Letter to Editor - Journal of Gastroenterology and Digestive Diseases (2016) Volume 1, Issue 1
HER2 Status Examination of Biopsy Specimens from Gastric Mucosa
Ooi Akishi*Department of Molecular and Cellular Pathology Graduate School of Medical Science, Kanazawa University, Ishikawa 920-8641, Japan
- *Corresponding Author:
- Ooi Akishi
Department of Molecular and Cellular Pathology
Graduate School of Medical Science, Kanazawa University
Ishikawa 920-8641, Japan
Tel: 81-76-265-2192; E-mail: aooi@med.kanazawa-u.ac.jp
Received date: June 17, 2016; Accepted date: July 19, 2016; Published date: July 22, 2016
Abstract
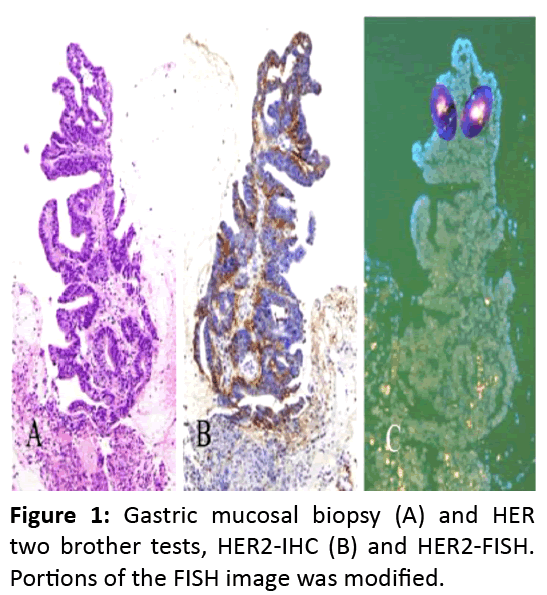
ERBB2 (also called HER2) is a 185 kDa transmembrane tyrosine kinase receptor. Overexpression as a result of HER2 gene amplification on chromosome 17q11.2-q12 has been observed in solid tumors including gastric and breast cancers1. HER2 is amplified in 7%-20% of gastric cancers2-5. Overexpressed HER2 is a therapeutic target of Trastuzumab, a humanized monoclonal antibody that binds to the extracellular juxtamembrane domain of ERBB2 and inhibits the proliferation and survival of HER2-overexpressing cancer cells6 (Figure 1).
Keywords
Erbb2, Gastric cancer, Mucosal biopsy, IHC, FISH
Letter to Editor
ERBB2 (also called HER2) is a 185 kDa transmembrane tyrosine kinase receptor. Overexpression as a result of HER2 gene amplification on chromosome 17q11.2-q12 has been observed in solid tumors including gastric and breast cancers [1]. HER2 is amplified in 7%-20% of gastric cancers [2-5]. Overexpressed HER2 is a therapeutic target of Trastuzumab, a humanized monoclonal antibody that binds to the extracellular juxtamembrane domain of ERBB2 and inhibits the proliferation and survival of HER2-overexpressing cancer cells [6] (Figure 1).
A critical factor in determining patient eligibility and predicting outcomes of this therapy is the intratumoral heterogeneity of HER2 amplification in gastric adenocarcinomas [7]. In adjuvant therapy, precise examination of the resected specimen was possible. In neoadjuvant therapy, endoscopic mucosal biopsy was the only test to know the eligibility of this molecular target study. Recently Ahn et al. compared HER2 expression in 702 paired biopsy and resection specimens of gastric cancer by immunohistochemistry (IHC) [8] . They concluded four fragments should be obtained to minimize the differences in HER2 scores between biopsy and resection specimens. In their study HER2 positivity was decided only by IHC; and their IHC images were consummate and their HER2 positive rates were well acceptable. However, it is well known that there are many unequivocal IHC judgements (2+) not only on surgical materials but also on biopsy specimens. Thus, I think when HER2-positive gastric cancer is suspicious, two brother’s tests, IHC and FISH, are mandatory.
References
- Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, et al. (2012) HER2 testing in gastric cancer: A practical approach.Mod Pathol.25: 637-650.
- Okines AF, Cunningham D (2012) Trastuzumab: a novel standard option for patients with HER-2-positive advanced gastric or gastro-oesophageal junction cancer. Therap AdvGastroenterol.5: 301-318.
- Kanta SY, Yamane T, Dobashi Y, Mitsui F, Kono K, et al. (2006) Topoisomerase IIalpha geneamplification in gastric carcinomas: correlation with the HER2 gene. Animmunohistochemical, immunoblotting, and multicolor fluorescence in situhybridizationstudy. Hum Pathol. 37: 1333-1343.
- Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, et al. (2002) Status of c-erbB-2 in gastricadenocarcinoma: Acomparative study of immunohistochemistry, fluorescence in situ hybridization and enzymelinkedimmuno-sorbent assay. Int J Cancer. 98: 833-837.
- Yano T, Doi T, Ohtsu A, Boku N, Hashizume K,et al. (2006) Comparison of HER2 gene amplification assessed byfluorescence in situ hybridization and HER2 protein expression assessed byimmunohistochemistry in gastric cancer. Oncol Rep. 15: 65-71.
- Hudis CA (2007) Trastuzumab: A mechanism of action and use in clinical practice. NEngl J Med. 357: 39-51.
- Tajiri R, Ooi A, Fujimura T, Dobashi Y, Oyama T, et al. (2014) Intratumoral heterogeneous amplification ofERBB2 and subclonal genetic diversity in gastric cancers revealed by multipleligation-dependent probeamplification and fluorescence in situ hybridization. Hum Pathol. 45: 725-734.
- Ahn S, Ahn S, van Vrancken M, Lee M, Yun Haet S, et al. (2015) Ideal number of biopsy tumor fragments forpredicting HER2 status in gastric carcinoma resection specimens. Oncotarget. 6: 38372-38380.