Research Article - Biomedical Research (2017) Volume 28, Issue 11
Expression and clinical significance of long non-coding RNA HOTAIR in endometrial carcinoma
Yanhua Zhang, Qinghua Zhang, Shumei Tuo, Xiaoli Wang*, Yan Wang, Shengju Hao, Jing Zheng and Bingxin Cai
Department of Gynecology, Maternal and Child Health Care Hospital of Gansu Province, Lanzhou, PR China
- *Corresponding Author:
- Xiaoli Wang
Department of Gynecology
Maternal and Child Health Care Hospital of Gansu Province, PR China
Accepted date: March 30, 2017
Abstract
This study aimed to investigate the expression and clinical significance of HOX Transcript Antisense RNA (HOTAIR) in endometrial carcinoma, and discuss its correlation with transforming growth factor- β1 (TGF-β1) in EC tissue. Sixty patients with EC were enrolled. The EC tissue and cancer adjacent tissue (control) were collected. The expressions of HOTAIR and TGF-β1 mRNA in EC and adjacent tissue were determined using real-time quantitative PCR. Results showed that, the relative expression levels of HOTAIR and TGF-β1 mRNA in EC tissue were significantly higher than those in adjacent tissue (P<0.05). The expression of HOTAIR mRNA was significantly correlated with pathological grade, muscular invasion depth and clinical stage of EC (P<0.05), and that of TGF-β1 mRNA was significantly correlated with muscular invasion depth and clinical stage of EC (P<0.05). In EC tissue the expressions of HOTAIR and TGF-β1 mRNA were positively correlated (r=0.676, P<0.05). There was significant difference of overall survival of patients between high and low HOTAIR expression groups (χ2=4.346, P<0.05). The abnormal expression of HOTAIR may be involved in the occurrence and development of EC. The expression of HOTAIR is positively correlated with TGF-β1 expression in EC tissue.
Keywords
Endometrial carcinoma, HOTAIR, TGF-β1, Correlation.
Introduction
Long non-coding RNAs (lncRNAs) are a class of RNAs of which the transcript length is more than 200 nt. lncRNAs do not encode the protein, but they regulate the gene expression from multiple levels (epigenetics, transcription, posttranscription, etc.). They play an important role in the occurrence and development of tumor [1,2]. HOX Transcript Antisense RNA (HOTAIR) is one of the firstly discovered lncRNAs, and has the regulation function of transcription [3]. Recent studies suggest that, there is abnormal expression of HOTAIR in a variety of tumors (liver cancer [4], colon cancer [5], breast cancer [6], lung cancer [7], pancreatic cancer [8], etc.). In addition, the amount of HOTAIR expression has important relation with the progression, metastasis and prognosis of tumor [9]. The in vitro study shows that, HOTAIR can promote the tumor cell proliferation, invasion and migration, and reduce the sensitivity of tumor to chemotherapy drugs [10]. Therefore, HOTAIR is expected to become a powerful prediction index and therapeutic target in diagnosis of tumor.
Endometrial Carcinoma (EC) is one of the three most common malignant tumors in female reproductive system. In some Europe and America countries, it has ranked the first in the tumors in obstetrics and gynecology [11]. In China, the incidence of EC is gradually increasing in recent years, which seriously harms the health of women [12]. The occurrence and development of EC is a complex pathological process which involves the participation of a large number of lncRNAs [13]. This study investigated the expression and clinical significance of long non-coding RNA HOTAIR in EC, and discussed its correlation with Transforming Growth Factor-β1 (TGF-β1) in EC tissue. The objective was to provide a basis for further investigating the role of HOTAIR in the diagnosis, treatment and prognosis of EC.
Subjects and Methods
Subjects
Sixty patients with EC confirmed in the Maternal and Child Health Care Hospital of Gansu Province from September 2015 to December 2016 were enrolled in this study. One EC tissue sample was collected from each patient, and the tissue 2 cm adjacent to EC tissue was used as the control. All specimens were stored in liquid nitrogen immediately after obtaining. The age of the patients ranged from 32-66 years old, with average of 50.7 ± 8.3 years. According to the pathological type, there were 42 cases of adenocarcinoma and 18 cases of other types. According to the pathological grade, there were 18, 25 and 17 cases with grade G1, G2 and G3, respectively. According to the muscular invasion depth, there were 39 cases with muscular invasion depth ≤ 1/2 and 21 cases with muscular invasion depth>1/2. According to the clinical stage of tumor, invasion depth, there were 36 cases with stage I and 24 cases with stage II. In addition, there were 48 cases with no lymph node metastasis and 12 cases with lymph node metastasis. All patients had not received chemotherapy or radiotherapy before surgery. This study was approved by the ethics committee of Maternal and Child Health Care Hospital of Gansu Province. Written informed consent was obtained from all participants.
Extraction of total RNA
EC or adjacent tissue sample (50-100 mg) was taken. The total RNA of sample was extracted using Trizol (Life Technologies Inc., CA, USA) in accordance with the instruction of kits. The OD260/OD280 value of RNA was measured using F-7000 ultraviolet spectrophotometer (Hitachi High-Technologies Corp., Tokyo, Japan), and the purity was analysed. The total RNA with OD260/OD280 value from 1.8 to 2.0 was considered as qualified. In addition, The 10 g/L agarose gel electrophoresis (Bio-Rad Laboratories, Inc., PA, USA) was performed on the extracted RNA sample. The 28 S and 18 S bands of ribosomal RNA were compared. The color intensity of 2: 1 (28 S: 18 S) by EB staining indicated that the extracted RNA was complete. Finally, the RNA sample was kept at -70°C in refrigerator for use. The entire total RNA extraction process was performed under RNase-free environment.
cDNA synthesis
The reverse transcription synthesis of cDNA was performed in 20 μL reaction system as follows: PrimeScript Buffer (5X), 4 μL; PrimeScript RT Enzyme Mix I, 1 μL; Oligo Dt Primer (50 μmol/L), 1 μL; Random 6 mers (100 μmol/L), 1 μL; total RNA, 13 μL. The operation was in accordance with the instruction of reverse transcription kits. The reaction condition was 37°C for 15 min, followed by 85°C for 5 s.
Real-time quantitative PCR
Real-time quantitative PCR was performed according the instruction of kits. The PCR system (15 μL for each sample) was as follows: Premix Ex Taq (2X), 7.5 μL; forward primer (10 μmol/L), 0.25 μL; reverse primer (10 μmol/L), 0.25 μL; cDNA (5 ng/μL), 3 μL; dH2O, 4 μL. Primers were designed and synthesized by Shanghai Invitrogen Biotechnology Co., Ltd. (Shanghai, China). The primers were as follows: HOTAIR: forward 5'-GGTAGAAAAAGCAACCACGAAGC-3'; reverse 5'-ACATAAACCTCTGTCTGTGAGTGCC-3'; TGF-β1: forward: 5'-CCAGA TTGAGACCCTCCTCA-3', reverse: 5'- ATGCAATGCTGTTCTTGCAG-3'; GAPDH (internal reference): forward 5'-CCGGGAAACTGTGGCGTGATGG-3', reverse 5'-AGGTGGAGGTATGGGTGTCGCTGTT-3', After initial denaturation of 15 min at 95°C, the PCR condition was as follows: HOTAIR: 50 cycles of 95°C for 15 s, 61°C for 15 s, and 72°C for 15 s; TGF-β1: 40 cycles of 95°C for 10 s, 58°C for 15 s, and 72°C for 10 s; GAPDH: 28 cycles of 94°C for 45 s, 59 °C for 30 s, and 72°C for 20 s. The relative expression level was determined using the 2-ΔΔCt analysis method [14].
Statistical analysis
All statistical analysis was carried out using SPSS19.0 software (SPSS Inc., Chicago, IL, USA). The measurement data were presented as mean ± SD, and were compared using t test. Pearson correlation analysis was performed on the correlation between HOTAIR and TGF-β1 expression in EC tissue. P<0.05 was considered as statistically significant.
Results
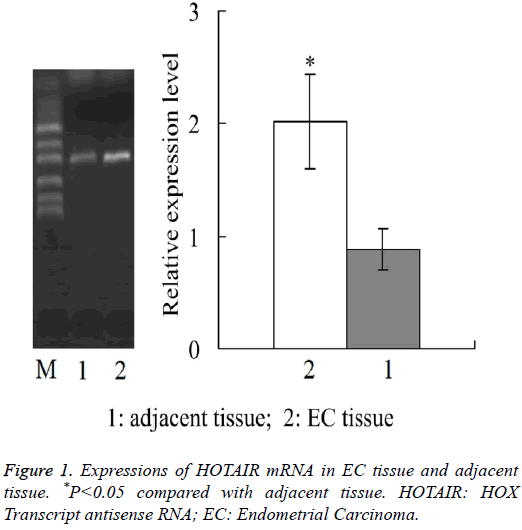
Expressions of HOTAIR mRNA in EC tissue and adjacent tissue
Real-time quantitative PCR showed that, the relative expression level of HOTAIR mRNA in EC tissue was 2.02 ± 0.42, which was significantly higher than 0.88 ± 0.18 in adjacent tissue (P<0.05) (Figure 1).
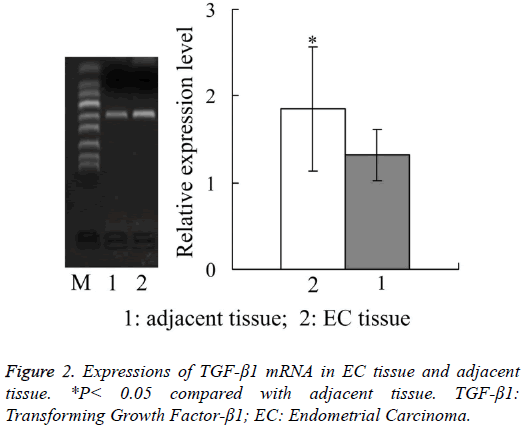
Expressions of TGF-β1 mRNA in EC tissue and adjacent tissue
Figure 2 showed that, the relative expression amount of TGF- β1 mRNA in EC tissue was 1.85 ± 0.71, which was also significantly higher than 1.32 ± 0.29 in adjacent tissue (P<0.05).
Correlation between HOTAIR mRNA expression and clinicopathological parameters in EC
Table 1 showed that, the expression level of HOTAIR mRNA was significantly correlated with pathological grade, muscular invasion depth and clinical stage of EC, respectively (P<0.05), with no significant correlation with patients age (>50 or ≤ 50 years), EC pathological type and lymph node metastasis, respectively (P>0.05).
| Clinicopathological parameter | n | HOTAIR mRNA expression | P |
|---|---|---|---|
| Age (years) | >0.05 | ||
| >50 | 43 | 2.13 ± 0.91 | |
| ≤ 50 | 17 | 1.96 ± 0.73 | |
| Pathological type | |||
| Adenocarcinoma | 42 | 1.92 ± 0.55 | >0.05 |
| Others | 18 | 2.19 ± 0.67 | |
| Pathological grade | <0.05 | ||
| G1 | 18 | 1.09 ± 0.52 | |
| G2 | 25 | 2.24 ± 0.88 | |
| G3 | 17 | 4.32 ± 0.93 | |
| Muscular invasion depth | <0.05 | ||
| ≤ 1/2 | 39 | 1.73 ± 0.58 | |
| >1/2 | 21 | 2.86 ± 0.61 | |
| Clinical stage | <0.05 | ||
| I | 36 | 1.61 ± 0.83 | |
| I+III | 24 | 3.26 ± 1.32 | |
| Lymph node metastasis | >0.05 | ||
| No | 48 | 1.94 ± 0.87 | |
| Yes | 12 | 2.19 ± 0.59 |
Table 1: Correlation between HOTAIR mRNA expression and clinicopathological parameters in EC.
Correlation between TGF-β1 mRNA expression and clinicopathological parameters in EC
As shown in Table 2, the expression level of TGF-β1 mRNA was significantly correlated with muscular invasion depth and clinical stage of EC, respectively (P<0.05), with no significant correlation with patients age (>50 or ≤ 50 years), EC pathological type, pathological grade and lymph node metastasis, respectively (P>0.05).
| Clinicopathological parameter | n | TGF-β1 mRNA expression | P |
|---|---|---|---|
| Age (years) | >0.05 | ||
| >50 | 43 | 1.89 ± 0.65 | |
| ≤ 50 | 17 | 1.68 ± 0.57 | |
| Pathological type | |||
| Adenocarcinoma | 42 | 1.92 ± 0.54 | >0.05 |
| Others | 18 | 1.79 ± 0.65 | |
| Pathological grade | >0.05 | ||
| G1 | 18 | 1.98 ± 0.61 | |
| G2 | 25 | 1.72 ± 0.71 | |
| G3 | 17 | 1.54 ± 0.57 | |
| Muscular invasion depth | <0.05 | ||
| ≤ 1/2 | 39 | 1.94 ± 0.64 | |
| >1/2 | 21 | 3.76 ± 0.94 | |
| Clinical stage | <0.05 | ||
| I | 36 | 1.53 ± 0.63 | |
| I+III | 24 | 2.95 ± 0.78 | |
| Lymph node metastasis | >0.05 | ||
| No | 48 | 1.66 ± 0.73 | |
| Yes | 12 | 2.13 ± 0.64 |
Table 2: Correlation between TGF-β1 mRNA expression and clinicopathological parameters in EC.
Correlation between HOTAIR mRNA and TGF-β1 mRNA expression in EC tissue
Pearson analysis showed that, in EC tissue the expression of HOTAIR mRNA and TGF-β1 mRNA were positively correlated (r=0.676, P<0.05).
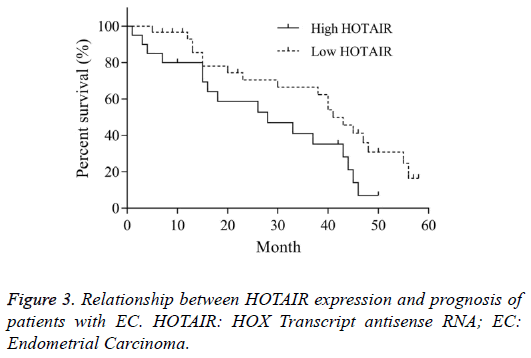
Relationship between HOTAIR expression and prognosis of patients with EC
According to the expression level of HOTAIR mRNA in EC tissue, the patients were divided into high HOTAIR and low HOTAIR groups, in which the HOTAIR mRNA level was higher and lower than the average value, respectively. The overall survival time of high HOTAIR group was 28 months (95% CI 0.35-1.32). The overall survival time of low HOTAIR was 41 months (95% CI 0.75-2.85). The Log-rank (Mantel- Cox) test in Kaplan-Meier method showed that, there was significant difference of overall survival between two groups (χ2=4.346, P<0.05, Figure 3). This indicated that, the high expression of HOTAIR was closely related with the prognosis of EC.
Discussion
EC is a kind of malignant epithelial tumor that occurs in the endometrium. The incidence of EC is increasing year by year. The diagnosis of EC is mainly based on the disease history, clinical symptoms and pathological outcome. However, the early symptoms of EC are not obvious, and it is easy to be misdiagnosed [15], so the survival rate of EC is not high [16]. The early diagnosis is helpful for the standardized treatment, and improvement of survival rate. At present, EC is still one of the gynecological malignant tumors which cause the death of patients. Therefore, in-depth understanding the pathogenesis of EC and early predicting its invasion and metastasis have important clinical importance for the early diagnosis of disease condition, formulation of treatment scheme, and comprehensive assessment of the prognosis [17].
HOTAIR is an important member of lncRNAs, and it is found to play an important role in the occurrence and development of tumors. Gupta et al. [3] found that, the expression of HOTAIR in primary loci and metastases loci of breast cancer tissue is significantly higher than the corresponding normal breast tissue. The high expression of HOTAIR is significantly related to the tumor size, clinical stage and survival time. In vitro studies have shown that, the up-regulation of HOTAIR can enhance the invasion and metastasis of breast cancer cells. Ishibashi et al. [18] found that, the expression of HOTAIR in hepatocellular carcinoma tissue is significantly higher than that in adjacent tissue, and the high expression of HOTAIR is significantly correlated with the tumor metastasis, recurrence and prognosis. Similarly, the up-regulated HOTAIR expression is found in pancreatic cancer [8], gastrointestinal stromal tumor [19], colorectal cancer [20], etc. Therefore, HOTAIR may serve as a cancer gene, which promotes the invasion and metastasis of tumor cells. However, the mechanism is not clear. Liu et al. [21] have found the up-regulated HOTAIR expression in cisplatin-resistant lung adenocarcinoma tissue. The possible mechanism is that, HOTAIR can regulate the expression of P21 in tumor tissue.
This study observed the expression of HOTAIR in EC tissue and adjacent tissue. Results found that, the expression level of HOTAIR mRNA in EC tissue was significantly higher than that in adjacent tissue (P<0.05). In addition, the expression of HOTAIR mRNA was significantly correlated with pathological grade, muscular invasion depth and clinical stage of EC, respectively (P<0.05). The study on overall survival showed that, the survival time in high HOTAIR group was significantly shorter than that in low HOTAIR group (P<0.05). It can be concluded that, HOTAIR plays an important role in the occurrence and development of EC and the high expression of HOTAIR was closely related with the prognosis of EC.
TGF-β1 is a kind of multifunctional polypeptide cytokines. It plays an important role in cell proliferation and differentiation, extracellular matrix formation, vascular cell growth, cell apoptosis, immune suppression and development of tumor [22]. TGF-β1 is highly expressed in a variety of tumors such as prostate cancer, colorectal cancer, gastric cancer, glioma and melanoma [23]. Results of this study found that, the relative expression amount of TGF-β1 mRNA in EC tissue was 1.85 ± 0.71, which was also significantly higher than 1.32 ± 0.29 in adjacent tissue (P<0.05). In addition, the expression level of TGF-β1 mRNA was significantly correlated with muscular invasion depth and clinical stage of EC, respectively (P<0.05).
Poly-comb repressive complex 2 (PRC2) is a member of genes in poly-comb protein family. It can maintain the gene transcription inhibition through modifying the chromatin structure. It is found that, HOTAIR can silence the transcription of HOXD site by linking PRC2 and histone methyltransferase LSD1 [24]. The cell cycle regulation-related genes, PRC2 target genes, are regulated by TGF-β1 [25]. Therefore, it is speculated that HOTAIR may has some correlation with TGF- β1. Results of this study found that, in EC tissue the expression of HOTAIR mRNA and TGF-β1 mRNA were positively correlated (r=0.676, P<0.05).
In conclusion, the abnormal expression of HOTAIR may be involved in the occurrence and development of EC. In addition, the expression of HOTAIR is positively correlated with TGF-β1 expression in EC tissue. This study has provided a basis for further investigating the role of HOTAIR in EC. This study still has some limitations. The sample size of this study is relatively small. Larger sample size will make the results more convincing. In our next studies, the sample size should be further increased for obtaining more satisfactory outcomes.
Funding
Gansu Provincial Youth Science and Technology Fund Project (No. 1506RJYA161).
References
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009; 10: 155-159.
- Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol 2012; 9: 703-719.
- Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010; 464: 1071-1076.
- Li H, An J, Wu M, Zheng Q, Gui X, Li T, Pu H, Lu D. LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2. Oncotarget 2015; 6: 27847-27864.
- Luo ZF, Zhao D, Li XQ, Cui YX, Ma N. Clinical significance of HOTAIR expression in colon cancer. World J Gastroenterol 2016; 22: 5254-5259.
- Milevskiy MJ, Al-Ejeh F, Saunus JM, Northwood KS, Bailey PJ, Betts JA, McCart Reed AE, Nephew KP, Stone A, Gee JM, Dowhan DH, Dray E, Shewan AM, French JD, Edwards SL, Clark SJ, Lakhani SR, Brown MA. Long-range regulators of the lncRNA HOTAIR enhance its prognostic potential in breast cancer. Hum Mol Genet 2016; 25: 3269-3283.
- Liu XH, Liu ZL, Sun M, Liu J, Wang ZX. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer 2013; 13: 464.
- Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 2013; 32: 1616-1625.
- Chen FJ, Sun M, Li SQ, Wu QQ, Ji L. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. MolCarcinog 2013; 52: 908-915.
- Shang C, Guo Y, Zhang H, Xue YX. Long noncoding RNA HOTAIR is a prognostic biomarker and inhibits chemosensitivity to doxorubicin in bladder transitional cell carcinoma. Cancer ChemotherPharmacol 2016; 77: 507-513.
- Buhtoiarova TN, Brenner CA, Singh M. Endometrial carcinoma: role of current and emerging biomarkers in resolving persistent clinical dilemmas. Am J ClinPathol 2016; 145: 8-21.
- Hu ZY, Tang LD, Zhou Q, Xiao L, Cao Y. Aberrant promoter hypermethylation of p16 gene in endometrial carcinoma. Tumour Biol 2015; 36: 1487-1491.
- Smolle MA, Bullock MD, Ling H, Pichler M, Haybaeck J. Long non-coding RNAs in endometrial carcinoma. Int J MolSci 2015; 16: 26463-26472.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402-408.
- Dijkhuizen FP, Mol BW, Brölmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer 2000; 89: 1765-1772.
- Tangjitgamol S, Srijaipracharoen S, Manusirivithaya S, Khunnarong J, Pataradool K, Thavaramara T. Endometrial carcinoma: clinical characteristic and survival rates by the new compared to the prior FIGO staging systems. J Med Assoc Thai 2013; 96: 505-512.
- Prat J. Prognostic parameters of endometrial carcinoma. Hum Pathol 2004; 35: 649-662.
- Ishibashi M, Kogo R, Shibata K, Sawada G, Takahashi Y, Kurashige J, Akiyoshi S, Sasaki S, Iwaya T, Sudo T, Sugimachi K, Mimori K, Wakabayashi G, Mori M. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep 2013; 29: 946-950.
- Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki Y, Nishida T, Bamba T, Kanda T, Ajioka Y, Taguchi T, Okahara S, Takahashi H, Nishida Y, Hosokawa M, Hasegawa T, Tokino T, Hirata K, Imai K, Toyota M, Shinomura Y. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res 2012; 72: 1126-1136.
- Xue Y, Gu D, Ma G, Zhu L, Hua Q. Genetic variants in lncRNA HOTAIR are associated with risk of colorectal cancer. Mutagenesis 2015; 30: 303-310.
- Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, De W, Wang Z, Wang R. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21WAF1/CIP1 expression. PLoS One 2013; 8: 77293.
- Fujii S, Maeda H, Tomokiyo A, Monnouchi S, Hori K, Wada N, Akamine A. Effects of TGF-β1 on the proliferation and differentiation of human periodontal ligament cells and a human periodontal ligament stem/progenitor cell line. Cell Tissue Res 2010; 342: 233-242.
- de la Cruz-Merino L, Henao-Carrasco F, Garcia-Manrique T, Fernandez-Salguero PM, Codes-Manuel de Villena M. Role of transforming growth factor beta in cancer microenvironment. ClinTranslOncol 2009; 11: 715-720.
- Kogo R , Shimamura T , Mimori K , Kawahara K , Imoto S , Sudo T , Tanaka F , Shibata K , Suzuki A , Komune S , Miyano S , Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 2011; 71: 6320-6326.
- Loewen G, Jayawickramarajah J, Zhuo Y, Shan B. Functions of lncRNA HOTAIR in lung cancer. J HematolOncol 2014; 7: 90.