Research Article - Biomedical Research (2017) Volume 28, Issue 8
Gastric damage related to radioactive iodine treatment and the protective effect of amifostine
Atakan Sezer1*, Mehmet Celik2, Ufuk Usta3 and Gulay Durmus Altun41Department of Surgery, Faculty of Medicine, Trakya University, Turkey
2Department of Endocrinology, Faculty of Medicine, Trakya University, Turkey
3Department of Pathology, Faculty of Medicine, Trakya University, Turkey
4Department of Nuclear Medicine, Faculty of Medicine, Trakya University, Turkey
Accepted date: January 21, 2017
Abstract
Introduction: The aim of this study was to evaluate the gastric damage related to high dose radioactive iodine treatment during thyroid cancer treatment in an experimental rat model and evaluate histopathologically the radioprotective effect of amifostine. Methods and findings: 40 adult rats were enrolled into the study. Radioactive iodine treatment with 30 mCi/kg I131 was applied via orogastric tube simulating radioiodine ablation treatment (RAI) in thyroid cancer in all rats. After RAI subjects were divided into 2 groups: Group Orogastric RAI (Group OG, n: 20): Rats received no radioprotective agent but serum physiologic. Group Amifostine RAI (Group AP, n: 20): Rats received 200 mg/kg amifostine intraperitoneally 30 minutes before I131 application. On the 1st, 3rd, 5th and 7th days of experiment rats were sacrificed for histopathological evaluation. Gastric tissues were excised and tissue damage was assessed by using histopathological scoring. Total pathology score revealed a significant change on the 7th day. The subjects in Group OG had histopathological changes, while only one subject in Group AP was found to have pathologic changes (X2=39.7 df (28), p=0.04). The common histopathological findings were striking mucosal and intraepithelial leucocytic infiltration together with thinning of the mucosa, regenerative changes, and reactive atypia. Conclusions: RAI treatment causes gastric mucosal injury and amifostine was found to decrease radioactive iodine treatment related gastric damage.
Keywords
Radioactive iodine treatment, Gastric damage, Amifostine, Thyroid
Introduction
Radioactive iodine (I131) treatment is applied in papillary and follicular thyroid carcinomas for the ablation of residual thyroid tissue and to treat tumoral foci and recurrences. Ablation has advantages also for early detection of recurrences by increasing the specificity of serum thyroglobulin (Tg) measurements and sensitivity of I131 whole body scintigraphy [1,2]. Following oral administration, I131 is rapidly absorbed from stomach and small intestine and then enters circulation, which may be followed by nausea and vomiting in thyroid cancer patients [3]. Up to date no clinical or experimental model investigated the damage of RAI on gastric tissues and symptomatic medications applied for gastrointestinal disorders. In current treatment protocols clinicians tended to use radioprotective agents to prevent acute and long-term negative effects of radiation on normal tissues. Amifostine, a cytoprotective agent, has been found to prevent radiation related damage during adjuvant treatments of cancer therapy [4-6]. Although there are studies reporting gastrointestinal side effects occurring during ablation treatment, histopathologic description of these side effects has not been made and moreover, preventive effects of radioprotective agents in such conditions have not been investigated. Therefore, this study aimed to evaluate the histopathologic damage of RAI treatment in gastric tissues and protective effect of amifostine during treatment period.
Material and Method
Study groups
40 adult rats with an average weight of 721 ± 111 gr were enrolled into the study. The study was approved by Local Ethics Committee. All rats were housed in Experimental Animals Laboratory under a 12:12-hour light/dark photoperiod at 22 ± 1°C temperature and 55% humidity and fed with et libitum. 24 h starvation was maintained except water to all experiment groups. All animals were applied orogastric radioactive iodine treatment with 30 mCi/kg I131 for thyroid ablation. Subjects were divided into 2 groups: Group Orogastric RAI (Group OG): 20 rats did not receive radioprotective agent, except same volume serum physiologic. Group Amifostine RAI (Group AP): 20 rats received 200 mg/kg amifostine intraperitoneally 30 minutes before I131 application. Rats were sacrified and tissues were removed under anesthesia in the 1st, 3rd, 5th and 7th days for histopathological examination to determine the effects of radioactive iodine on stomach. Gastric tissue damage was evaluated by histopathological scoring.
Histopathologic evaluation
During histopathological evaluation the rats’ stomachs were fixed in buffered neutral 10% formaldehyde for 24 h. The tissues were sampled circularly from the transition of corpus and antrum and they were subjected to alcohol processing for 12 h. Five micrometer thick sections were obtained from the paraffin embedded tissues. The hematoxylin-eosin stained slides were evaluated 2 times under light microscope (Olympus BX51, Japan) by a pathologist in a blinded manner. So as to evaluate the leucocytic infiltration of the mucosal layer of the stomachs quantitatively, neutrophil leucocytes were counted in 5 consequent high power fields (HPF=40X objective) and then the sum of the leucocyte number was divided into 5 to get the mean value of the neutrophil leucocytes per HPF. After counting the leucocytes, whole specimen on the slide was examined in detail and further mucosal pathologies were noted, if present. Pathological changes were visually scored as acute inflammatory reaction (0-2), regenerative change (0-1) and reactive atypia (0-1), lymphoid aggregate (0-1) and mucosal thinning (0-1) and all scores were calculated; a total pathology score was obtained for each case.
Statistical method
Leucocyte count was calculated as mean ± SD for statistical analysis. Nonparametric ANOVA was used for multi group analysis. Pathological changes including acute inflammatory reaction, regenerative change and reactive atypia, lymphoid aggregate, mucosal thinning and a total pathology score was obtained for each case, and then results obtained were analyzed statistically. Chi-square test was used for the evaluation of the differences of the scores between the groups. The differences were considered significant when the probability was less than 0.05.
Results
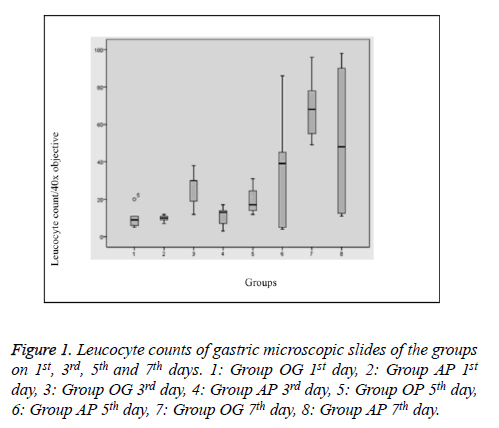
Histopathological evaluation was performed on 40 rats and no rat died during the study. The leucocyte counts are given in “Table 1 and Figure 1”. On the first day, leucocytes mostly tended to accumulate in the small blood vessels and the margination of the leucocytes was prominent. However, those leucocytes were not taken into consideration during the counting process and only the leucocytes in the lamina propria and mucosal epithelium were counted. The leucocytes in the first day specimens were mostly located in the very basal part of the mucosa and they were found only in lamina propria without any intraepithelial infiltration. The third day specimens showed similar findings with the first day specimens when absence of additional pathologies was taken into consideration. Leucocytes in those specimens also tended to locate basely, however, leucocytes infiltrating the upper portions of the mucosa were also present in some slides. Leucocyte accumulation in small vessel lamina was not as striking as in the first day group, rather they were mostly located in lamina propria of the mucosa. In some slides of the fifth day groups, some degree of mucosal damage and prominent intraepithelial infiltration could be observed which were more prominent and striking in all of the non-treated specimens of the 7th day group (Group OG). Most of that sample revealed striking mucosal and intraepithelial leucocytic infiltration together with thinning of the mucosa, regenerative changes and reactive atypia. Those findings were somewhat similar to two cases of treatment group of the 7th day.
| 1st day | 3rd day | 5th day | 7th day | |
|---|---|---|---|---|
| Group OG Mean ± SD Min-Max |
10.2 ± 5.9 5-20 |
25.8 ± 10.3 12-38 |
19.2 ± 8.2 12-31 |
69.2 ± 18.7 49-96 |
| Group AP Mean ± SD Min-Max |
9.8 ± 1.9 7-12 |
10.8 ± 5.6 3-17 |
35.8 ± 33.8 4-86 |
51.2 ± 45.2 11-98 |
| p | NS | 0.04 | 0.02 | 0.07 |
Table 1. Leucocyte counts of gastric microscopic slides of the groups on 1st, 3rd and 5th and 7th days.
Pathological examination revealed acute inflammatory changes only in 2 cases, which showed no difference between the groups and the days (X2=15,9 df (14), p=0.31). There was no difference between amifostine and ablation groups in terms of regeneration and reactive atypia evaluation on the 1st, 3rd and 5th days.
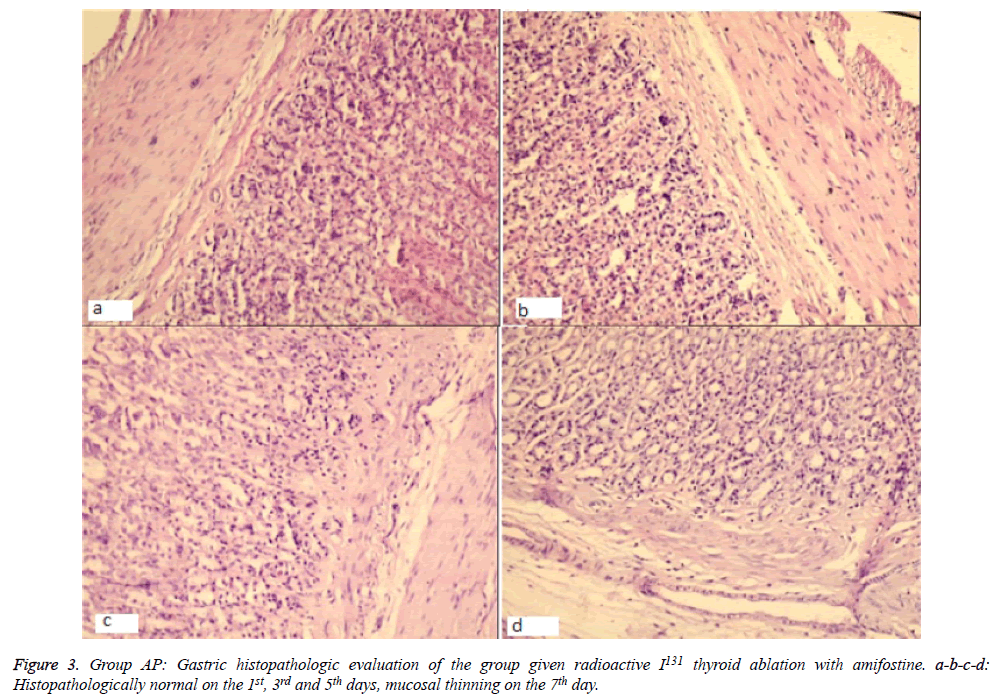
Significantly, histopathological changes significantly on day 7 between Group OG and AP. The histopathological results revealed evidence of regenerative and mucosal changes. Amifostine application generated a significant difference with respect to regeneration and reactive atypia (X2=29 df (24), p=0.01). Regenerative changes were observed in 3 subjects of Group OG, while only 1 case in Group AP had findings suggesting regeneration. Mucosal thinning was observed on the 5th day in Group AP (in 2 of 5 cases) and on the 7th day in Group OG (3 cases) and Group AP (1 case). There was no difference between the groups in terms of mucosal thinning (X2=12,1 df (7), p=0.09). Lymphoid aggregate was only observed in one subject of Group OG on the 3rd day, therefore, it was not considered in statistical evaluation. Evaluation of total pathology score revealed a significant change on the 7th day; except one subject, all cases in Group OG had changes, while only one case in Group AP was found to have pathologic changes (X2=39,7 df (28), p=0.04) (Figures 2 and 3).
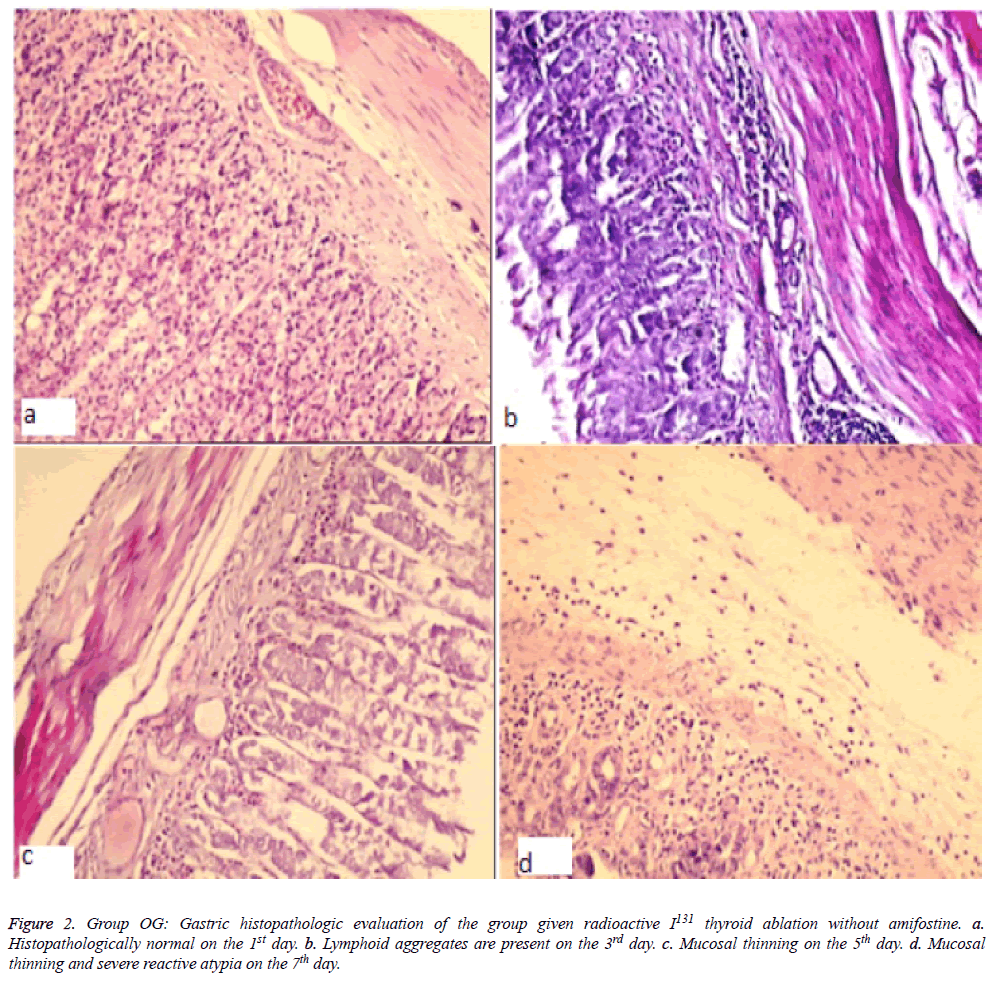
Figure 2: Gastric histopathologic evaluation of the group given radioactive I131 thyroid ablation without amifostine. a. Histopathologically normal on the 1st day. b. Lymphoid aggregates are present on the 3rd day. c. Mucosal thinning on the 5th day. d. Mucosal thinning and severe reactive atypia on the 7th day.
Discussion
In current study, the damage of radioiodine ablation on gastric tissue was shown in histopathologic examination and the protective effect of amifostine was evaluated. We hypothesized that amifostine may attenuate the gastric damage during RAI treatment. Radioactive iodine is a radiopharmaceutical agent used in the diagnosis and treatment of thyroid diseases and radioactive iodine treatment was first used during 1940’s [7-9]. Radioactive iodine treatment is administered via oral way in the form of capsule or liquid. Following rapid and total absorption, radioiodine is taken by thyroid as in case of inorganic iodine, rapidly concentrated by thyroid follicular cells, organified and stored in colloid. Once radioactive iodine is localized in thyroid gland, distribution of radioactive dose depends on the energy and type of the beam and the distribution of iodine among follicles [10,11]. Low dose radioactive iodine is used in the treatment of hyperthyroidism, while high dose is given for differentiated thyroid cancer ablation and treatment of metastases [12,13]. Differentiated thyroid cancers and the metastasis of them concentrate RAI, and RIT is beneficial in these tumors [14,18]. On the other way RIT may has systemic effects such as hair loss, xerophthalmia, loss of taste and smell, nasal pain and epistaxis, sialoadenitis, radiation pneumonitis and pulmonary fibrosis, bone marrow suppression, aplastic anemia and leukemia, impaired spermatogenesis, transient ovarian insufficiency, and mostly nausea and vomiting [16-18]. Once I131 is ingested, it is quickly absorbed from stomach and small intestines, enters into systemic circulation, concentrated and secreted from active gastric mucosa. When RAI dose less than 30 mCi is directly given to stomach, no symptoms occur. Nausea and vomiting secondary to radiation gastritis occur just after RIT and resolve within few days [14-16]. However, to the best of our knowledge, there is no published data in the literature presenting acute effects of ablative radioiodine-131 on gastrointestinal damage demonstrated with histopathology. In our study a marked ablative radioiodine-131 induced gastrointestinal toxicity consist of mucosal damage and prominent intraepithelial infiltration was observed in all of the non-treated specimens of the 7th day group after a single dose of 30 mCi/kg. Dixon et al. [19] described the pathophysiology of acute gastritis as edema of lamina propria, vascular congestion, intact epithelium, scattered neutrophils, and hemorrhage in mucosa. Those findings of current study in mucosal damage in gastric tissue were similar with acute gastritis. Throughout the findings of current study radioiodine-131 causes gastric mucosal damage similar with acute gastrititis, which may cause nausea and vomiting during RIT. The pathophysiologic mechanism of RAI damage over gastric mucosa may be related with gastric radioiodine uptake due to sodium iodine simporter (NIS), a membrane glycoprotein that mediates active iodide uptake in the thyroid gland and several extrathyroidal tissues. As very well documented by Wyszomirska [20] ionizing radiation acts with tissues and causes cell damage in terms of three mechanisms consist of with free radicals or a direct damage to proteins in physical, biological, and chemical phases in the pathways of DNA control and repair. I131 radiates β and gamma beams and once radioactive iodine is localized in thyroid gland destruction of follicular cells is a result of β particle radiation. Specifically I131 is accumulated from the extravascular/extracellular space into the parietal cells by the NIS is the same mechanism responsible for the accumulation of iodine by the thyroid gland and the damage of RIT on gastric mucosa may be related with I131 accumulation throughout gastric tissues. Since 1949, many investigators have been studying on preventive effects of various chemical compounds against radiation related damage. Data obtained from experimental animals suggest that these agents may decrease mortality when they are given prior to lethal dose radiation exposure. This preventive effect is promising in that radiation related damage may be decreased in humans and it may offer an option for prophylactic treatment [21]. As a result of factors such as environmental conditions, diet and biological alterations among living creatures, it is difficult to make a realistic prediction about the diversity and extent of ionizing radiation related health problems. In addition, total dose of radiation exposed, dose given in each session, extension of exposure time, linear energy transfer, defense mechanisms of organism, concomitant exposure to chemical carcinogens and other toxins that may activate protooncogenes and many other accompanying factors complicate making realistic predictions about ionizing radiation related damage. However, the most important biologic and cellular alteration is the production of molecules called free radicals that carry an unpaired electron in one of its orbits [21-23]. Radioprotective mechanisms are described as inactivation of free radical products, hydrogen atom binding to target molecules, generation of complex disulphide compounds, and deceleration of cell division and induction of the development of hypoxia in tissues. Radioprotective agents are thiol compounds, other sulphur compounds, pharmacological agents such as anesthetic drugs, analgesics and tranquilizers and other radioprotective compounds such as WR-1065, WR-2721, vitamin C, vitamin E and glutathione [21]. In current study, amifostine was used as cytoprotective agent. Amifostine is a cysteamine like molecule, which has been found to be effective in preventing tissue damage related to radiotherapy (RT) and chemotherapy [24-26]. Preclinical studies conducted on animals have shown preventive effect of amifostine against lethal dose radiation [4,5]. Normal tissues, which are known to be protected by amifostine, are kidneys, lungs, esophagus, peripheral nerves, bone marrow, small intestine, large intestine, immune system, salivary glands, oral mucosa, heart, genetic material and testis. Extended clinical uses of amifostine include radioprotection, chemoprotection, tumor sensitization, bone marrow stimulation, radioprevention and chemoprevention [4,6,25].
Aktoz et al. [27] investigated radioiodine-induced kidney damage and protective effect of amifostine in an experimental rat study. The authors reported that RIT causes renal tubular damage statistically significant compared to sham group and the group of rats, which were treated with amifostine, has statistically less renal tubular damage. Joseph et al. [28] studied the effect of amifostine on the salivary gland of rats after therapeutic radioiodine exposure and the authors showed histologically the protective effect of amifostine in this experimental model. In current study, the histopathological damage of high-dose radioiodine therapy in gastric tissues was firstly reported and also the protective effect of amifostine with respect to regeneration and reactive atypia in mucosal surfaces.
The main limitation of the study is being an experimental animal study, which may not reflect or simulate the anatomy, physiology, and regulation of gene expression between two species. Although based on these experimental results, prospective clinical trials are needed to highlight the effect of role of acute and chronic effects of radioiodine-131exposure and protective role of amifostine.
In conclusion, in this study which aimed to histopathologically determine protective effects of amifostine in stomach against radioactive iodine treatment, amifostine was found to be effective in preventing RIT related gastric damage. To the best of our knowledge, there is no published data in the literature about acute and early protective role of amifostine on gastric damage.
Declaration of Interests
The authors declare no conflict of interests.
References
- Mazzaferri EL. Thyroid remnant 131I ablation for papillary and follicular thyroid carcinoma. Thyroid 1997; 7: 265-271.
- Verburg FA, de Keizer B, Lips CJ, Zelissen PM, de Klerk JM. Prognostic significance of successful ablation with radioiodine of differentiated thyroid cancer patients. Eur J Endocrinol 2005; 152: 33-37.
- Mendoza A, Shaffer B, Karakla D, Mason ME, Elkins D, Goffman TE.l. Quality of life with well-differentiated thyroid cancer: treatment toxicities and their reduction. Thyroid 2004; 14: 133-140.
- Bohuslavizki KH, Brenner W, Klutmann S, Kröger S, Buchert R, Bleckmann C. Radioprotection of salivary glands by amifostine in high-dose radioiodine therapy. J Nucl Med 1998; 39: 1237-1242.
- Bohuslavizki KH, Klutmann S, Jenicke L, Brenner W, Feyerabend B, Henze E. Radioprotection of salivary glands by S-2-(3-aminopropylamino)- ethylphosphorothioic (amifostine) obtained in a rabbit animal model. Int J Radiat Oncol Biol Phys 1999; 45: 181-186.
- Uguzalp-Kaldir M, Yürüt-Çaloglu V, Cosar-Alas R, Çermik T, Altaner S, Eskiocak S. Radyasyona bagli olusan karaciger ve böbrek toksitesini önlemede amifostinin rolü. Türk Onkoloji Dergisi 2007; 22: 105-17.
- Teelucksingh S, Singh V, Balkaran B. Use of radioiodine in adolescent Graves' disease. Ann Trop Paediatr 2001; 21: 335-338.
- Huysmans DA, Hermus AR, Corstens FH, Kloppenborg PW. Long-term results of two schedules of radioiodine treatment for toxic multinodular goitre. Eur J Nucl Med 1993; 20: 1056-1062.
- McDougall IR. Management of thyroid cancer and related nodular disease. (1stedn), Springer-Verlag, London, 2006: 172.
- Rivkees SA, Sklar C, Freemark M. Clinical review 99: The management of Graves' disease in children, with special emphasis on radioiodine treatment. J Clin Endocrinol Metab 1998; 83: 3767-3776.
- Bruger M, Member S. On the excretion of iodine in the saliva. Am J Physiol 1943; 139: 212-216.
- Huysmans DA, Hermus AR, Corstens FH, Barentsz JO, Kloppenborg PW. Large, compressive goiters treated with radioiodine. Ann Intern Med 1994; 121: 757-762.
- Burch HB, Solomon BL, Cooper DS, Ferguson P, Walpert N, Howard R. The effect of antithyroid drug pretreatment on acute changes in thyroid hormone levels after I- 131 ablation for Graves disease. J Clin Endocrinol Metab 2001; 86: 3016-3021.
- Özcan Z, Özkiliç H. Iyi diferansiye tiroid kanserlerinin tedavi ve takibinde nükleer tibbin rolü. T Klin J Endocrin 2003; 1: 37-45.
- Akin A. I-131 ile tiroid kanserlerinin tedavisi. Hatemi H (Editör). Nükleer Tip Yilligi I.’de 1.baski Istanbul: Emek Matbaacilik Ltd.Sti, 1987: 116-127.
- Nostrand DV. Side effects of 131I for ablation and treatment of well-differentiated thyroid carcinoma. In: Wartofsky L, Nostrand DV, (Eds.). Thyroid Cancer A Comprehensive Guide to Clinical Management. (2ndedn), Humana Pres, 2006; pp. 459-485.
- Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med 1998; 39: 1551-1554.
- Kita T, Yokoyama K, Higuchi T, Kinuya S, Taki J, Nakajima K. Multifactorial analysis on the short-term side effects occurring within 96 h after radioiodine- 131 therapy for differentiated thyroid carcinoma. Ann Nucl Med 2004; 18: 345-349.
- Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161-81.
- Wyszomirska A. Iodine-131 for therapy of thyroid diseases. Physical and biological basis. Nucl Med Rev Cent East Eur 2012; 15: 120-123.
- Kelle I. Radyoprotektif Etkili Ajanlar. Dicle Tip Dergisi 2008; 35 :69-76.
- Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003; 189: 1-20.
- Wasserman T. Radioprotective effects of amifostine. Semin Oncol 1999; 26: 89-94.
- Capizzi RL. Clinical status and optimal use of amifostine. Oncology (Williston Park) 1999; 13: 47-59.
- Karadayi B, Akmansu M, Dirier A, Akdemir Ö. Amifostinin sitoprotektif etkinliginin kantitatif tükürük bezi sintigrafisi ile degerlendirilmesi. Dicle Tip Dergisi 2005; 32: 183-189.
- Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J. Clinical practice guidelines for the prevention and treatment of cancer therapy induced oral and gastrointestinal mucositis. Cancer 2004; 9: 2026-2046.
- Aktoz T, Durmus-Altun G, Usta U, Torun N, Ergulen A, Atakan IH. Radioiodine-induced kidney damage and protective effect of amifostine: Anexperimental study. Hippokratia 2012; 16: 40-45.
- Joseph LJ, Bhartiya US, Raut YS, Hawaldar RW, Nayak Y, Pawar YP. Radioprotective effect of Ocimum sanctum and amifostine on the salivarygland of rats after therapeutic radioiodine exposure. Cancer Biother Radiopharm 2011; 26: 737-43.