Research Article - Biomedical Research (2017) Volume 28, Issue 1
Expressing the quantity variants of fibroblast growth factors-21 (FGF-21) in mice with congenital hypothyroidism
Jian-li Liu1, Rong-xiu Zheng1*, Xiu-hua Dai2, Lan-ying Wang21Department of Paediatrics, General Hospital of Tianjin Medical University, No. 154 Anshan Road, HePing District, Tianjin, PR China
2Maternal and Child Health Care Hospital of Tangshan, No.14 South Construction Road, Hebei Tangshan, PR China
- *Corresponding Author:
- Rong-xiu Zheng
Department of Pediatrics
General Hospital of Tianjin Medical University
PR China
Accepted date: May 23, 2016
Abstract
Thyroid hormone is essential for metabolism, nerve excitability and physical development. Congenital hypothyroidism leads to dysfunction of neuronal migration for thyroid hormone deficiency, and even delays psychomotor formation and development in serious congenital hypothyroidism. Congenital hypothyroidism presents a higher rate of incidence (1/2,500) in infants and is the most common causes of mental retardation. Fibroblast growth factors-21 (FGF-21) is an endogenous metabolism regulate factor that increases energy consumption, reduces blood lipid, triglyceride, low density lipoprotein levels, enhances the capacity of fat cells to absorb glucose and suppresses glucagon secretion. The purpose of this study was to investigate the changes of FGF-21 level and the efficacy of FGF-21 for mice with congenital hypothyroidism. The therapeutic efficacy of FGF-21 was conducted in an intravenous injection manner in a congenital hypothyroidism mouse model. The therapeutic outcomes of FGF-21 for mice with congenital hypothyroidism were assessed by animals’ behaviours. Our results found that FGF-21 levels were significantly lower both in serum and thyroid in congenital hypothyroidism mouse model than that in healthy subjects (**P<0.01). We observed that T4 and TSH were up-regulated in thyroid after FGF-21 treatment. The serum levels of FGF-21 and FGF-21 concentration in thyroid were higher than those in control groups (**P<0.01) after treatment with FGF-21. The serum levels of FGF-21 in thyroid was and positively related with thyroid stimulating hormone (TSH) and thyroxine (r=0.832, **P<0.01; r=0.381, **P<0.01; r=0.733, respectively). Multiple linear regression analysis showed that in hypothyroidism, FGF-21 was decreased and independent factors influencing serum TSH levels. Taken together, we provided evidence for protein diagnosis and potential efficacy of FGF-21 for congenital hypothyroidism.
Keywords
Congenital hypothyroidism, Fibroblast growth factors-21, Thyroid, Thyroxine.
Introduction
Congenital hypothyroidism is a kind of disease that causes insufficiency physiological effect and metabolism due to the reduction of thyroid hormone synthesis and secretion [1,2]. The critical importance of congenital hypothyroidism therapy has now been brought to the forefront in most industrialized parts of the world [3]. Congenital hypothyroidism is preventable though early detection and seasonable treatments. Despite the optimal therapy and the susceptive in clinical diagnosis and recognition for patients, more and more measures and methods programs for the detection and treatment of congenital hypothyroidism are urging needed [1,2,4,5]. Fibroblast growth factors-21 [FGF-21], one atypical member of the fibroblast growth factors [FGF] family, is a multifunctional protein predominantly secreted by adipose tissue, pancreas and liver [6,7]. FGF-21 is predominantly identified as a momentous controller and regulator of glucose and lipid metabolism as well as long-term energy balance [8,9]. FGF-21 has been found association with various human diseases and metabolic syndrome including the age, obesity, and type 2 diabetes mellitus and congenital hypothyroidism [10-12]. Previous study has showed that FGF-21 resulted in insulin resistance by inhibiting activation of NF-κB [13]. In addition, FGF-21 also played an endocrine hormone role in blocking somatic growth, which led to growth hormone resistance [14]. However, the mechanisms of endocrine hormone associated with starvation were not well understood and clearly elaborated.
In recent years, FGF-21 has developed a promising metabolic regulator and a hopeful potential drug for various human diseases including type 2 diabetes and caducity [15,16]. FGF-21 has been developed to be associated with glucose metabolism, hyperglycaemia and thermogenesis, which was performed as a hormone like manner [17]. In addition, the upstream and downstream signal pathways of FGF-21 were still keep unknown and being investigated in numerous studies. The relationships between FGF21 and thyroid hormone in metabolic actions were few reported and relation between congenital hypothyroidism and FGF-21 in previous study showed that expression of FGF-21 was up-regulated in patients with congenital hypothyroidism suggested that the metabolic regulation of FGF-21 and thyroid hormone may be closely related [18]. However, there was no study to investigate the correlation between FGF21 and thyroid function in an original congenital hypothyroidism mouse model. Furthermore, the therapeutic effects of FGF-21 for mice with congenital hypothyroidism were never studied.
In this present study, we investigated and the relationship between FGF-21 and thyroid hormone. We also studied the therapeutic effects of FGF-21 in a mouse model of congenital hypothyroidism through intravenous injection of FGF-21. To investigate the function and levels changes of FGF-21 in mice with congenital hypothyroidism, the concentration and therapeutic effects of FGF-21 were studied in mice with congenital hypothyroidism. The date analysed the correlation between FGF-21 and congenital hypothyroidism. This is a primary study and our results demonstrate that FGF-21 promoted the congenital hypothyroidism mice to secret thyroxine to recover cellular metabolism and increase vitality of thyroid cells.
Materials and Methods
Cell culture
HT-ori3 cells were obtained from the American Type Cell Culture (Rockville, MD), and cultured in DMEM (Gibco, CA, USA) with 10% foetal calf serum (FBS).
Standard protocol approvals, registrations, and patient consents
The animals’ study was administrated in Guide of Chinese animals’ experiments. All operations were carried out in strict accordance with the recommendations in the Use of Laboratory Animals of the National Institutes of Health. All surgery and blood collection were performed under sodium pentobarbital anaesthesia and analgesia, respectively. We exerted the greatest efforts to minimize suffering for experimental animals.
RNA extraction and real-time quantitative PCR (RT-qPCR)
Total RNAs were extracted from thyroid gland and HT-ori3 cells by using RNA easy Mini Kit (QIAGEN, Gaithersburg, MD). Expressions of FGF-21 in HT-ori3 cells and thyroid gland were measured by applying a RT-qPCR kit (QIAGEN, Gaithersburg, MD). All primers were synthesized by Invitrogen. The amplified PCR products were quantified by measuring the calculated cycle thresholds (Ct) of β actin mRNA. Relative mRNA expression changes were calculated by 2-ΔΔCt. The results in this study are expressed as the n-fold way compared to control.
Enzyme-linked immuno sorbent assay (ELISA)
Serum levels of TSH, T4, thyroxine and FGF-21 were determined by RIA with Coat-A-Count reagents as described previous [19] and FGF-21 protein detection kit.
Treatment administration of FGF-21
FGF-21 (once-daily) was administered intravenously injection on a continuous 30-day administration preclinical schedule. Treatment of FGF-21 will be terminated when unacceptable toxicity, progressive disease or death occurred. Doses of FGF-21 confirmation were conducted with experiment proteinuria and hypertension as well as continued until the MTD was determined. In addition, no food was forbidden for 2 hours following administration of FGF-21 once-daily. The score of mice was descripted in previous study [20].
Evaluation of toxicity
Toxicity of FGF-21 was assessed by using the National Congenital Hypothyroidism Institute Common Toxicity Criteria. Biochemical profile measurement of blood pressure and urinalysis were performed every two days during congenital hypothyroidism treatment periods. Electrocardiograms and biochemical detection were performed every three days. A DLT was defined as any of the following drug-related toxicities in previous study [21].
Animal study
Heterozygous Titf1+/− mice and heterozygous Pax8+/− mice were originally generated in a mixed genetic background to generate double heterozygote for both Titf1 and Pax8-null mutations (DHTP) mice according to previous study [22]. All mice were assigned Salac Experimental Animals Co., Ltd. Animals were housed in a conventional facility with a 12 h light, 12 h dark cycle and were supplied with standard rodent food. Genotypes were determined by PCR using genomic DNA isolated from the tail snips as described [23]. FGF-21 was administered daily by intravenously injection with PBS as control. Different serums from mice were collected from caudal vein of at least three mice for hormone, blood lipid, triglyceride, low density lipoprotein measurements. Animal experiments were conducted in accordance with the ethical guidelines of the Endocrine Society and approved institutional protocols at the National Institutes of Health.
Statistical methods
All data were presented as means and SEM. Statistical significance of differences between mean values was assessed by Student’s t test for unpaired data. Comparisons of data between multiple groups were performed with analysis of variance (ANOVA). Robust nonparametric Hodges-Lehmann estimates of median drugs treatment effects and 95% confidence interval are provided. Responder rates and treatment adverse events were analysed by χ2 test. P<0.05 was considered statistically significant.
Results
RT-qPCR analysis of gene expression in thyroid
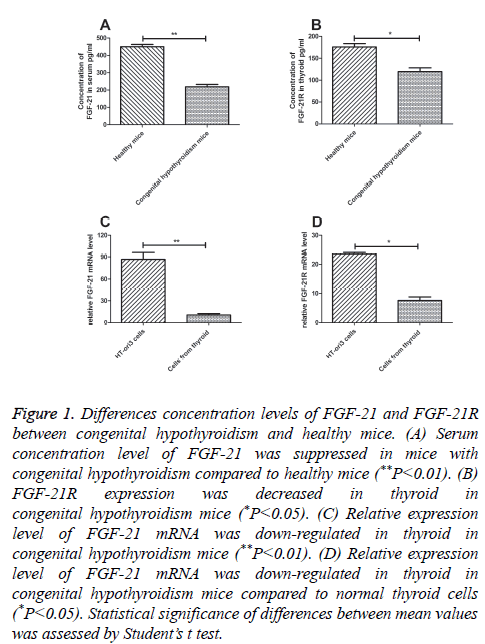
RT-qPCR was used to analyse the function of FGF-21 both in cultured thyroid cells and thyroid from mice with congenital hypothyroidism. The mRNA expression levels of FGF-21 and FGF-21 receptor (FGF-21R) were detected in this study. The results in Figure 1A showed that FGF-21 expression was decreased in thyroid in mice with congenital hypothyroidism (P=0.0075). Coincidentally, FGF-21R mRNA expression levels in thyroid also showed a decreased regulation in mice with congenital hypothyroidism (Figure 1B, P=0.036). In addition, normal human thyroid cell line HT-ori3 was used to analyse expression levels of FGF-21 and FGF-21R. FGF-21 expression level was well-balanced in HT-ori3 cells compared to mice with congenital hypothyroidism by analysis RT-qPCR (Figure 1C, P=0.0018). However, expression level of FGF-21R was more affluent in HT-ori3 cells than in thyroid in mice with congenital hypothyroidism (Figure 1D, P=0.0014). These results suggested that FGF-21 and FGG-21R expression levels were down-regulated in mice with congenital hypothyroidism.
Figure 1: Differences concentration levels of FGF-21 and FGF-21R between congenital hypothyroidism and healthy mice. (A) Serum concentration level of FGF-21 was suppressed in mice with congenital hypothyroidism compared to healthy mice (**P<0.01). (B) FGF-21R expression was decreased in thyroid in congenital hypothyroidism mice (*P<0.05). (C) Relative expression level of FGF-21 mRNA was down-regulated in thyroid in congenital hypothyroidism mice (**P<0.01). (D) Relative expression level of FGF-21 mRNA was down-regulated in thyroid in congenital hypothyroidism mice compared to normal thyroid cells (*P<0.05). Statistical significance of differences between mean values was assessed by Student’s t test.
Analysis of FGF-21, triglyceride, LDL-cholesterol and serum cholesterol concentration levels in serum in mice with congenital hypothyroidism
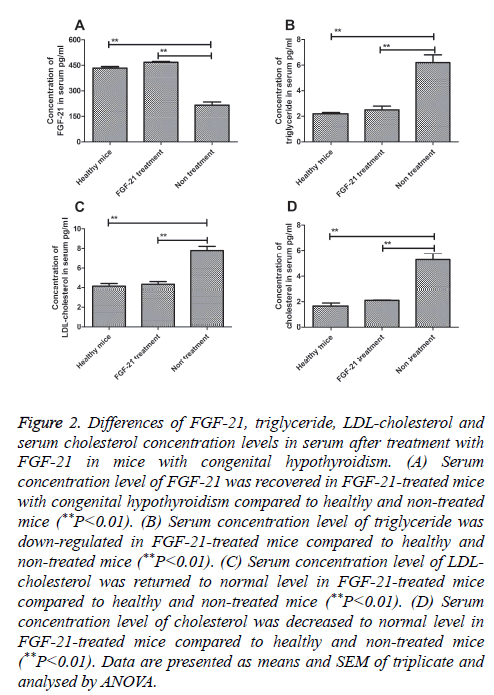
In order to confirm the expression and function of FGF-21 for congenital hypothyroidism, serum from mice with congenital hypothyroidism was used to detect FGF-21 expression by using ELISA as normal mice as control. Our result in Figure 2A showed that levels of FGF-21 in FGF-21-treated and healthy mice was increased compared to non-treated mice (P=0.0037 and P=0.0048, respectively). We observed that FGF-21 level recovered normal level after FGF-21 treatment compared to healthy mice. In addition, serum levels of triglyceride, LDL-cholesterol and serum cholesterol were also analysed prior and post treatment of FGF-21. The results in Figures 2B-2D showed that triglyceride, LDL-cholesterol and serum cholesterol were significantly up-regulated in serum after FGF-21 treatment compared to non-treated mice (**P<0.01, FGF-21-treated and healthy mice vs. non-treated mice). We found FGF-21 serous concentration recovered normal levels in congenital hypothyroidism mice treated by FGF-21 at MTD compared to healthy mice. These date indicated that FGF-21 showed a positive regulation of triglyceride, LDL-cholesterol and serum cholesterol.
Figure 2: Differences of FGF-21, triglyceride, LDL-cholesterol and serum cholesterol concentration levels in serum after treatment with FGF-21 in mice with congenital hypothyroidism. (A) Serum concentration level of FGF-21 was recovered in FGF-21-treated mice with congenital hypothyroidism compared to healthy and non-treated mice (**P<0.01). (B) Serum concentration level of triglyceride was down-regulated in FGF-21-treated mice compared to healthy and non-treated mice (**P<0.01). (C) Serum concentration level of LDL-cholesterol was returned to normal level in FGF-21-treated mice compared to healthy and non-treated mice (**P<0.01). (D) Serum concentration level of cholesterol was decreased to normal level in FGF-21-treated mice compared to healthy and non-treated mice (**P<0.01). Data are presented as means and SEM of triplicate and analysed by ANOVA.
Therapeutic effects of FGF-21 in in mice with congenital hypothyroidism
Median overall duration of treatment was 14 days. The cohorts of dose were 0.06, 0.12, 0.20, 0.28 and 0.40 mg for mice, respectively. In our results, 0.20 mg dose of FGF-21 once daily was identified as the MTD. The lowest-dose cohort of FGF-21 demonstrated the fewest treatment-related adverse events. The most common treatment-related advert events were fatigue, constipation, hypertension, proteinuria, decreased appetite, and weight decreased (Table 1, the overall incidence ≥ 10%).
| Total (n=60) | FGF-21 0.06-0.12 mg (n=20) | FGF-21 0.20-0.28 mg (n=20) | FGF-21 0.40 mg (n=20) | |
| Adverse events | ||||
| Hypertension | 9 | 1 | 3 | 5 |
| Proteinuria | 12 | 2 | 3 | 7 |
| Fatigue | 7 | 2 | 2 | 3 |
| Constipation | 13 | 3 | 3 | 7 |
| Weight decreased | 7 | 2 | 2 | 3 |
| Decreased appetite | 10 | 2 | 3 | 5 |
Table 1: Treatment-related adverse events of FGF-21 with an overall incidence ≥ 10%.
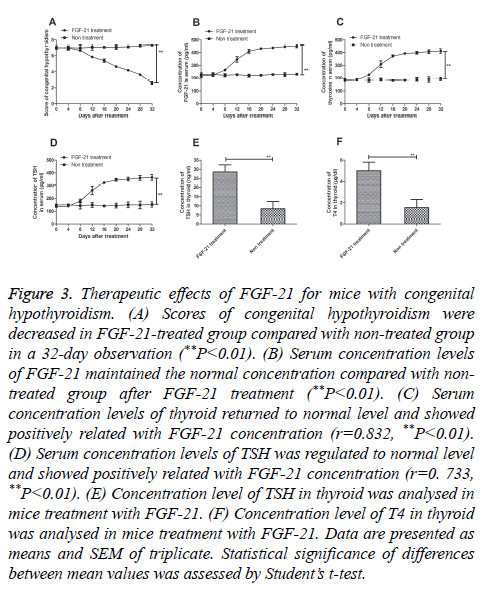
In this study, a MDT dose of FGF-21 was used to cure mice congenital hypothyroidism. The results in Figure 3A showed that congenital hypothyroidism was relieved after treatment with FGF-21 on day 32 (P=0.0044). We also analysed the FGF-21 levels after FGF-21 treatment. Our date in Figure 3B exhibited that FGF-21 could maintain a normal state after a 32- day treatment (P=0.0061). In addition, the result in Figures 3C and 3D showed that expression levels of thyroxine (P=0.0075) and thyroid stimulating hormone (TSH) (P=0.0086) were up-regulated in FGF-21-treated mice. Furthermore, we analysed T4 and TSH expression levels in thyroid after FGF-21 treatment. As shown in Figure 3E demonstrated that TSH concentration level was recovered the normal level after FGF-21 treatment as non-treated mice as control (P=0.0038). The result in Figure 3F showed that T4 was reached 6 μg/dl that was significant difference with non-treated mice with congenital hypothyroidism (P=0.0035). These results suggest that FGF-21 presented beneficial outcomes for mice with congenital hypothyroidism.
Figure 3: Therapeutic effects of FGF-21 for mice with congenital hypothyroidism. (A) Scores of congenital hypothyroidism were decreased in FGF-21-treated group compared with non-treated group in a 32-day observation (**P<0.01). (B) Serum concentration levels of FGF-21 maintained the normal concentration compared with nontreated group after FGF-21 treatment (**P<0.01). (C) Serum concentration levels of thyroid returned to normal level and showed positively related with FGF-21 concentration (r=0.832, **P<0.01). (D) Serum concentration levels of TSH was regulated to normal level and showed positively related with FGF-21 concentration (r=0. 733, **P<0.01). (E) Concentration level of TSH in thyroid was analysed in mice treatment with FGF-21. (F) Concentration level of T4 in thyroid was analysed in mice treatment with FGF-21. Data are presented as means and SEM of triplicate. Statistical significance of differences between mean values was assessed by Student’s t-test.
Pharmacokinetics of FGF-21 in congenital hypothyroidism mice
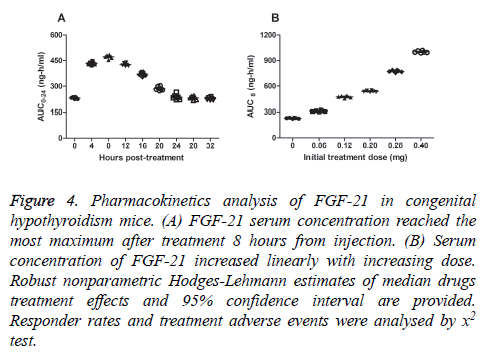
Blood samples obtained from 40 mice with congenital hypothyroidism were used for Pharmacokinetics analysis after the last dose of FGF-21. FGF-21 was absorbed rapidly at MDT dose within 4 hours. As shown in Figure 4A, serum concentration of FGF-21 reached the most maximum after treatment 8 hours. We observed mice Cmax concentrations of FGF-21 increased linearly with the increasing dose (Figure 4B). Our study showed that the median t½ of FGF-21 was ranged from 6.4 to 10.4 hours at the MDT dose (0.20 mg). The time of FGF-21 action lasted 24 hours and terminal elimination by estimating properly treated at the MDT dose. There is no drug accumulation after mice received MDT dosing (0.20 mg) by observing the Cmax values at steady state after the last dose of FGF-21. These date suggested that FGF-21 could preserve efficient concentration and functional time.
Figure 4: Pharmacokinetics analysis of FGF-21 in congenital hypothyroidism mice. (A) FGF-21 serum concentration reached the most maximum after treatment 8 hours from injection. (B) Serum concentration of FGF-21 increased linearly with increasing dose. Robust nonparametric Hodges-Lehmann estimates of median drugs treatment effects and 95% confidence interval are provided. Responder rates and treatment adverse events were analysed by x2 test.
Discussion
In this preclinical study, we demonstrated that the serum concentration level of FGF-21 was significantly decreased in mice with congenital hypothyroidism compared to healthy mice. We observed that levels of triglyceride, LDL-cholesterol and serum cholesterol were also relatively higher in the congenital hypothyroidism mice compared to healthy mice. Serum concentration levels of FGF-21, thyroxine and TSH were increased and reached normal level after treatment with FGF-21 as well as decreasing triglyceride, LDL-cholesterol and serum cholesterol, which suggested that treatment of FGF-21 for mice with congenital hypothyroidism presented beneficial effects of thyroid hormone, TSH triglyceride, LDL-cholesterol and serum cholesterol on FGF-21 metabolism dependently of dyslipidaemia induced by hypothyroidism. The MTD and the most common treatment-related advert events at MTD dose of FGF-21 for mice were also tested. The results exhibited well tolerate for FGF-21 in DHTP mice in our study. Congenital hypothyroidism is rare disease that thyroid hormone biosynthesis by thyroid secrets inadequately due to defective stimulation of a normal thyroid gland by TSH [24]. Congenital hypothyroidism was also reported as an isolated occurrence, or more frequently associated with additional pituitary hormone defective with or without associated extra pituitary abnormalities [25]. In addition, the European Society for Paediatrics Endocrinology has revised previous guidelines upon clinical and therapeutic schemes on congenital hypothyroidism in 2014 [26,27]. Treatment of congenital hypothyroidism has been became the focus for scientists and clinicians. New-born screening for congenital hypothyroidism are pivotal technique for preventing and treating of severe congenital hypothyroidism (TSH>40 mU/L). Therefore, treatments should be performed immediately as soon as the diagnosis of congenital hypothyroidism [28,29]. Hence, new efficient and advance treatments required to be developed and realized. FGF-21 is multifunctional protein predominantly secreted by adipose tissue, pancreas and liver, which increases energy consumption, reduces blood lipid, triglyceride, low density lipoprotein levels. Previous study has been showed that plasma FGF-21 levels were increased in patients with hypothyroidism independently of lipid profile [18]. Effects of thyroxine treatment on histology and behaviour by using the methimazole model of congenital hypothyroidism in the rat have been studied in previous study and initiated at P3 partially protected learning deficits seen following the imposition of complex operant response rules [29]. In this study, mouse model of congenital hypothyroidism was used to analyse FGF-21 expression level and the therapeutic effects of FGF-21 on metabolism dependently of dyslipidaemia induced by hypothyroidism were studied in a 32-day short observation. However, the animals’ results concluded a reverse conclusion with previous clinical trial of that FGF-21 concentration level was down-regulated and as well as triglyceride, LDLcholesterol and serum cholesterol in mice with congenital hypothyroidism. Although the comprehensible mechanisms of plasma FGF-21 concentration changes are not well understood, FGF-21 may be regulate NF-κB signal pathway to increase vitality of thyroid cells in hypothyroidism described in previous study [30]. However, the treatment of FGF-21 for mice with hypothyroidism indeed presented acceptable and slight treatment-related advert events during the treatment. FGF-21 was associated with the pathways of energy metabolism and this study presented a new insight of FGF-21 in treatment of thyroid hormone deficiently, which enhanced better explanation of FGF-21 polyfunctionality and preclinical efficacy for thyroid physiology. In addition, our experimental results suggested that FGF-21 might serve as a more comprehensive and tolerate drug for patients with mild congenital hypothyroidism. Furthermore, our date exhibited that FGF-21 up-regulated T4 and TSH concentration levels in thyroid through thyroid hormone replacement or thyroid hormone resistant.
In this preclinical study, dose of FGF-21 at the MTD and lower-dose cohort was associated with manageable toxicity. Treatment-related adverse events were relative minor and FGF-21 showed well tolerate for experimental mice [31]. Fatigue, constipation, hypertension, proteinuria, decreased appetite, and weight decreased, as well as gastrointestinal toxicity were low probability. Proteinuria, hypertriglyceridemia, diarrhoea, and fatigue were the most common treatment-related adverse events in mice after FGF-21 treatment.
In conclusion, in the present study, FGF21 served as a potential candidate for congenital hypothyroidism therapy by identifying serum level of FGF-21, which may be a protein of diagnosis and prognosis for patients with congenital hypothyroidism. However, this hypothesis of FGF-21 is needed for further assessment.
References
- Dalili S, Rezvany SM, Dadashi A. Congenital hypothyroidism: a review of the risk factors. Acta medica Iranica 2012; 50: 735-739.
- Rovet J, Daneman D. Congenital hypothyroidism: a review of current diagnostic, treatment practices in relation to neuropsychological outcome. Paediatric drugs 2003; 5: 141-149.
- Veisani Y, Sayehmiri K, Rezaeian S, Delpisheh A. Congenital hypothyroidism screening program in iran; a systematic review, metaanalysis. Iranian journal of pediatrics 2014; 24: 665-672.
- Hrytsiuk I, Gilbert R, Logan S, Pindoria S, Brook CG. Starting dose of levothyroxine for the treatment of congenital hypothyroidism: a systematic review. Arch Pediatr Adolesc Med 2002; 156: 485-491.
- Cameo T, Gumer LB, Williams KM, Gomez J, McMahon DJ, Oberfield SE. A retrospective review of new-born screening for congenital hypothyroidism, newborn thyroid disease at a major medical center. Clinical pediatrics 2013; 52: 1054-1058.
- Eto K. FGF-21, a newcomer in the field of hypertension research. J hum h tens 2013; 27: 343-344.
- Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21), its relation to obesity, metabolic syndrome,, non-alcoholic fatty liver in children: a longitudinal analysis. J Clin Endocrinol Metab 2012; 97: 2143-2150.
- Suomalainen A, Elo JM, Pietilainen KH. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet neurology 2011; 10: 806-818.
- Lin Z, Wu Z, Yin X. Serum levels of FGF-21 are increased in coronary heart disease patients, are independently associated with adverse lipid profile. PloS one 2010; 5: 15534.
- Ren G, Yin J, Wang W, Li L, Li D. Fibroblast growth factor (FGF)-21 signals through both FGF receptor-1, 2. Science China Life sciences 2010; 53: 1000-1008.
- Kharitonenkov A, Dunbar JD, Bina HA. FGF-21/FGF-21 receptor interaction, activation is determined by betaKlotho. J Cell Physiol 2008; 215: 1-7.
- Kharitonenkov A, Shiyanova TL, Koester A. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627-1635.
- Salehi MH, Kamalidehghan B, Houshm, M, Aryani O, Sadeghizadeh M, Mossalaeie MM. Association of fibroblast growth factor (FGF-21) as a biomarker with primary mitochondrial disorders, but not with secondary mitochondrial disorders (Friedreich Ataxia). Mol Biol Rep 2013; 40: 6495-6499.
- Gahete MD, Cordoba-Chacon J, Luque RM, Kineman RD. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocr J 2013; 154: 263-269.
- Matuszek B, Lenart-Lipinska M, Duma D, Solski J, Nowakowski A. Evaluation of concentrations of FGF-21 - a new adipocytokine in type 2 diabetes. Endokrynologia Polska 2010; 61: 50-54.
- Yu Y, Bai F, Wang W. Fibroblast growth factor 21 protects mouse brain against d-galactose induced aging via suppression of oxidative stress response, advanced glycation end products formation. Pharmacol Biochem Behav 2015; 133: 122-131.
- Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG: Brown adipose tissue responds to cold, adrenergic stimulation by induction of FGF21. Mol Med 2011; 17: 736-740.
- Lee Y, Park YJ, Ahn HY. Plasma FGF21 levels are increased in patients with hypothyroidism independently of lipid profile. Endocr J 2013; 60: 977-983.
- Hashimoto K, Tagami T, Yamakage H. Serum free thyroxine levels is associated with the efficacy of weight reduction therapy in obese female patients. Endocr J 2015; 63: 221-229.
- Barone B, Lopes CL, Tyszler LS. Evaluation of TSH cut-off value in blood-spot samples in neonatal screening for the diagnosis of congenital hypothyroidism in the Programa "Primeiros Passos" - IEDE/RJ. Arquivos brasileiros de endocrinologia e metabologia 2013; 57: 57-61.
- Boss DS, Glen H, Beijnen JH. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer 2012; 106: 1598-1604.
- Amendola E, De Luca P, Macchia PE. A mouse model demonstrates a multigenic origin of congenital hypothyroidism. Endocr J 2005; 146: 5038-5047.
- Friedrichsen S, Christ S, Heuer H. Expression of pituitary hormones in the Pax8-/- mouse model of congenital hypothyroidism. Endocr J 2004; 145: 1276-1283.
- Schoenmakers N, Alatzoglou KS, Chatterjee VK, Dattani MT. Recent advances in central congenital hypothyroidism. J endocr 2015; 227: 51-71.
- Noseda C, Putet G. Congenital chylothorax, hypothyroidism: a case report, a review of the literature. Archives de pediatrie organe officiel de la Societe francaise de pediatrie 2009; 16: 1470-1473.
- Calderoni P, Mignani G, Innao V. Congenital hypothyroidism (description of a clinical case, review of the literature). La Chirurgia degli organi di movimento 1988; 73: 389-392.
- Nagasaki K, Minamitani K, Anzo M. Guidelines for Mass Screening of Congenital Hypothyroidism (2014 revision). Clin pedi endocrinology, case reports, clinical investigations Clin Pediatr Endocrinol 2015; 24: 107-133.
- Peroni E, Vigone MC, Mora S. Congenital hypothyroidism treatment in infants: a comparative study between liquid, tablet formulations of levothyroxine. Horm Res Paediatr 2014; 81: 50-54.
- Hare E, Kim EM, Page D, Reid R. Effects of thyroxine treatment on histology, behavior using the methimazole model of congenital hypothyroidism in the rat. Neuroscience 2015; 285: 128-138.
- Liu MH. FGF-21 alleviates diabetes-associated vascular complications: Inhibiting NF-kappaB/NLRP3 inflammasome-mediated inflammation? Int J Cardiol 2015; 185: 320-321.
- Koransky R, Ferastraoaru D, Jerschow E. Single nonsteroidal anti-inflammatory drug induced serum sickness-like reaction to naproxen in a patient able to tolerate both aspirin, ibuprofen. J Allergy Clin Immunol Pract 2016; 4: 160-161.