Research Article - Journal of Dermatology Research and Skin Care (2023) Volume 7, Issue 2
Evaluation of the serum 25-hydroxyvitamin d levels and body mass index in patients with familial and non-familial psoriasis and their association with disease severity
Muhsin A Al-Dhalimi1*and Haider A Rudha2
1Department of Dermatology, University of Kufa, Najaf, Iraq.
2Department of Dermatology, Alsader Teaching hospital, Iraq.
- Corresponding Author:
- Muhsin A Al-Dhalimi
Department of Dermatology
University of Kufa, Najaf, Iraq.
E-mail: muhsin.aldhalimi@uokufa.edu.iq
Received: 06-Mar-2023, Manuscript No. AADRSC-23-93569; Editor assigned: 08-Mar-2023, PreQC No. AADRSC-23-93569(PQ); Reviewed: 21-Mar-2023, QC No AADRSC-23-93569; Revised: 24-Mar-2023, Manuscript No. AADRSC-23-93569(R); Published: 31-Mar-2023, DOI:10.35841/aadrsc-7.2.140
Citation: Al-Dhalimi MA, Rudha HA. Evaluation of the serum 25-hydroxy Vitamin D levels and body mass index in patients with familial and non-familial psoriasis and their association with disease severity. Dermatol Res Skin Care. 2023;7(2):140
Abstract
Background: Psoriasis is a common skin disease characterized by hyperproliferation of keratinocytes, poor differentiation, and altered immune system. Vitamin D deficiency and obesity may considered as independent risk factors for psoriasis.
The Objective: is to evaluate serum status of 25 hydroxyvitamin D and the body mass index in familial and nonfamilial chronic plaque psoriatic patients and their correlation with the severity of the disease.
Patients and Methods: This cross-sectional study had been conducted in the Department of Dermatology and Venereology – Al-Sader Medical Hospital in province of AL-Najaf, for the period March 2020 to October 2020. A total of 120 patients with chronic plaque psoriasis were included, divided into two groups according to the presence or absence of a positive family history of psoriasis in their first-degree relatives (60 patients for each group).
Results: A high prevalence of vitamin D deficiency (<20 ng/ml) was shown in both groups (83.4% in the familial vs. 93.3% in the non-familial group). No significant differences in the mean of vitamin D between the two groups were observed. Psoriasis was significantly more severe in the familial group. There was no significant weak negative relationship between psoriasis severity and vitamin D level in both groups.
Conclusions: A low level of vitamin D was found in psoriatic patients regardless of the family history of the disease. Body mass index in psoriatic patients not related to family history or severity of the disease. Psoriatic patients with a family history had an earlier onset and more severe disease.
Keywords
Psoriasis, Familial, Non-familial, Vitamin D, PASI score.
Introduction
Psoriasis is a common immune-mediated polygenic skin disorder with different environmental triggering factors [1]. It is characterized by sharply demarcated erythematous scaly plaques that are most commonly distributed symmetrically involving the elbows, knees, scalp and lower back, but any skin surface can be involved, which can affect quality of life and considered as stigmata [2].
The exact etiology of psoriasis is still unknown. Psoriasis is an immune-mediated inflammatory skin disease with a complex etiology involving the interaction between environmental and genetic risk factors as disease initiating event [3].
As a result, some studies have suggested that there are two types of psoriasis vulgaris, patients with early-onset psoriasis, a positive family history of psoriasis and expression of HLACw6 as having type I psoriasis and those with late-onset disease, no family history and lack of expression of HLACw6 as having type II psoriasis [4,5]. Patients with type one psoriasis have more severe disease in comparison to type two [6].
Vitamin D is a fat-soluble vitamin that has been found in all cells of the human body. The main source of vitamin D is dermal synthesis by the effect of Ultra Violet B-light (UVB), while the skin considers one of the targets of action of Vitamin D [7]. Keratinocytes have a unique feature because they are considered the main source of Vitamin D and also a responder to its active form. Vitamin D regulates the proliferation and differentiation of keratinocytes in a dose-dependent relationship [8]. The antiproliferative effect is mediated by decreased expression of c-myc and cyclin D and increased expression of cell cycle inhibitor proteins, while differentiation of keratinocytes is promoted by increasing the synthesis of the structural components of the cornified cell envelope, as well as increasing the intracellular calcium level through induction of the calcium receptor. Furthermore, vitamin D helps regulate the synthesis of glycosylceramides, which is essential for the integrity and permeability in the stratum corneum [9]. On the other hand, many studies revealed that vitamin D regulates keratinocyte apoptosis in a dose-dependent manner; this can be mediated by the effect of vitamin D on decreasing the expression of Bcl-xL (anti-apoptotic protein) which leading to the completion of the apoptotic process [10].
The effect of vitamin D on psoriatic patients is not yet complexly clear; some studies show that keratinocytes and fibroblasts from psoriatic patients may respond differently to vitamin D compared to normal controls. Fibroblasts from psoriasis patients had partial resistance to Vitamin D3, suggesting that there may be a biochemical defect inherent in the dermal fibroblasts of psoriatic patients [11,12].
The main cells involved in the pathogenesis of psoriasis are lymphocytes, dendritic cells, and keratinocytes, all of which contain VDR. Furthermore, the keratinocyte is considered the site of production and activation of vitamin D at the same time, therefore, vitamin D could play a role in the disease pathogenesis.
Psoriasis is considered Th-1 and Th-17 mediated disease; Vitamin D can inhibit the cytokines that are required for the activation of Th-1 and Th-17. Meanwhile, it stimulates the Th2 cell to produce IL-10, which is an anti-inflammatory cytokine that inhibits the expression of inflammatory cytokines in psoriasis that include IL-2, IL-6, IL-8, and finally γ-interferon. In dendritic cells, Vitamin D suppresses the expression of major histocompatibility complex (MHC) class-II molecules and costimulatory molecules, which leading to the prevention of T-cell activation [13].
Psoriasis is now considered as a systemic inflammatory disorder, associated with inflammatory arthritis, cardiovascular disease, and metabolic syndrome. Recent research suggests that vitamin D can improve metabolic syndrome. Vitamin D has been proposed to be hidden and stored in the abundance of adipose tissue in metabolic syndrome, with decreased circulating levels [14].
The efficacy of phototherapy treatment for psoriasis is well known since decades, and one of the postulated mechanisms of phototherapy is the increase of endogenous vitamin D synthesis [15]. Furthermore, topical vitamin D analogue has been used as a first-line treatment for localized disease for many years [16]. However, the use of a systemic vitamin D3 supplement has been investigated in several studies suggesting that this therapeutic alternative is safe and effective [17,18].
Other studies assessed the relationship between Vitamin D level and psoriasis [19-22]. In a recent meta-analysis, 25-hydroxyvitamin D levels were demonstrated to be lower in patients with psoriasis and that there is a small but statistically significant negative correlation between 25(OH) D levels and psoriasis severity [23].
Occurrence of psoriasis in family members of the first degree indicates a genetic predisposition, while vitamin D and obesity are considered triggering factors. The purpose of this study is to evaluate the relationship between the serum vitamin D level and body mass index in patients with familial and non-familial psoriasis and its correlation with disease severity.
Patients and methods
Study Design: This cross-sectional study was carried out in the dermatology department of Al-Sader Medical City in Al- Najaf province during the period between March 2020 and October 2020.
Ethics Statement: Verbal consent was obtained from all patients after an explanation of the nature of the study and ethical approval was obtained from the Scientific Committee of Dermatology and Venereology of the Iraqi council for medical specialization.
Patient selection: In this study a total of 120 adult patients with chronic plaque psoriasis were included, divided into two groups according to the presence or absence of a positive family history of psoriasis in their first degree relatives. Those with positive family history were considered a familial group, while those with negative family history regarded as nonfamilial group [24]. Each group consisted of 60 patients. The familial group was included (38 males and 22 females), while the non-familial group was (32 males and 28 females). The age of the participants ranged from (18-70) years. Full patient history was collected with respect to age, sex, job (indoors or outdoors), estimated time spent outdoors (in hours/week) [25], residence, family history, duration of the disease, the age at onset of psoriasis, drug administration, smoking and pastmedical. The information put together in a questionnaire sheet form involved subjects according to the patient's interview.
Inclusion criteria: Age between 18-70 years, clinically diagnosed patients with chronic plaque psoriasis depending on clinical morphology.
Exclusion criteria
1. Patients who received topical vitamin D analogue or any systemic medication for psoriasis treatment in the last month.
2. Patients taking a vitamin D supplement or those who have treated with phototherapy in the past month.
3. Patients with a medical history of renal, hepatic, thyroid, parathyroid, gastrointestinal disease (concomitant inflammatory bowel diseases).
4. Patients with chronic inflammatory diseases such as multiple sclerosis, inflammatory bowel disease, rheumatoid arthritis, lupus erythematosus, cutaneous lymphoma, and nonmelanoma skin cancer, or any other cancer.
5. Pregnancy and lactating women.
6. Patients taking drugs that might affect vitamin D status such as antiepileptic drugs, bisphosphonates, systemic corticosteroids, and calcium supplements.
Examination and Classification
Patients who met the inclusion and exclusion criteria were carefully examined for scales, erythema, induration, and sites of involvement to identify the Psoriasis Area and Severity Index (PASI). The patients were divided into three groups according to the PASI score into:
• Mild chronic plaque psoriasis < 10 PASI score.
• Moderate chronic plaque psoriasis 10-20 PASI score.
• Severe chronic plaque psoriasis > 20 PASI score.
The weight and height of the patients were measured, and their Body Mass Index (BMI) (kg/m2) was calculated dividing the weight in kilograms by the square of the height in meters. Skin phenotypes were identified according to the Fitzpatrick scale. General examination of the patients was performed to find signs of nail and joint involvement. All samples were collected in the sunny season to avoid seasonal variation in vitamin D level. All participants were resident in the same geographic zone (middle of Iraq) to avoid geographic variation in the level of vitamin D.
Two ml of venous blood were taken from participants with tourniquet, then blood was collected in vacutainer tube, centrifugation was carried out for 3 minutes where 20 units of serum were taken to measure the 25-hydroxyvitamin D level by the VIDAS® (BIOMerieux, inc. France) using the Enzyme Linked Fluorescent Assay techniques and automatically calculate the 25-hydroxyvitamin D concentration of each sample in 30 minutes.
Statistical analysis
Statistical analysis was performed using SPSS version 20. Mean+/- SD was used to represent numerical variables, while frequency, bar charts, and percentage were used for categorical variables. The Chi-square test and the Fisher exact test were used to assess the relationships between categorical variables.
Students t-test and ANOVA were used to compare means of two groups or more, respectively. Pearson correlation used to assess the correlation between two numerical variables. A P value equal to or less than 0.05 was considered statistically significant.
Results
In this study, a total of 120 patients with chronic plaque psoriasis were included, 70 males (58.3%) and 50 females (41.7%). The age of the participants ranged from 18-70 years, with a mean age of 40.8 years. Fitzpatrick’s skin phenotype ranged from type III to type V. They had suffered from psoriasis for 9.1±8.1 years. The mean age at the time of the onset of psoriasis was 31.5 years; the mean PASI value was 14.38, while the mean body mass index was 26.69 kg/m2. Of these 120 patients with psoriasis, 15% had nail psoriatic changes, and 21.7% were smoker. Table 1 showed the main characteristic information of the investigated sample.
| Variable | Number | Percentage | |
|---|---|---|---|
| Gender | Male | 70 | 58.3% |
| Female | 50 | 41.7% | |
| Age group | 18-29 | 18 | 15.0% |
| 30-39 | 44 | 36.7% | |
| 40-49 | 28 | 23.3% | |
| 50-59 | 14 | 11.7% | |
| 60-69 | 16 | 13.3% | |
| Skin phenotype | Type III | 22 | 18.3% |
| Type IV | 80 | 66.7% | |
| Type V | 18 | 15.0% | |
| Smoking | Yes | 26 | 21.7% |
| No | 94 | 78.3% | |
| Nail involvement | Yes | 18 | 15.0% |
| No | 102 | 85.0% | |
| BMI | Normal | 48 | 40.0% |
| Overweight | 38 | 31.7% | |
| Obese | 34 | 28.3% |
Table 1: The demographical features of the enrolled patients
The patients were divided into two groups: the first group consists of 60 psoriatic patients (28 males and 22 females) with a positive family history (familial group); while the second group consists of 60 psoriatic patients (32 males and 28 females) with a negative family history of psoriasis (nonfamilial group). Age ranged from (18 to 70) years, with the mean age of (39.5 ± 12.6) years for the familial group and (42.1 ± 13.1) years for the the non-familial group. The two groups did not differ significantly in age, gender, Fitzpatrick skin phototype, jobs, estimated time spent outdoors, smoking habit, and body mass index. The demographical characteristics of both groups were demonstrated in (Table 2).
| Family history | Total No. |
P | |||
|---|---|---|---|---|---|
| Positive (30) No.(%) |
Negative (30) No.(%) |
||||
| Gender | Male | 28 (54.3%) | 32 (45.7%) | 35 | 0.4 |
| Female | 22 (44%) | 28 (56%) | 25 | ||
| Age group | 18-29 | 10 (55.6%) | 8 (44.4%) | 18 | 0.3 |
| 30-39 | 26 (59%) | 18 (41%) | 44 | ||
| 40-49 | 8 (28.6%) | 20 (71.4%) | 28 | ||
| 50-59 | 10 (71.4%) | 4 (28.6%) | 14 | ||
| 60-69 | 6 (37.5%) | 10 (62.5%) | 16 | ||
| Skin phenotype | Type III | 10 (45.5%) | 12 (54.5%) | 11 | 0.9 |
| Type IV | 42 (52.5%) | 38 (47.5%) | 80 | ||
| Type V | 8 (44.4%) | 10 (55.6%) | 18 | ||
| Smoking | Yes | 12 (46.2%) | 14 (52.8%) | 26 | 0.8 |
| No | 48 (51.1%) | 46 (48.9%) | 92 | ||
| Nail involvement | Yes | 12 (66.7%) | 6 (33.3%) | 18 | 0.3 |
| No | 48 (47.1%) | 52 (52.9%) | 102 | ||
| Job | Outdoor | 28 (46.7%) | 32 (53.3%) | 60 | 0.6 |
| Indoor | 32 (53.3%) | 28 (46.7%) | 60 | ||
| BMI | Normal | 20 (41.7%) | 28 (58.3%) | 48 | 0.3 |
| Overweight | 18 (47.4%) | 20 (52.6%) | 38 | ||
| Obese | 22 (64.7%) | 12 (35.3%) | 34 | ||
Table 2: Demographic features of both groups participated in the study.
Association between Vitamin-D and psoriasis in both groups
The mean serum 25-hydroxyvitamin D level was (12.9 ± 6.8) ng/ml for the familial group and (11.8 ± 5.6) ng/ml for the non-familial group, so that the P-value=0.5 which means there is no significant difference between both groups. In addition, there was no significant difference in estimated time outside in both groups.
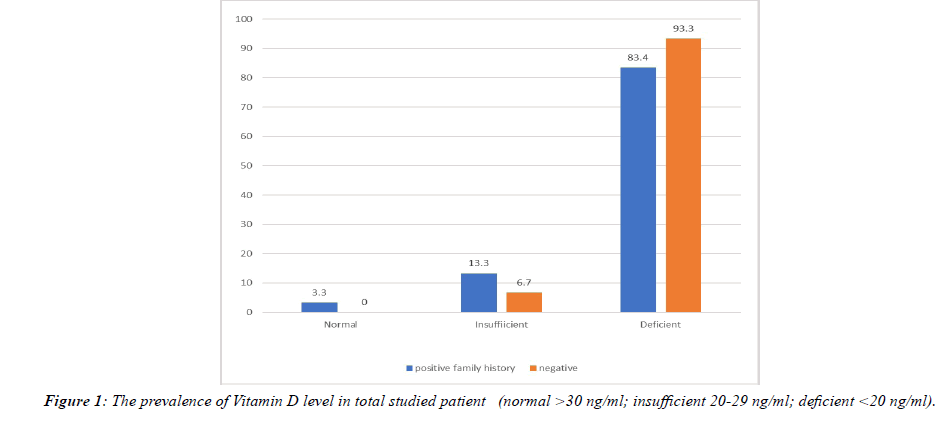
Although there is no significant difference between the two groups, interestingly the serum vitamin D level was deficient in 93.3% of the non-familial group and 83.4% of the familial group (only one patient had a sufficient level within the familial group) (Figure 1).
The prevalence of vitamin D insufficiency and deficiency (<30 ng/ml) in both groups was 98.3%, the total mean in both groups was (12.38 ± 6.2) ng/ml.
Comparison between PASI score in the familial and non-familial groups
The mean Psoriasis Area Severity Index score (PASI) for the familial group was (17.9 ± 10.7), while in non-familial group it was (10.8 ± 11.15); so there is a significant difference between the two groups (P value=0.01).
Examination of the relationships of severity of psoriasis assessed by PASI score and family history of psoriasis revealed that 74.2% of the non-familial group had mild disease; while 68.8% of the familial group had severe disease. The statistical difference between the two groups was highly significant (P value=0.0001) as shown in (Table 3).
| Family history | Total | p | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| PASI score | Mild (< 10) |
No. | 16 | 46 | 62 | 0.0001 |
| % | 25.8% | 74.2% | 100.0% | |||
| Moderate (10-20) |
No. | 22 | 4 | 26 | ||
| % | 84.6% | 15.4% | 100.0% | |||
| Severe (>20) |
No. | 22 | 10 | 32 | ||
| % | 68.8% | 31.2% | 100.0% | |||
| Total | No. | 60 | 60 | 120 | ||
| % | 50.0% | 50.0% | 100.0% | |||
Table 3: Comparison by mean ± SD of 25-hydroxyvitamin D level in both groups
Comparison of body mass index in the familial and the nonfamilial groups
Although more than half of the patients (58.3 %) with negative family history of psoriasis had a normal body mass index and approximately (64.7 %) of those with positive family history had obesity, the difference between the two group was not statistically significant (p value 0.3). Furthermore, the comparison of the BMI mean in both groups was not significant, as shown in (Table 4 & 5).
| Family history | Total | p | |||
|---|---|---|---|---|---|
| Positive(30) No. (%) | Negative (30) No. (%) | ||||
| BMI | Normal | 20 (41.7%) | 28 (58.3%) | 48 (100%) | 0.3 |
| Overweight | 18 (47.4%) | 20 (52.6%) | 38 (100%) | ||
| Obese | 22 (64.7%) | 12 (35.3%) | 34 (100%) | ||
Table 4:Comparison by mean ± SD of PASI score in both groups.
| Family history | mean | SD | p | |
|---|---|---|---|---|
| Body Mass Index | Positive | 27.4 | 4.7 | 0.2 |
| Negative | 25.9 | 3.8 |
Table 5: Comparison of psoriasis severity in both groups
The relationship between psoriasis severity and Vitamin D level in both groups
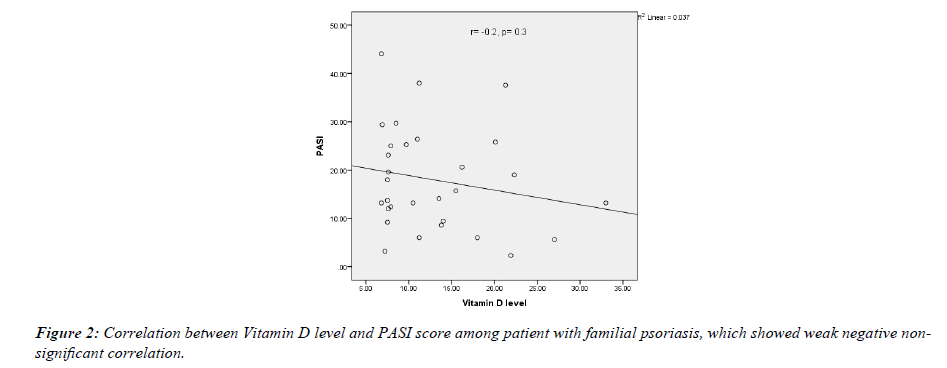
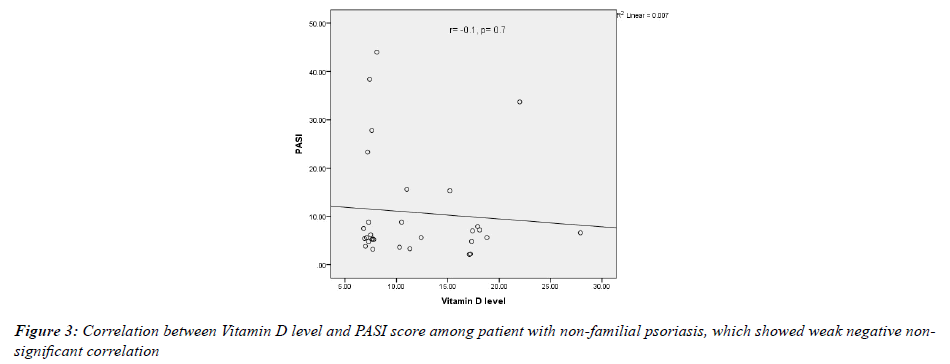
In both groups, the PASI score was inversely correlated with serum 25-OHD concentration but not significantly (in the familial group r=-0.2, p value=0.3; while in the non-familial group r=-0.1, p-value=0.7) as shown in (Figures 2 & 3).
Association between body mass index and severity of psoriasis
Although the mean PASI score was higher in obese psoriatic patients than in patients with normal BMI (18.7 ± 12.3 vs 12.5 ± 11.5) and approximately 62.5% of normal BMI had mild disease, while 47.1% of obese patients had severe disease, there was no significant association between BMI and PASI score (P value=0.2) (Table 6).
| PASI | Total | P | |||||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||||
| BMI | Normal | No. | 15 | 5 | 4 | 24 | 0.2 |
| % | 62.5% | 20.8% | 16.7% | 100.0% | |||
| Overweight | No. | 10 | 5 | 4 | 19 | ||
| % | 52.6% | 26.3% | 21.1% | 100.0% | |||
| Obese | No. | 6 | 3 | 8 | 17 | ||
| % | 35.3% | 17.6% | 47.1% | 100.0% | |||
| Total | No. | 31 | 13 | 16 | 60 | ||
| % | 51.7% | 21.7% | 26.7% | 100.0% | |||
Table 6: Family history status by body mass index
Relationship between vitamin D level and body mass index
There was no significant difference in the mean vitamin D level between normal, overweight and obese, psoriatic patients; where the p value=0.8 as shown in Table 7.
| BMI | p | |||
|---|---|---|---|---|
| Normal (a) | Overweight (b) | Obese (c) | ||
| The mean of Vitamin D level | 12.7±5.8 | 11.5±5.6 | 12.9±7.6 | 0.8 |
| (a) BMI= (18.5-24.9)kg/m2 (b) BMI= (25- 29.9) kg/m2 (c) BMI= >30 kg/m2 |
||||
Table 7:Comparison by mean±SD of body mass index in both groups
Association between Vitamin D level and patient sex
There was no significant difference in the mean vitamin D level between male and female (12.28 ng/ml vs 12.52 ng/ml) where p=0.9.
Discussion
Psoriasis is a common chronic multifactorial disease. The exact etiology of psoriasis is still unknown. Genetic susceptibility and various endogenous and exogenous triggering environmental factors are essential in its pathogenesis.
Previous studies have reported a relationship between vitamin D deficiency and psoriasis. In a large meta-analysis study, a significant relationship between low 25 hydroxyvitamin D levels and psoriasis have been concluded, thus low vitamin D levels might be one of the risk factor for psoriasis [26].
In the present study, 60 psoriatic patients with positive family history of psoriasis were compared with other 60 psoriatic patients without family history of psoriasis, there were no significant differences in age, sex, skin phenotypes and estimated time outside; demonstrating that the level of 25-hydroxyvitamin D was deficient (<20 ng/ml) in both groups in 88.33% of patients without significant statistical difference in mean between the two groups; in fact, only one patient (1.66%) of the participants in both groups had normal vitamin D level (>30 ng/ml). The prevalence of vitamin D deficiency in the non-familial group was higher than in the familial group (93.3% vs. 83.4%). Although the difference was not significant (p value=0.5), the mean of vitamin D was slightly lower in the non-familial group (11.8 ± 5.6 ng/ml vs 12.9 ± 6.8 ng/ml). We consider that all samples were collected during the sunny season in Iraq and from the same geographic region to avoid variation in vitamin D level due to these reasons. Accepting the limitation that the control group is absent in this research, our analysis also confirms that patients with psoriasis are a risk group for vitamin D deficiency and insufficiency. This may indicate that vitamin D deficiency is a risk factor for psoriasis regardless of family history.
Pavlov et al. evaluated 92 patients with psoriasis in a crosssectional study. Dividing them into two groups according to the age at the onset of psoriasis (<40 year for type-1 psoriasis, >40 year for type-2 psoriasis; family history wasn’t recorded) for the level of 25 hydroxyvitamin. They found that the mean level of Vitamin D in psoriatic patients was 12.07 ng/ml and there was no significant difference between the two groups. They also found that the prevalence of vitamin D deficiency and insufficiency (level<30 ng/ml) was 96.7% and only 3.3% had a normal level of vitamin D [27]. These results were consistent with our results, which found that most of the patients have a vitamin D level below 30 ng/ml (98.3%) and the mean in both studies was convergent.
The relationship between vitamin D and psoriasis was evaluated in several case-control studies, and most of them showed a significant association between psoriasis and Vitamin D deficiency.
Chandrashekar, et al. [28], Orgaz- Molina, et al. [29], Mattozzi, et al. [30] all show that the Vitamin D level significantly lower in psoriatic group than control.
Al Mutairi, et al. examined the level of vitamin D in 100 patients with psoriasis and the number of healthy controls. The researchers found that vitamin D concentration was (11.8 ± 3.7) ng/ml in patients, and (21.4 ± 7.8) ng/ml in healthy controls (p<0.0001) and vitamin D deficiency (serum vitamin D levels of <20 ng/ml) was detected in 87% of patients with psoriasis and 56% of the controls. In the present work, the prevalence of vitamin D deficiency was slightly more than the results of (88.33% vs 87%), and may be related to sample size differences and types of food intake. The mean of vitamin D in our research is slightly higher (12.38 ± 6.2 vs 11.8 ± 3.7) ng/ml, this difference may be attributed to the relatively small sample size and all patients in the present study were taken during the sunny season, while in their research the samples were collected throughout the year.
Gisondi, et al. evaluated the level of vitamin D in 145 patients with psoriasis and 141 healthy controls, the mean values of the psoriasis group was (20.7 ± 11.3) ng/ml while (37.1 ± 27.6) ng/ ml in the control group (p-value 0.001). Their results showed that vitamin D deficiency is significantly more prevalent in patients with psoriasis (57.8% vs 29.7%) [31]. They found that the level of vitamin D varies depending on the season during which blood sampling was taken; with highest level was in summer and the lowest in winter (27 ± 14.5 vs 16 ± 10.5) ng/ml. In the present research, we overcome this variation by taking all sample within the sunny season of Iraq (spring and summer).
On the other hand, Mallick, et al. alevaluated 70 psoriatic patients with the the same numbers of the healthy control group for the level of vitamin D. They found that the mean of 25 hydroxyvitamin D in patients with psoriasis is lower compared to controls (17.1 ± 9.1 vs 18.3 ± 10.7) but it is not statistically significant (P=0.66) [32]. Another study by Ardakani et al. investigated 110 patients with psoriasis and 110 matched controls for the prevalence of vitamin D deficiency in psoriasis and control. Although the results were not significant with regard to vitamin D deficiency (<20ng/ml) 64.5% of psoriatic patients vs 60% of controls (P-value=0.45), severe deficiency (<8 ng/ml) was significantly associated with psoriasis patients (P-value=0.014), this finding was attributed to sun avoidance behaviours, lack of vitamin D food enrichment and clothing habits in their cultures [33].
Solmaz, et al. studied 1393 psoriatic patients with or without psoriatic arthritis to determine the impact of having a family history on disease features. They concluded that patients with a positive family history had an earlier age at onset of psoriasis (29 ± 14.8 year vs. 31 ± 14.9 year; p=0.007), while statistical difference were observed between both groups with respect to body mass index (28.4 ± 5.5 kg/m2 vs 28.0 ± 5.1 kg/m2; p=0.316). These results were consistent with our results regarding the significant age difference at the onset of psoriasis in the familial and non-familial groups (26.8 ± 8.7 year vs 36.28 ± 11.12 year; P=0.001) and the body mass index in both groups that didn’t show significant differences (27.4 ± 4.7 kg/m2 vs 25.9 ± 3.8 kg/m2; p=0.2). The present study had shown greater significance regarding age of onset (P=0.001) compared to the study by Solmaz, et al. (p=0.007), this variation may be related to the difference in sample size, ethnic diversity, and differences in inclusion criteria between both studies.
Dividing patients into two groups according to the age at onset of psoriasis less than 40 years (early onset psoriasis) and more than 40 years (late onset psoriasis), showed that about 70% of the patient had early onset psoriasis and the family history was positive in 60% of them while only 30% of the patients their age at the time of onset of psoriasis was above 40 years, the family history was positive in only 28% of them. In addition, the present study, the results showed that the disease is more severe in the familial group with the mean PASI score was (17.9 ± 10.7 vs 10.8 ± 11.5) and the p value was significantly different (p=0.01), these results were consistent with Henseler and Christophers who concluded that early onset psoriasis associated with family history and has a more severe course of disease [34]. A similar result also showed in Chularojanamontri et al. [35,36].
The current work showed that there was a negative correlation between vitamin D level and PASI score, but this relationship was weak and non-significant in both groups (in the familial group r= -0.2, p value= 0.3; while in the non-familial group r=-0.1, p-value=0.7). This result was consistent with the results of Ricceri, et al. and El Farargy, et al., where they found this correlation between vitamin D level and PASI score. In Ricceri, et al., serum 25-hydroxy (OH) vitamin D concentration was significantly and negatively correlated with PASI (r = -0.88; P=0.001) while El Farargy, et al., also found a significant negative correlation (r=-0.680, P<0.001) [37,38]. In the present study the P value was not significant and the relationship was weaker, which may be related to the differece in sample size.
Interestingly, examination of the severity of psoriasis in association with body mass index showed that there was a weak positive correlation (r=0.18) with non-significant p value (p=0.2), where the mean PASI score was 12.5 ± 11.5, 13.01 ± 10.07 and 18.7 ± 12.3 in normal, overweight and obese patients, respectively. However, the prevalence of psoriatic patients who had severe disease (PASI>20) was higher in obese patients 47.1%, while mild disease (PASI<10) was higher in normal BMI 62.5%. These results were similar to the results of Sobhan and Farshchian, where they surveyed 42 patients with psoriasis in Iran and found the mean BMI of patients with mild, moderate and severe psoriasis was 25.86 ± 5.93, 30.85 ± 3.77 and 26.96 ± 5.68 kg/m2, respectively (P=0.096), which showed no significant difference [39]. These results are also consistent with El-Komy, et al., in a recently published large retrospective observational study in Egypt that observed 2534 patients to identify the clinical and epidemiological characteristics of psoriasis. They did not detect any correlation between body mass index and PASI score (P=0.849) [40]. On the other hand many studies showed that the relationship between psoriasis severity and obesity is significant, in one systemic review published by Felming et al. reviewing 134 823 psoriasis patients included in nine different articles revealed a significant association of increased psoriasis severity with higher BMI in seven of nine articles [41]. The current study examined the relationship between obesity and the severity of psoriasis, and the result didn’t show a significant relationship between the severity of the disease and mean BMI. However, the disease was more severe in obese patients. This variation in the result may be related to the difference in sample size and ethnic factors.
Furthermore, our result showed that there was no relationship between vitamin D level and body mass index with the mean of vitamin D was 12.7 ± 5.8, 11.5 ± 5.6, 12.9 ± 7.6 ng/ml in normal, overweight and obese patients, respectively (p=0.8), this result may be related to the fact that the prevalence of vitamin D deficiency was quite high in almost all the samples examined. Similar finding was recorded by Pavlov et al., which couldn’t find a significant correlation between body mass index and 25- hydroxyvitamin D (r=-0.09). In Hamza and Hasan, another study surveyed the association between vitamin D level and body mass index in Erbil, Iraq. Out of 200 childbearing age females participated in the study, vitamin D deficient individuals were more likely to be in obese individual (43.8%) compared to overweight (28.8%), normal weight (39%) and underweight (20%), but the level of difference was not significant (P=0.205) [42].
Comparison of serum vitamin D level in male and female didn’t show significant differences in mean of vitamin D (12.28 ng/ml vs 12.52 ng/ml) where p = 0.9. A slight difference in the mean vitamin D level according to sex was also observed in Al-Hilali, who studying 300 individuals for the prevalence of Vitamin D deficiency in Karbala- Iraq. Dividing them into four groups according to gender and age. The mean of Vitamin D was relatively convergent between both genders within the same age group [43]. In another large retrospective study on 10823 individuals (2782 male and 8043 female) living in Erbil-Iraq, their results showed that 76.9% of the examined population had Vitamin D deficiency (<20 ng/ml) and there were no differences in the mean vitamin D level between male and female within the study population (15 ± 16.8 vs 14.6 ± 22.1 ng/ml; P>0.05) [44]. These results were consistent with our results, and we may attributed these results because Iraq climate is very hot and dry during sunny seasons, so the population of both sexes does not spend their time outdoors. In addition to indoor life, the poor diet in vitamin D and the inaccessibility of vitamin D-fortified diet and clothing habits are substantial factors.
Lastly, the main limitation of this study is the absence of a control group, single centre study and the small number of sample sizes.
References
- Bolognia JL, Jorizzo JL, Schaffer JV, et al. Bolognia Textbook of Dermatology. 4th ed. ELSEVIER; 2018. 138-47 p.
- Kang S, Amagai M, Bruckner AL, et al. Fitzpatrick’s Dermatology in General Medicine. 9th ed. Mc Graw Hill Book Company; 2019. 458-94 p.
- Singh S, Pradhan D, Puri P, et al. Genomic alterations driving psoriasis pathogenesis. Gene. 2019;683:61-71.
- Barker JN. Genetic aspects of psoriasis. Clin Exp Dermatol. 2001;26(4):321-5.
- Christophers E HT. Psoriasis type-I and II as subtypes of nonpustular psoriasis. Semin Dermatol. 1992;1::261-6.
- Solmaz D, Bakirci S, Kimyon G, et al. Impact of Having Family History of Psoriasis or Psoriatic Arthritis on Psoriatic Disease. Arthritis Care Res. 2020;72(1):63-8.
- Mostafa WZ, Hegazy RA. Vitamin D and the skin: Focus on a complex relationship: A review. J Adv Res. 2013;6(6):793–804.
- Umar M, Sastry KS, Al Ali F, et al. Vitamin D and the pathophysiology of inflammatory skin diseases. Skin Pharmacol Physiol. 2018;31(2):74-86.
- Barrea L, Savanelli MC, Di Somma C, et al. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev Endocr Metab Disord. 2017;18(2):195-205.
- Fukuya Y, Higaki M, Higaki Y, et al. Effect of vitamin D3 on the increased expression of bcl-xL in psoriasis. Arch Dermatol Res. 2002;293(12):620-5.
- MacLaughlin JA, Gange W, Taylor D, et al. Cultured psoriatic fibroblasts from involved and uninvolved sites have a partial but not absolute resistance to the proliferation-inhibition activity of 1, 25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1985;82(16):5409-12.
- Smith EL, Walworth NC, Holick MF. Effect of 1α, 25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986;86(6):709-14.
- Mihaela Z, Nicolae-Alexandru G, Sergiu C, et al. The association between vitamin d values and psoriasis: A literature review. ARS Medica Tomitana. 2020;25(4):193-9.
- Kuang Y, Xiao Y, Fang Z, et al. Association of serum Vitamin D with psoriasis and effect modification by central obesity. Front Med. 2020;7:1-6.
- Al-Mutairi N, Shaaban D. Effect of narrowband ultraviolet B therapy on serum vitamin D and cathelicidin (LL-37) in patients with chronic plaque psoriasis. J Cutan Med Surg. 2014;18(1):43-8.
- Ighani A, Partridge ACR, Shear NH, et al. Comparison of Management Guidelines for Moderate-to-Severe Plaque Psoriasis: A Review of Phototherapy, Systemic Therapies, and Biologic Agents. J Cutan Med Surg. 2019;23(2):204-21.
- Lourencetti M, De Abreu MM. Use of active metabolites of Vitamin D orally for the treatment of psoriasis. Rev Assoc Med Bras. 2018;64(7):643–8.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatolog Treat. 2013;24(4):261–7.
- Al-Mutairi N, El Eassa B, Nair V. Measurement of vitamin D and cathelicidin (LL-37) levels in patients of psoriasis with co-morbidities. Indian J Dermatol Venereol Leprol. 2013;79(4):492–6.
- Maleki M, Nahidi Y, Azizahari S, et al. Serum 25-OH Vitamin D level in psoriatic patients and comparison with control subjects. J Cutan Med Surg. 2016;20(3):207–10.
- Zuchi MF, Azevedo P de O, Tanaka AA, et al. Serum levels of 25-hydroxy vitamin D in psoriatic patients. An Bras Dermatol. 2015;90(3):430–2.
- El-Moaty Zaher HA, El-Komy MHM, Hegazy RA, et al. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69(5):840-2.
- Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43(5):529-35.
- Bayaraa B, Arima H, Imafuku S. Body mass index in psoriatic patients with or without familial psoriasis. J Dermatol. 2020;47(4):402-4.
- Cargill J, Lucas RM, Gies P, et al. Validation of brief questionnaire measures of sun exposure and skin pigmentation against detailed and objective measures including vitamin D status. Photochem Photobiol. 2013;88(1):219-26.
- Pitukweerakul S, Thavaraputta S, Prachuapthunyachart S, et al. Hypovitaminosis D is Associated with Psoriasis: A Systematic Review and Meta- Analysis. Kansas J Med. 2019;12(4):103–8.
- Pavlov SI, Ivanova II, Gerova D. Vitamin D status in patients with psoriasis. Scr Sci Med. 2016;48(1):50-4.
- Chandrashekar L, Krishna Kumari GR, Rajappa M, et al. 25-hydroxy Vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72(2):56-60.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-Hydroxyvitamin D in psoriatic patients: A case-Control study. J Am Acad Dermatol. 2012;67(5):931–8.
- Mattozzi C, Paolino G, Salvi M, et al. Correlation between plasmatic levels of Vitamin D and PASI score. G Ital di Dermatologia e Venereol. 2018;153(2):155-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166(3):505–10.
- Mallick YA, Jiwani A. Serum Concentration of 25-Hydroxy Vitamin D in Patients with Chronic Plaque Psoriasis: A Case Control Study. J Dow Univ Heal Sci. 2020;14(2):47-53.
- Ebrahimzadeh Ardakani M, Afkhami-Ardekani M, Taghizadeh Yazdi MR, et al. Vitamin D Deficiency and Psoriasis: A Cross-Sectional study of Iranian Population in Yazd Province. Iran J diabetes Obes. 2020;12(3):151-5.
- Henseler T, Christophers E. Psoriasis of early and late onset: Characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13(3):450-6.
- Ferrándiz C, Pujol RM, García-Patos V, et al. Psoriasis of early and late onset: A clinical and epidemiologic study from Spain. J Am Acad Dermatol. 2002;46(6):867-73.
- Chularojanamontri L, Kulthanan K, Suthipinittharm P, et al. Clinical differences between early- and late-onset psoriasis in Thai patients. Int J Dermatol. 2015;54(3):290-4.
- Ricceri F, Pescitelli L, Tripo L, et al. Deficiency of serum concentration of 25-hydroxyvitamin D correlates with severity of disease in chronic plaque psoriasis. J Am Acad Dermatol. 2013;68(3):511-2.
- El-Farargy S, Ghanayem NM, Elrashidy AM. Correlation between vitamin D serum level and severity of psoriasis. Menoufia Med J. 2020;33(3):1016-20.
- Sobhan M, Farshchian M. Associations between body mass index and severity of psoriasis. Clin Cosmet Investig Dermatol. 2017;10:493-8.
- El-Komy MHM, Mashaly H, Sayed KS, et al. Clinical and epidemiologic features of psoriasis patients in an Egyptian medical center. JAAD Int. 2020;1(2):81-90.
- Fleming P, Kraft J, Gulliver WP, et al. The relationship of obesity with the severity of psoriasis: A systematic review. J Cutan Med Surg. 2015;19(5):450-6.
- Mohammed AAAG, Al-aaragi ANH, Merzah MA. The association between Vitamin D Level and Body Mass Index in a Sample of Childbearing Age Women in Erbil. Med J Babylon. 2018;15(2):164-8.
- Al-Hilali KA. Prevalence of hypovitaminosis D in adult Iraqi people including postmenopausal women. Sci Res J. 2016;4:53-62.
- Abdulrahman RM, Rahman BMA. Prevalence of vitamin D level in the serum of patients living in Erbil city, Iraq, referred to private clinical laboratory and effect of age and sex on it. J Biol Res. 2018;91(1):8-11.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref