Research Article - Journal of Pathology and Disease Biology (2022) Volume 6, Issue 4
Extra pulmonary manifestations in covid-19: A review on histopathological alterations.
Nilanjana Bhattacharyya Nath1*, Anjali Smita2, Abhijit Dutta3
1Department of Biotechnology, Swami Vivekananda Institute of Modern Science, Kolkata, West Bengal, India.
2Department of Zoology, Nirmala College, Ranchi, Jharkhand, India.
3Department of Zoology, Ranchi University, Ranchi, Jharkhand, India.
- Corresponding Author:
- Nilanjana Bhattacharyya Nath
Department of Biotechnology
Swami Vivekananda Institute of Modern Science
Kolkata, West Bengal, India, US
E-mail: nbn0305@gmail.com
Received: 17-Jun-2022, Manuscript No. AAPDB-22-66863; Editor assigned: 20-Jun-2022, PreQC No. AAPDB-22-66863(PQ); Reviewed: 27-Jun-2022, QC No. AAPDB-22-66863; Published: 05-Jul-2022, DOI: 10.35841/aapdb-6.4.116
Citation: Nath NB, Smita A, Dutta A. Extra pulmonary manifestations in covid-19: A review on histopathological alterations. J Pathol Dis Biol. 2022;6(4):116
Abstract
COVID-19 is a major health issue, responsible for more than three million deaths worldwide as of June 2021. COVID-19 is most well-known for causing acute respiratory pathology, but it can also result in several extra pulmonary manifestations. In serious condition, SARS-CoV-2 causes a systemic disease, with possible cardiovascular, gastrointestinal, hepatobiliary, thrombotic, neurological, renal, dermatologic, reproductive, and psychologic manifestations. Histopathological alterations are mostly found within lungs and blood vessels. Recent investigations through full autopsy or minimally invasive autopsy of COVID-19 patients revealed that SARS-CoV-2 hijacked angiotensin converting enzyme-2 (ACE2) for entry into target cells to cause severe pathologic changes in the lungs and multiple extra pulmonary organs/tissues. To understand its morbidity, mortality and the pathogenesis, histopathological findings are essential. The histopathological features of COVID-19 significantly help therapists to improve disease treatment and outcome. So, more studies and evidence on tissue samples are required to establish the degree of involvement of other organs and tissues which are indeed affected by COVID-19. In this article, we have tried to provide comprehensive information regarding the multisystemic involvement of COVID-19 based on the evidence and experiences from the researchers worldwide.
Keywords
COVID-19, ACE2, Histopathological alterations, Extra pulmonary manifestations.
Introduction
Coronavirus disease 2019 (COVID-19) is a severe acute respiratory syndrome, caused by coronavirus-2 (SARSCoV- 2) which is zoonotic in origin and most spread through respiratory droplets and has caused a big threat to humankind [1]. First detected in December 2019 in Wuhan (China) and declared by World Health Organization (WHO) a pandemic in March 2020. Till now it presents a great challenge for the healthcare communities across the globe.
Coronaviruses envelop a single-stranded large RNA. It is a virus that infects humans as well as a wide range of animals. Their spherical morphology with a core shell and glycoprotein projections form their envelope and gives them a “crown-like” appearance; therefore, they are termed coronaviruses [2]. The genome size ranges from 27 to 32 kB, which is mostly the largest genome among all RNA viruses. Their four main structural genes encode the nucleocapsid protein (N), the spike protein (S), the small membrane protein (SM), and the membrane glycoprotein (M) [3].
The spike protein of the virus, through its Receptor Binding Domain (RBD) attaches to a human cell surface receptor Protein - Angiotensin Converting Enzyme-2 (ACE-2), encoded by the ACE2 gene, followed by its priming through auxiliary protein TMPRSS2 (trans membrane protease, serine 2). The TMPRSS2 is a cell-surface protein, expressed by epithelial cells of specific tissues including those in the aero digestive tract. ACE-2, which acts as a viral host cell entry receptor, distributed all over the organs. SARS- CoV-2 in severe cases affects entire body - the kidneys, the heart and blood vessels, the liver, the pancreas and regulates alterations in circulating lymphocytes and the immune system [4-6]. From the human protein atlas dataset [7], the maximum expression of the ACE- 2 receptors is present in small intestine, duodenum, and colon, followed by kidney, testis, gall bladder, heart, thyroid gland, adipose tissue, rectum, and Lungs.
Fundamental in the pathophysiology of multi-organ injury caused by SARS-CoV-2 infection include: (i) direct virusmediated cell damage; (ii) dysregulation of the renin– angiotensin–aldosterone system (RAAS) as a consequence of the down regulation of ACE2 related to viral entry; (iii) endothelial cell damage with subsequent inflammation and generation of a thrombotic milieu; and (iv) dysregulation of the immune response and cytokine release syndrome [8].
Primarily identified as a typical pneumonia known as COVID pneumonia, it has developed into severe acute respiratory distress syndrome (ARDS), a multi-organ dysfunction with associated fatality. Other extra pulmonary conditions include thrombotic complications, myocardial dysfunction and arrhythmia, acute coronary syndromes, acute kidney injury, gastrointestinal symptoms, hepatocellular injury, hyperglycemia and ketosis, neurologic illnesses, ocular symptoms, and dermatologic complications [8].
The aim of this review is to describe the main histopathological alterations and the immunopathological mechanisms underlying COVID-19, with particular attention to extra pulmonary manifestations.
Pathophysiology
SARS-CoV-1 and SARS-CoV-2 have the same mechanism of action; both can cause rapid production of multiple cytokines in body fluids following infection, leading to acute respiratory distress and multiple organ failure. This is the main reason most COVID-19 patients have mild symptoms at the onset of the disease, while conditions of a few affected patients are suddenly worsened after being diagnosed in hospital. This severity may be related to the excessive production of cytokines after the disease, leading to ‘cytokine storm’ in the body. The association of viral infection with any comorbidity such as hypertension, diabetes and renal failure has shown more serious form of clinical presentations such as respiratory failure to multiple organ failure [9]. Post-mortem studies have shown pulmonary, renal, and small vessel injury with particles resembling virus observed in the kidney by electron microscopy [10-12]. Benjamin T Bradley done post- mortem examinations on fourteen people who died with COVID-19 at the King County Medical Examiner’s Office (Seattle, WA, USA) and Snohomish County Medical Examiner’s Office (Everett, WA, USA) in negative-pressure isolation suites during February and March 2020. Tissue examination done by light microscopy, immunohistochemistry, electron microscopy, and quantitative RT-PCR. Coronavirus-like particles were detected in the respiratory system, kidney, and gastrointestinal tract. Lymphocytic myocarditis was observed in one patient with viral RNA detected in the tissue. All patients were median aged (range 42–84) with different comorbidities (hypertension, chronic kidney disease, obstructive sleep apnea and metabolic disease including diabetes and obesity). The pathological findings identified in the COVID-19 suggested that SARS-CoV-2 can widely spread in the epithelial lining of the respiratory tract, digestive tract, distal convoluted tubules of the kidney, the sweat glands of the skin and testicular epithelium including spermatogonia and Sertoli cells [13]. SARS-CoV-2 can bind angiotensin converting enzymes 2 (ACE2) receptors, expressed in multiple extra pulmonary tissues and this can lead to a direct viral tissue damage [8,14,15].
Histopathological findings in liver
COVID-19 primarily causes pulmonary injury, but has been implicated to cause hepatic injury, both by serum markers and histologic evaluation. ACE2 expressed highly in the endothelial layer of small blood vessels, but not in the sinusoidal endothelium. ACE2 cell surface receptor was expressed highly in cholangiocytes (59.7%) than hepatocytes (2.6%) [16]. The level of ACE2 in cholangiocytes was like that in type 2 alveolar cells of the lungs suggesting that the liver could be another potential target for viral RNA.
Xu first reported the post-mortem findings of a COVID-19 patient and stated micro vesicular steatosis and mild inflammatory infiltrates in the hepatic lobule and portal tract. Stephen studied histology of liver of forty patients who died of complications of COVID-19 [17]. Infected patients showed elevated level of hepatic enzymes, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Macro vesicular steatosis was detected in thirty patients (75%). Mild lobular necro inflammation and portal inflammation were present in twenty cases each (50%). Tian reported the post-mortem liver biopsies in four patients with COVID-19 showing mild sinusoidal dilatation and focal macro vesicular steatosis [11]. There was mild lobular lymphocytic infiltration, which was not the normal feature of portal areas (Figure 1). Viral RNA was isolated from liver tissue in one of the patients through RT- PCR. Histopathological examinations showed hepatocyte degeneration with lobular focal necrosis, congestion of hepatic sinuses with microthrombus, fibrosis of portal tract, proliferation of portal vein branches, and mononuclear leukocyte and neutrophil infiltration within the portal area [18,19]. Sonzogni also found alteration of vascular structure, both acute (thrombosis, luminal ectasia) and chronic (fibrous thickening of vascular wall or phlebosclerosis, and abnormal asset of portal intrahepatic system) [20]. They observed typical arrangement of intrahepatic blood vessels with CD34 staining, decorating a Periportal network of sinusoidal vessels, which may show increased arterial pressure.
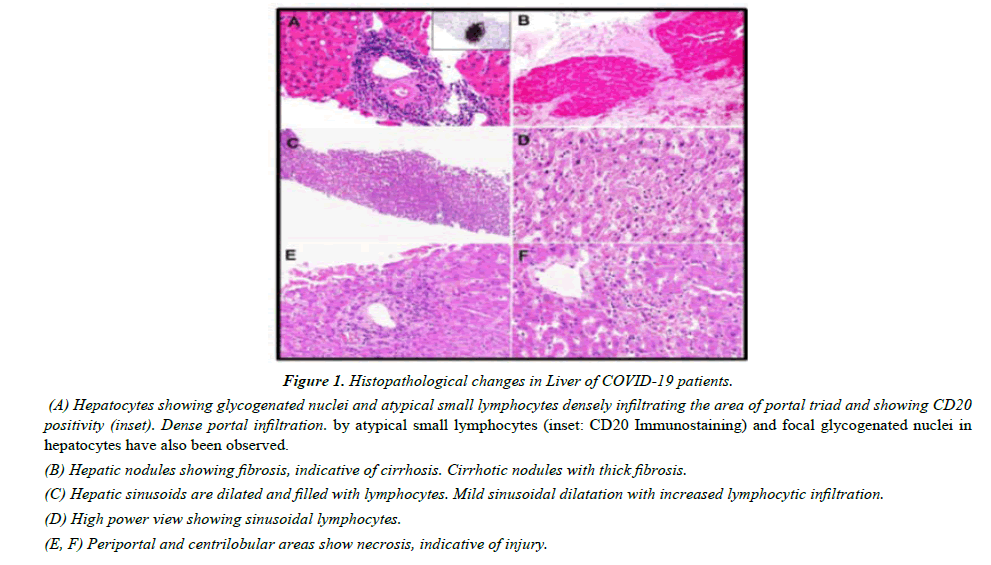
Figure 1: Histopathological changes in Liver of COVID-19 patients.
(A) Hepatocytes showing glycogenated nuclei and atypical small lymphocytes densely infiltrating the area of portal triad and showing CD20 positivity (inset). Dense portal infiltration. by atypical small lymphocytes (inset: CD20 Immunostaining) and focal glycogenated nuclei in hepatocytes have also been observed.
(B) Hepatic nodules showing fibrosis, indicative of cirrhosis. Cirrhotic nodules with thick fibrosis.
(C) Hepatic sinusoids are dilated and filled with lymphocytes. Mild sinusoidal dilatation with increased lymphocytic infiltration.
(D) High power view showing sinusoidal lymphocytes.
(E, F) Periportal and centrilobular areas show necrosis, indicative of injury.
Though autopsies had limitations, death may occur long interval after the acute liver injury and the histological changes. At the time of the acute injury, it would be important to perform a liver biopsy for better histopathological study. However, liver biopsies are not performed in COVID-19 patients. Fiel presented the report of two liver biopsies of COVID 19 patients without pulmonary injury [21]. The first one was a 63-year- old post–liver transplant patient with different comorbidities (chronic renal failure, hypertension, and diabetes) reported following manifestations:- abnormal liver enzymes (alanine aminotransferase level - 1761 U/L, bilirubin, and an alkaline phosphatase level-1568 U/L). Significant bile duct injury was also identified. The second patient was a 36-year-old woman without other known comorbidities developed high aminotransferase levels and jaundice. Biopsy performed after one week of hospital admission showed histopathological findings like the first patient, with acute hepatitis and prominent bile duct injury. Though researchers suggested that COVID is responsible for the association of viral infection and biochemical liver injury and the histologic findings of acute hepatitis and bile duct injury.
Histopathological findings in gastrointestinal tract
Common gastrointestinal symptoms of COVID-19 positive patients reported were diarrhea, anorexia, nausea, vomiting, abdominal pain, and gastrointestinal bleeding during the onset and after hospitalization [18]. Microscopically, the gastrointestinal tract may show epithelial damage, endothelitis and ischemic enterocolitis [21,22]. There is few evidence of stenosis of the small intestine. Gastric tissue may show epithelial degeneration, necrosis and shedding of the mucosa with the presence of dilated and congested small blood vessels in lamina propria and sub mucosa. Endocrine pancreas may show evidence of tissue degradation [23].
The lower GI (LGI) tract is more involved than upper GI (UGI). Viral RNA detected in GI tissue by RT-PCR correlates with disease severity and is more dependable than RNA detected in the stool [24]. Although most cases had neither significant gross nor histological pathological changes in esophagus, stomach nor duodenum [23]. Barton has described gaseous stomach, punctate gastric and duodenal hemorrhages, and multifocal gastric hemorrhage in COVID patients [10].
Histopathological findings in kidney
Though respiratory and immune systems are the major targets of Coronavirus (COVID-19), acute kidney injury and proteinuria was also observed. Su Hua [25] reported twentyfive kidney abnormalities in twenty-six autopsies of patients with COVID-19. Out of twenty-six, nine patients showed clinical signs of kidney injury which included systemic hypoxia, abnormal coagulation, and drug or hyperventilationrelevant rhabdomyolysis.
They also observed increased serum creatinine and/or newonset proteinuria. Electron microscopic study showed clusters of coronavirus-like particles with distinctive spikes in the tubular epithelium and podocytes. The diameter of virus-like particle varied from about 65 nm to 136 nm, with distinctive spikes, around 20 to 25 nm, presenting in a solar “corona” appearance. Immunostaining with SARS-CoV nucleoprotein antibody was also positive in tubules.
ACE2 attached to the cell membranes of cells in the lungs, arteries, heart, kidney, and intestines. Cell entry of corona virus depends on binding of the viral spike (S) proteins to cellular receptors and on S protein priming by host cell proteases. SARS-CoV2 uses the SARS-CoV receptor ACE2 for entry and the serine protease, TMPRSS2, for S-protein priming. Xu found that ACE2 and TMPRSS2 are significantly co-expressed in podocytes and proximal convoluted tubules which provide direct viral involvement of the kidneys.
Akalin reported that transplanted kidney patients have an increased risk from Covid-19. In New York, the Montefiore Medical Center reported twenty-six transplant patients with Covid-19 those who had secondary risk factors. An extremely high early mortality (28% at 3 weeks) was found among kidney transplant recipients with Covid-19. Two thirds of the patients died [26].
Clinically, the incidence of acute kidney injury in COVID-19 varies from 0.9% to 29% with new onset proteinuria. The significant microscopic changes were proximal tubule injury with loss of brush border, swollen glomerular endothelial cells, presence of thrombus in the capillaries, tubular epithelial cell oedema and vacuolar degeneration (Figure 2A & B). Yao mentioned the occasional findings include segmental fibrin thrombus, podocyte vacuolation, focal segmental glomerulosclerosis and shrinkage of capillary loops and accumulation of plasma in Bowman’s space (Figure 2G & H) [9].
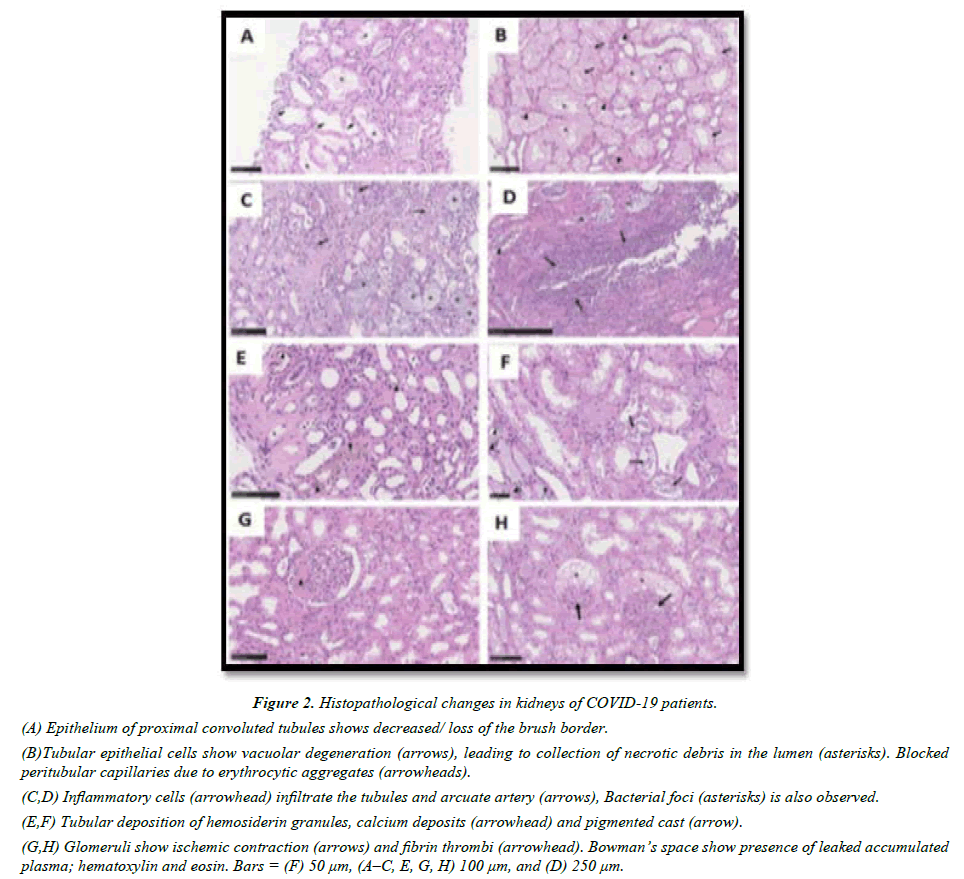
Figure 2: Histopathological changes in kidneys of COVID-19 patients.
(A) Epithelium of proximal convoluted tubules shows decreased/ loss of the brush border.
(B)Tubular epithelial cells show vacuolar degeneration (arrows), leading to collection of necrotic debris in the lumen (asterisks). Blocked peritubular capillaries due to erythrocytic aggregates (arrowheads).
(C,D) Inflammatory cells (arrowhead) infiltrate the tubules and arcuate artery (arrows), Bacterial foci (asterisks) is also observed.
(E,F) Tubular deposition of hemosiderin granules, calcium deposits (arrowhead) and pigmented cast (arrow).
(G,H) Glomeruli show ischemic contraction (arrows) and fibrin thrombi (arrowhead). Bowman’s space show presence of leaked accumulated plasma; hematoxylin and eosin. Bars = (F) 50 μm, (A–C, E, G, H) 100 μm, and (D) 250 μm.
Histopathological findings in Heart
Several researchers reported cardiovascular damages in COVID-19 patients including myocardial infarction, acute coronary syndrome, cardiomyopathy, arrhythmias, and cardiogenic shock. Especially in pre-existing cardiovascular diseases, myocardial injury occurred in more than 20% of hospitalized patients infected with corona virus. Bengaluru presented a case study of eighteen patients with ST segment elevation on ECG that showed six patients had coronary obstruction leading to myocardial infarction, two were diagnosed with myocardial infarction as wall abnormalities on echocardiogram and ten had non-coronary myocardial injury [27]. Cardiac arrhythmias and ventricular arrhythmias have been frequently reported during COVID-19 disease, 17% among hospitalized patients and 44% among those admitted in intensive care units. Few case reports described the occurrence of myocarditis as the first manifestation of COVID-19 even in absence of respiratory symptoms or radiological features of interstitial pneumonia.
Histopathological findings in nervous system
Histopathological examination of the brain showed no infiltration of inflammatory cells or neural cell degeneration though neurological symptoms in COVID-19 have been frequently reported. Autopsy findings of a Covid-19 patient showed haemorrhagic white matter lesions with axonal injuries and Perivascular acute disseminated encephalomyelitis (ADEM)-like appearance as well as neocortical microscopic infarcts were also observed [27].
Dizziness and headache were most common CNS manifestations, while taste and smell impairment were the most common peripheral nervous system symptoms [28]. Occasional neurological symptoms were stroke, acute encephalopathy, convulsions, ataxia, or nerve demyelination found in some patients. Autopsied brain tissue displayed signs of acute hypoxic ischemic injury like hyperaemia, oedema, and neuronal degeneration [29]. A post-mortem examination study in COVID-19 patients showed widespread brain lesions [30].
Histopathological findings in skin
The virus reaches the cutaneous tissue through the blood vessels where the ACE-2 can easily bind to the viral spike protein and facilitate viral invasion into the skin tissue. Like other viral infection, a COVID-19 patient may also show signs of erythematous rash, dermatitis, urticaria, chicken pox-like vesicles purpuric papulovesicular rash which may even be painful, pseudo-chilblains on fingertips and toes, macular/ maculopapular exanthems, livedo reticularis lesions, and petechiae (Figure 3). Histopathological findings of biopsy samples of infected persons showed superficial and deep perivascular dermatitis, blood vessels surrounded by lymphocytes, focal acantholytic suprabasal clefts, dyskeratotic and ballooning herpes-like keratinocytes, necrosis of keratinocytes, mucin deposition in the dermis and hypodermis, and nests of Langerhans cells within the epidermis [31,32].
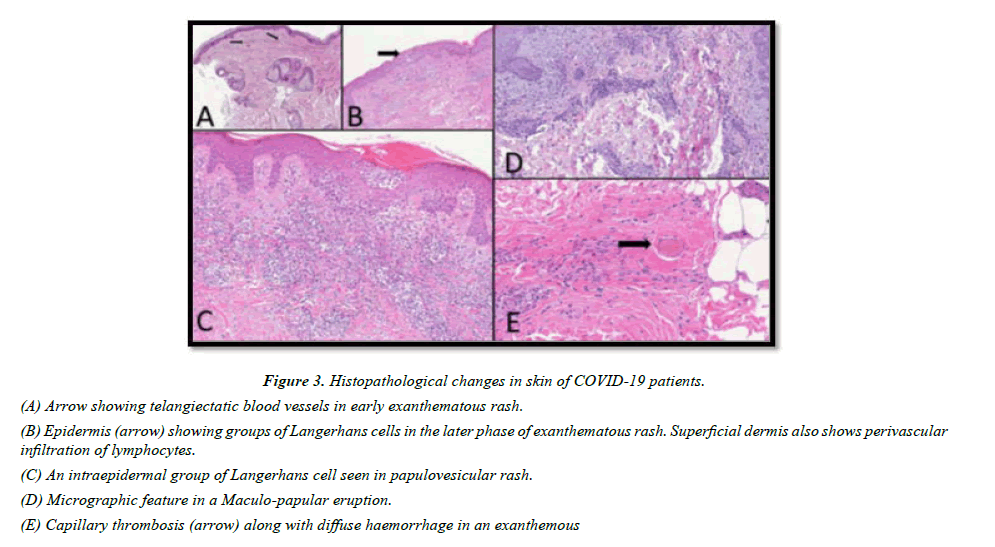
Figure 3: Histopathological changes in skin of COVID-19 patients.
(A) Arrow showing telangiectatic blood vessels in early exanthematous rash.
(B) Epidermis (arrow) showing groups of Langerhans cells in the later phase of exanthematous rash. Superficial dermis also shows perivascular infiltration of lymphocytes.
(C) An intraepidermal group of Langerhans cell seen in papulovesicular rash.
(D) Micrographic feature in a Maculo-papular eruption.
(E) Capillary thrombosis (arrow) along with diffuse haemorrhage in an exanthemous
Histopathological findings in Spleen and lymph nodes
Researchers stated that viral nucleocapsid protein (NP) could be seen in splenic tissue. Histopathologic examinations of some autopsy samples showed reduction of cell composition, atrophy of white pulp, neutrophil and plasma cell infiltration, reduction, or absence of lymph follicles, increase in red pulp to white pulp proportion, reduction of T and B cells due to necrosis and apoptosis, and atrophy of corpuscles in the spleen of infected cases [33]. Other than these symptoms, congestion and haemorrhagic appearance were also visualized in the spleen.
Histopathological findings in placenta & testis
SARS-Cov-2 alter placenta in pregnant women. Pathologic findings of biopsy samples revealed low grade fetal vascular malperfusion, intramural fibrin deposition, intramural nonocclusive thrombi, and meconium macrophages. Fetal thrombotic vasculopathy has also been reported [34].
ACE2 is also present in seminiferous tubules, Leydig cells, Sertoli cells and spermatogonia. When viral protein bound with the testicular cells expressing ACE2 receptor, it not only damages the testicular tissue but also forms a potential shelter for the virus. Different studies showed that Sertoli cells are more susceptible than germ cells as more than 90% Sertoli cells expressed ACE-2 receptors. All COVID-19 infected testes demonstrated extensive germ cell destruction and decreased spermatogenesis in the seminiferous tubules [13].
The basement membrane becomes thickened with peritubular fibrosis. Sertoli cells showed swelling, vacuolation and cytoplasmic rarefaction. One of the evident phenomena in the COVID-19 testis is leucocyte infiltration (Figure 4D). These cells could affect the function of Leydig cells and thus directly decrease the production of testosterone (Figure 4C). These infiltrated cells the lymphocytes and histiocytes also damage the blood–testis barrier and destroy the seminiferous tubules directly (Figure 4E & F). Like other viruses like HIV, mumps and hepatitis B virus, SARS-CoV- 2 also may lead to activation of inflammatory cytokines, which may initiate the autoimmune response as well as may result in the testicular damage leading to infertility and sterility. These symptoms may further increase the chances of testicular tumours [35].
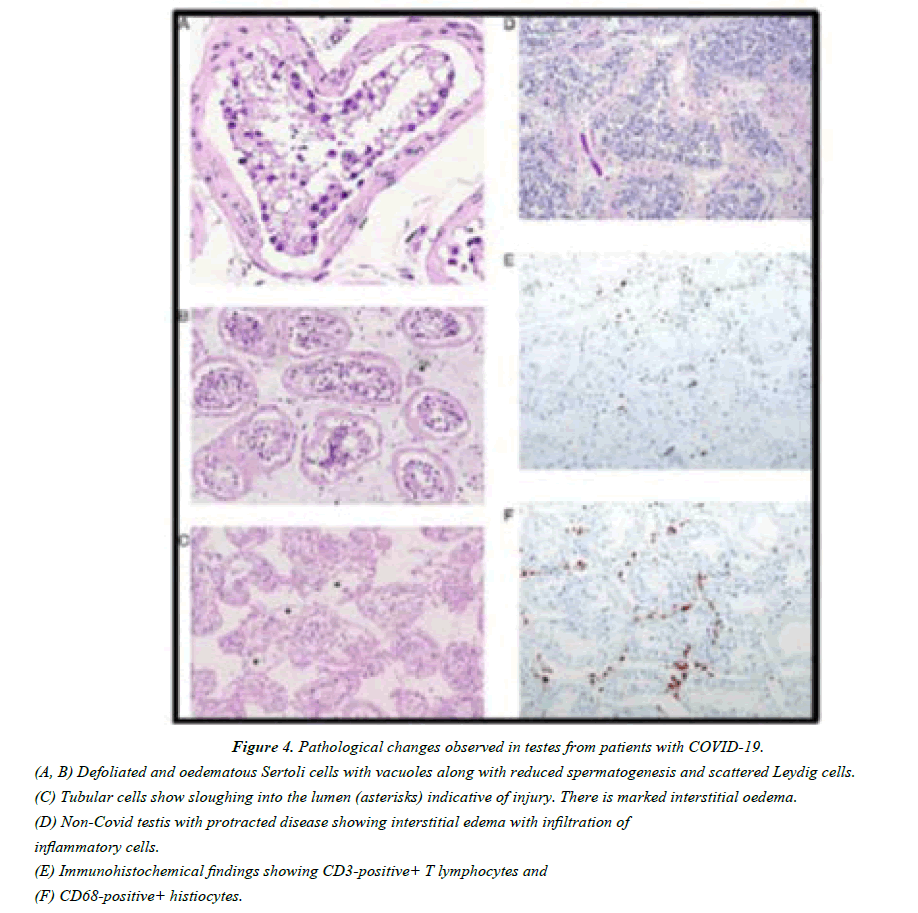
Figure 4: Pathological changes observed in testes from patients with COVID-19.
(A, B) Defoliated and oedematous Sertoli cells with vacuoles along with reduced spermatogenesis and scattered Leydig cells.
(C) Tubular cells show sloughing into the lumen (asterisks) indicative of injury. There is marked interstitial oedema.
(D) Non-Covid testis with protracted disease showing interstitial edema with infiltration of inflammatory cells.
(E) Immunohistochemical findings showing CD3-positive+ T lymphocytes and
(F) CD68-positive+ histiocytes.
RT-PCR result was not found any positive report for the semen or testicular biopsy specimen. So, it can be suggested that it will not be transmitted through the sexual route [36].
Conclusion
Varga published endothelial infection by the virus & stated that SARS-CoV-2 infection facilitates the induction of endothelitis in several organs as a direct consequence of viral involvement. SARS-CoV-2 infects the host using the angiotensin converting enzyme 2 (ACE2) receptor which is expressed in several organs including the lung, heart, kidney, intestine & testis more often if comorbidities are present [22]. Thus, no vascular tissue is safe.
In this article, we try to describe the histopathological changes caused by COVID-19 and special emphasis with extra pulmonary manifestations. This description is up to date as of now, but every day new experiences are pouring from different parts of the world, newer knowledge and information will appear by the time this article is published.
References
- Wang W, Yoneda M. Determination of the optimal penetration factor for evaluating the invasion process of aerosols from a confined source space to an uncontaminated area. Sci Total Environ. 2020;740:140113.
- Velavan TP, Meyer CG. The COVID?19 epidemic. Trop Med Int Health. 2020;25(3):278.
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3(1):237..
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. lancet. 2020;395(10223):497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med2020; 8: 420–22.
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033-4.
- The Human Protein Atlas. Tissue expression of ACE2 - Summary.
- Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017-32.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Chin J Pathol. 2020;49(5):411-7.
- Barton LM, Duval EJ, Stroberg E, et al. Covid-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725-33.
- Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007-14.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-3.
- Deshmukh V, Motwani R, Kumar A, et al. Histopathological observations in COVID-19: a systematic review. J Clin Pathol. 2021;74(2):76-83.
- Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215-20.
- Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221-4.
- Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. Biorxiv. 2020.
- Lagana SM, Kudose S, Iuga AC, et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33(11):2147-55.
- Sonzogni A, Previtali G, Seghezzi M, et al. Liver and COVID 19 infection: a very preliminary lesson learnt from histological post-mortem findings in 48 patients.
- Li J, Fan JG. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8(1):13.
- Fiel MI, El Jamal SM, Paniz-Mondolfi A, et al. Findings of hepatic severe acute respiratory syndrome Coronavirus-2 infection. Cell Mol Gastroenterol Hepatol. 2021;11(3):763-70.
- Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Internal Med. 2020;173(4):268-77.
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endothelitis in COVID-19. The Lancet. 2020;395(10234):1417-8.
- Yao XH, He ZC, Li TY, et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30(6):541-3.
- Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microb Infec. 2020;9(1):727-32.
- Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Intern. 2020;98(1):219-27.
- Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475-7.
- Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with Covid-19—a case series. New Engl J Med. 2020;382(25):2478-80.
- Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-90.
- Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018-27.
- Remmelink M, De Mendonça R, D’Haene N, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Critic Care. 2020;24(1):1-0.
- Gianotti R., Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm. Venereol. 23;100(8):adv00124.
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain?like lesions: lights and shadows on the relationship with COVID?19 infection. J Eur Acad Dermatol Enereol. 2020;34(11):2620-9.
- Tabary M, Khanmohammadi S, Araghi F, et al. Pathologic features of COVID-19: A concise review. Pathol Res Prac. 2020;216(9):153097.
- Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatric Dev Pathol. 2020;23(3):177-80.
- Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410-6.
- Song C, Wang Y, Li W, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod. 2020;103(1):4-6.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref