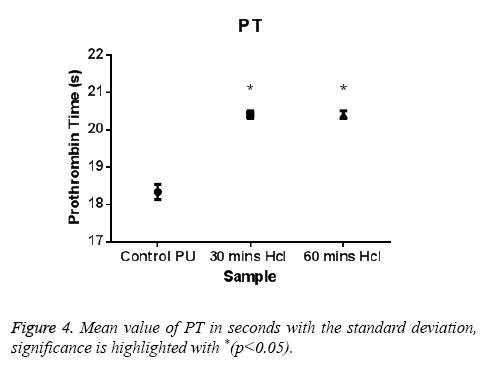
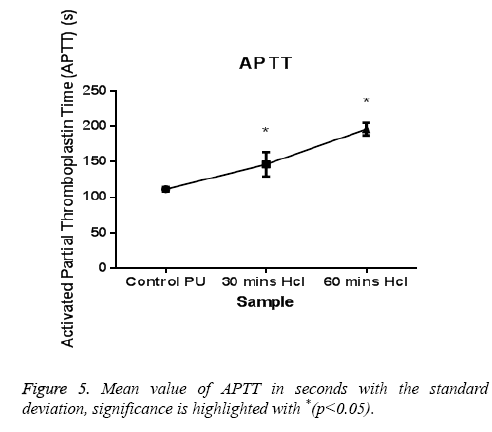
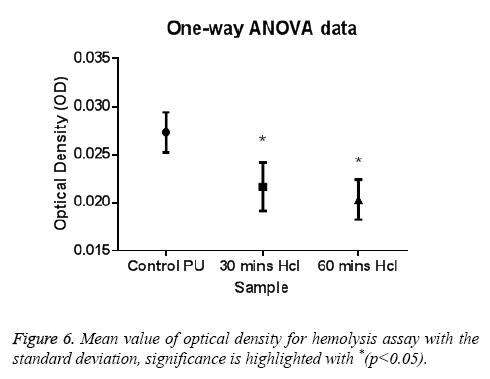
The PT and APTT, which reveals the intrinsic and extrinsic pathway respectively, showed a positive increase in time for the treated samples compared with the control. The results of both PT and APTT were summarized in Figure 4 and Figure 5. Prothrombin time was increasing from 18.3 sec for control to 20.4 sec for 30 min treated sample. The prothrombin time doesn’t have much significant change between 30 min treated and 1 h treated samples which suggest that the prothrombin time reaches an equilibrium from which it doesn’t increase or decrease. Whereas, APTT also showed an increase from 111 sec for control to 146 s for 30 min and an increase to 196 s in case of 1 h treated sample. Statistical analysis of the control sample with the treated ones using one-way ANOVA indicated significant differences (P<0.05) between them for both PT and APTT times. Further hemolysis assay defines the damage done to the RBC by the polymer. The decrease in OD value demonstrates the less number of damaged RBC’s in the treated samples compared to untreated samples. The results of hemolysis assay of hydrochloric treated samples and pure PU samples were summarized in Figure 6 and showed that the treated samples interact with the RBC in a much better fashion than the control.
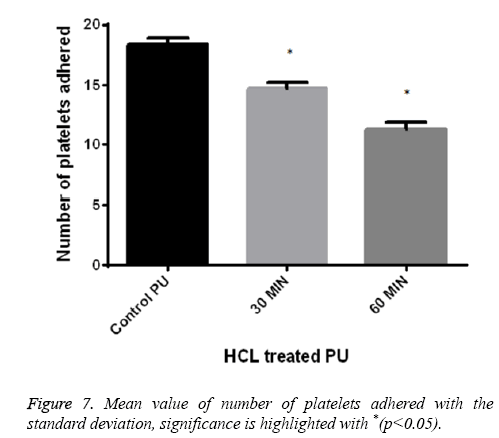
Platelet adhesion analysis shows that the number of platelets attached to the 60 min treated samples was found to be 11-12 and for 30 min treated samples it was observed 14-15 in number respectively. But for control the number of platelets attached to its surface was greater in number of about 18-20 and was represented in Figure 7. This signified that the treated surface of the polymer makes it adaptable for the RBC and platelet to interact with the surface.
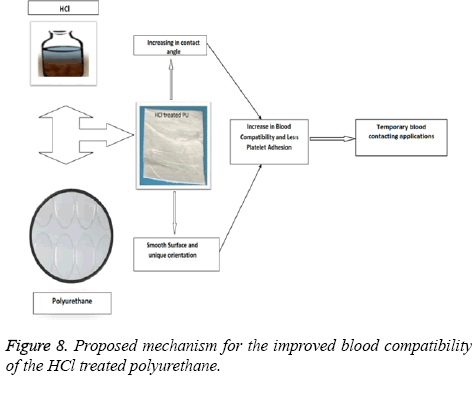
In this work we had found significant changes in the morphological properties like surface roughness and wettability as observed in the SEM and contact angle measurements. Although chemical nature of the surface may have an alleged role, it has been observed from our previous study that HCl treated mPE does not alter the chemical nature significantly [33]. Hence we surmise that the HCl treated PU may not have any significant changes in the chemical nature. Initially when the surface was exposed to the HCl it resulted in the smoother surface with unique orientations. This in turn causes the changes in the wettability of the surface. From our contact angle measurements it has been inferred HCl treated PU approaching to be hydrophobic. These changes may be attributed for the observed increase in the blood compatibility and decrease platelet adhesion. The above mechanism is illustrated in Figure 8. Jansen et al prepared porous polymeric scaffolds based on 1-vinyl-2-pyrrolidinone (NVP) and n-butyl methacrylate (BMA) and had reported that the prepared nanocomposites (50-50 wt%) showed hydrophobic nature and also enhanced the cell proliferation [34]. And in another study, Ajith et al. used Polyoxymethylene (POM) and hydroxyapatite nanoparticles (nHA) for preparing nanocomposites and was reported that the surface of developed nanocomposites was found to be hydrophobic and showed enhanced protein absorption compared to control. Since the HCl modified polyurethane showed hydrophobic nature which might favours the enhanced cell proliferation and protein absorption suitable for tissue engineering applications [35]. As a whole, hydrochloric acid treatment on the polyurethane under sterilized conditions have brought above surface changes in favourable for the use as cardiovascular implants or in tissue engineering.
Conclusion
To conclude, the polyurethane was treated with hydrochloric acid in this study. It was found that overall surface roughness of the hydrochloric acid treated polyurethane have decreased resulting in decrease in overall surface roughness. This was ascertained by surface characterization studies like contact angle and scanning electron microscope. Similarly, the blood compatibility of the polyurethane was also found to be improved determined by the blood compatibility assays like PT, APTT, hemolysis assay and platelet adhesion studies.
Acknowledgements
This work was supported partly by the Ministry of Higher Education Malaysia with the Grant Number: Q.J130000.2545.12H80.
References
- Bret DU, Lakshmi SN, Cato TL. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys 2011; 49: 832-864.
- Zhu Y, Gao C, He T, Shen J. Endothelium regeneration on luminal surface of polyurethane vascular scaffold modified with diamine and covalently grafted with gelatin. Biomater 2004; 25: 423-430.
- Wang DA, Feng LX, Ji J, Sun YH, Zheng XX, Elisseeff JH. Novel human endothelial cell-engineered polyurethane biomaterials for cardiovascular biomedical applications. J Biomed Mater Res A 2003; 65: 498-510.
- Paulina AZ, Beata B, Tomasz C. Artifical heart: Hydrophobic coating to make it more natural. Challengers Mod Technol 2011; 2: 15-19.
- Katherine DK, Todd WP, Jeffrey OH, Scott AG, Aaron SG. Synthesis and characterization of segmented poly (esterurethane urea) elastomers for bone tissue engineering. Acta Biomater 2007; 3: 475-484.
- Guan J, Sacks MS, Beckman EJ, Wagner WR. Biodegradable poly(ether ester urethane) urea elas-tomers based on poly(ether ester) triblock copolymersand putrescine: synthesis, characterization and cyto-compatibility. Biomater 2004; 25: 85-96.
- Joseph TP, Thomas G, Mark WM, Spencer C, Yuji N, Toshiharu S, Christopher KB. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regen Med 2012; 7: 409-419.
- Leena PA, Arvind S, Anand R. In vitro hemocompatibility testing of UV-modified hyaluronan hydrogels. Biomater 2006; 27: 1416-1424.
- Yoshihisa K, Yoshimura A, Shibamori Y, Fuchigami K, Kubota N. Polymer surface modification by using microwave plasma Irradiation. J Solid Mech Mater Eng 2012; 6: 654-659.
- Thevenot P, Hu W, Tang L. Surface chemistry influences implant biocompatibility. Curr Top Med Chem 2008; 8: 270-280.
- Ponsonnet L, Reybier K, Jaffrezic N, Comte V, Lagneau C, Lissac M, Martelet C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behavior. Mater Sci Eng C 2003; 23: 551-560.
- Mirzadeh H, Dadsetan M, Sharifi-Sanjani N. Platelet adhesion on laser-induced acrylic acid-grafted polyethylene terephthalate. J Appl Polym Sci 2002; 86: 3191-3196.
- Hegemann D, Brunner H, Oehr C. Plasma treatment of polymers for surface and adhesion improvement. Nucl Instrum Methods Phys Res B 2003; 208: 281-286.
- Borcia C, Borcia G, Dumitrascu N. Surface treatment of polymers by plasma and UV radiation. Romanian J Phys 2011; 56: 224-232.
- Borcia G, Anderson CA, Brown NMD. Dielectric barrier discharge for surface treatment: application to selected polymers in film and fibre form. Plasma Sources Sci Technol 2003; 12: 335-344.
- Gopferich A, Peter SJ, Lucke A, Lu L, Mikos AG. Modulation of marrow stromal cell function using poly (D, L-lactic acid)-block-poly (ethylene glycol)-monomethyl ether surfaces. J Biomed Mater Res 1999; 46: 390-398.
- Mao C, Liang CX, Mao YQ, Li L, Hou XM. Modification of polyethylene with Pluronics F127 for improvement of blood compatibility. Colloids Surf B Biointerfaces 2009; 74: 362-365.
- Palmer RP, Cobbold AJ. Thermal and mechanical properties of oriented polyethylene treated with nitric acid. Makromol Chem 1964; 74: 174.
- Cagiao ME , Rueda DR, Balta Calleja EJ. Hardness of nitric acid treated polyethylene followed by recrystallization. Colloid Polym Sci 1987; 265: 37-41.
- Taite LJ, Yang P, Jun HW, West JL. Nitric oxide-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J Biomed Mater Res B Appl Biomater 2008; 84: 108-116.
- Avaialble at http://www.entecpolymers.com/products/pellethane/
- Mohandas H, Sivakumar G, Palaniappan K, Jaganathan SK, Supriyanto E. Microwave assisted surface modification of metallocene polyethylene for improving blood compatibility. Biomed Res Int 2013; 2013: 1-7.
- Muthu VV, Saravana KJ, Ida IM. Unravelling the potential of nitric acid as a surface modifier for improving the hemocompatibility of metallocene polyethylene for blood contacting devices. PeerJ 2016; 4: 1388.
- Ishihara K, Tanaka S, Furukawa N, Kurita K, Nakabayashi N. Improved blood compatibility of segmented polyurethanes by polymeric additives having phospholipid polar groups. I. Molecular design of polymeric additives and their functions. J Biomed Mater Res 1996; 32: 391-399.
- Skarja GA, Woodhouse KA. Synthesis and charac-terization of degradable polyurethane elastomers con-taining an amino acid-based chain extender. J Biomater Sci Polym Ed 1998; 9:271-295.
- Yeganeh H, Jamshidi H, Jamshidi S. Synthesis andproperties of novel biodegradable poly(e-caprolac-tone)/poly(ethylene glycol)-based polyurethane elas-tomers. Polym Int 2007; 56: 41-49.
- Rochotzki R, Nitschke M, Artz M, Meischsner J. Plasma modification of polymer films studied by Ellipsometry and Infrarot Spectroscopy. Physica Status Solidi (a) 1994; 145: 289-297.
- Van Wachem PB, Beugeling T, Feijen J, Bantjes A, Detmers JP, Aken WG. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomater 1985; 6: 403-408.
- Arima Y, Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomater 2007; 28: 3074-3082.
- Chang HI, Wang Y. Cell responses to surface and architecture of tissue engineering scaffolds. Intech 2011.
- Bahram SJ, Julien P, Rong Q, Shuling G, Eric H, Gschweng B, Stiles K, Tzy M, Owen N, Witte X, Bruce D, Hong W. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc National Acad USA 2008; 105:14459-14464.
- Gong F, Lu Y, Guo H, Cheng S, Gao Y. Hyaluronan immobilized Polyurethanes as a Blood Contacting Material. Int J Polym Sci 2010; 807935: 1-8.
- Saravana KJ, Hemanth M, Gunalan S, Palaniappan K, Theertha S, Sruthi AV, Selvakumar M, Eko S. Enhanced blood compatibility of metallocene polyethylene subjected to hydrochloric acid treatment for cardiovascular implants. Biomed Res Int 2014; 963149.
- Jansen EJP, Sladek REJ, Bahar H, Yaffe A, Gijbels M J, Kuijer R, Bulstra SK, Guldemond N A, Binderman NV, Koole LH Jul. Hydrophobicity as a design criterion for polymer scaffolds in bone tissue engineering. Biomater 2005; 26: 4423-4431.
- Ajith JJ, Alagar M. Fabrication of bioactive polyoxymethylene nanocomposites for bone tissue replacement. Macromol Symp 2012; 320: 24-37.