Research Article - Biomedical Research (2017) Volume 28, Issue 5
Evaluating the effect of peroral bentonite administration on iron disposition and mortality in rat model of acute iron toxicity
Demir O1*, Kaplan YC2, Gokalp Yildiz F3, Aslan O4, Karadas B2, Gelal A5, Yigitbasi T6, Dost T7, Birincioglu M71Department of Pharmacology, Izmir University, Izmir, Turkey
2Department of Pharmacology, Izmir Katip Celebi University, Izmir, Turkey
3Provincial Health Directorate of Aydin, Aydin, Turkey
4Diyarbakır Maternity and Children Hospital, Clinical Chemistry Laboratory, Diyarbakir, Turkey
5Department of Pharmacology, Dokuz Eylul University Faculty of Medicine, Izmir, Turkey
6Department of Biochemistry, Izmir Katip Celebi University, Izmir, Turkey
7Department of Pharmacology, Adnan Menderes University, Aydin, Turkey
- *Corresponding Author:
- Omer Demir
Department of Pharmacology, Faculty of Medicine
Izmir University, Turkey
Accepted date: October 15, 2016
Abstract
Activated charcoal lacks efficacy in preventing the absorption of iron. We hypothesized that bentonite, which is an inert natural adsorbent, may decrease the iron absorption and ameliorate the toxic manifestations in rats after iron poisoning. For the pharmacokinetic experiments, rats were randomized into three groups which received iron (as ferrous sulfate)+distilled water (Control), iron+bentonite (B5 (five-fold higher than the iron dose)), iron+bentonite (B10 (ten-fold higher than the iron dose)) via gavage. The blood samples were taken for six hours. The area under the plasma concentration-time curve (AUC) (0-360) (min μg/dl), peak concentration of iron in serum (Cmax) and time to reach Cmax (tmax) were calculated from the individual serum concentration-time profiles. For the survival part, rats were randomized into three groups and received (LD50), iron+distilled water (Control (s)), iron +bentonite (Bs5 (fivefold bentonite)) and iron+bentonite (Bs10) via gavage. Rats were observed for 72 hours. Iron levels showed a wide variation, and no significant differences in AUC (0-360), Cmax and tmax were detected. However, the mortality rate was significantly lower (9.1%) in the Bs10 group, as compared to Control (s) (55.6%) groups. Bentonite was found to be effective in preventing mortality in the rat model of acute iron toxicity.
Keywords
Iron, Poisoning, Pharmacokinetics, Survival, Toxicology, Bentonite
Introduction and background
Unintentional poisoning with iron or iron containing products is still an important issue particularly in young children [1,2]. Decontamination options are limited with gastric lavage and whole bowel irrigation since activated charcoal is demonstrated to be ineffective in previous studies [3]. Parenteral deferoxamine, which is the preferred antidote in severe cases, is not free of drawbacks such as high cost, lack of availability in hospitals and adverse effects with prolonged use [3,4]. Therefore, the search for an effective and orally active adsorbent for iron is important.
Bentonite, the clay from volcanic origin that contains montmorillonite, is a physiologically inert substance [5,6]. It is ubiquitous in many places among the world and has been used in the production of cement, adhesives, ceramics and even rocket engine nozzles. Bentonite has a surface area of 250-800 m2/g which provides a high adsorptive capacity for cations [6-8]. It has also been used as an oil purifier and -is effective in adsorbing protein molecules from aqueous solutions [8]. Similar to activated charcoal, it has been suggested as a gastric decontaminant in paraquat poisonings [9] and was shown to be effective in adsorbing lithium in vitro [10].
This two-step study was aimed to investigate whether bentonite might be effective in preventing the absorption of iron and improve survival rates in the rat model of acute iron toxicity.
Materials and Methods
Chemicals
Ferrous sulfate heptahydrate (FeSO4-7H2O, Sigma-Aldrich Chemie GmbH, F8048) was used as the iron salt. Bentonite was gifted from Amcol Minerals Europe Ltd./Amcol Mineral Madencilik Istanbul-Turkey. Ketamine (Ege Vet. Hayvancilik San. ve Tic. Ltd. Sti. Izmir-Turkey/Alfasan International Woerden-Nederland) and xylazine (Ege Vet. Hayvancilik San. ve Tic. Ltd. Sti. Izmir-Turkey/Alfasan International Woerden- Nederland) were used for anaesthesia.
Animals
Our study was performed in accordance with the guidelines provided by the Local Ethics Committee of Animal Experiments of the Adnan Menderes University School of Medicine in Aydin, Turkey (Approval number: B. 30.2. ADU. 0.00.00.00/050.04/2010/004). Female Wistar rats, 240.9±18 g, 8-weeks old, were obtained from the Experimental Animal Production and Experimental Research Laboratory of the Adnan Menderes University. The rats were housed in a temperature-controlled room (22ºC) with 12 h light-dark cycles and received a standard diet and water ad libitum. All rats were fasted for 12 hours before the experiments.
Study design
This study was composed of two sections. In the first part, the aim was to evaluate the effect of bentonite on iron absorption. The latter part was designed to observe the effect of bentonite on survival rates in a rat model of iron toxicity.
Pharmacokinetic experiment
On experiment day, rats were randomized into three groups. After reversible intraperitoneal anaesthesia with ketamine (50 mg.kg-1) and xylazine (5 mg.kg-1), the right jugular vein was cannulated with polyethylene tubing (PE-50 Cannula Tubing, Commat Ltd., Ankara-Turkey). The cannula was then fixed onto the back of the neck of the animal for blood sampling.
Iron, as ferrous sulphate (760 mg.kg-1, equivalent to 153 mg.kg-1 elemental iron, 750 μl) was administered via oral gavage. Five minutes later, the Control group received distilled water (1250 μl), and the bentonite groups received bentonite in doses which are 5 or 10 fold of the elemental iron dose (B5, 765 mg.kg-1 or B10, 1530 mg.kg-1, 1250 μl), respectively. The elemental iron doses for the pharmacokinetic and survival experiments were based on a previous study by Berkovitch et al. [11].
The platform on which the rat was attached to was set at a 45 degree angle with horizontal axis to avoid aspiration during gavage. Blood samples (250 μl) were withdrawn through the inserted cannula before (0) and at the 30th, 60th, 90th, 120th, 180th and 360th minutes of ferrous sulphate administration. Equal amounts of saline (250 μl) were administered after each sampling to replace blood volume. Serum samples were separated by immediate centrifugation at 4000 rpm for 10 min. Aliquots of serum were stored at -20°C until analysis.
Survival experiment
This part of the study was planned to evaluate whether bentonite might improve the survival rates in a rat model of iron toxicity. On experiment day, rats were again randomized into three groups and all rats received ferrous sulphate (1013 mg.kg-1, equivalent to 204 mg.kg-1 elemental iron, 750 μl). Five minutes later, the control group received distilled water (Control (s), 1250 μl) and the bentonite groups received bentonite in doses which were 5 (Bs5) or 10 (Bs10) fold of the elemental iron dose (1020 mg.kg-1 or 2040 mg.kg-1, 1250 μl), respectively. Because iron toxicity has also a late phase which may be delayed for up to 2-4 days, the rats were observed for 72 hours [5].
Analysis
Biochemical analysis: The iron assay (Multigent, Abbott Diagnostics, IL, 60064, USA) used aims direct colorimetric determination of iron without deproteinization in serum or plasma. The assay utilizes an acidic media (pH=4.8) to release ferric ion from transferrin which is subsequently converted to the ferrous form by the action of hydroxylamine hydrochloride. Released ferrous iron reacts with Ferene-S (3-(2-pyridyl))-5, 6- bis[2-(5-furylsulfonic acid)]-1, 2, 4-triazine) to produce a colored iron-Ferene-S complex of which absorbance is measured at 604 nm in Architect c16000 Chemistry Analyser (Architect, Abbott Diagnostics, IL, 60064, USA) [12,13].
Intraassay Coefficients of Variance (CV) values of the serum samples were 0.8% and 0.7% for the mean values of 64.3 μg/dl and 226.4 μg/dl, respectively. Inter-assay CV values were 2.0% and 1.5% for the mean values of 57.4 μg/dl and 212.4 μg/dl, respectively.
Pharmacokinetic analysis: The peak concentration of iron in serum (Cmax) and time to reach Cmax (tmax) were determined directly from the individual serum concentration-time profiles. Area under the plasma concentration-time curve (AUC (0-360) (min μg/dl)) were calculated using PK Functions for Microsoft Excel (Allergan, CA 92606, USA).
Statistical analysis: The statistical calculations were performed using Graph Pad Prism 4 software (CA, 92037, USA). Pharmacokinetic data were reported as mean ± SD. One-way Analysis of Variance (ANOVA) for repeated measures and unpaired Mann-Whitney U test was used to assess the serum iron concentrations at predetermined time points within and between the Control, B5 and B10 groups, respectively.
The mortality rates of the Control (s), Bs5 and Bs10 groups were assessed by Fisher’s exact test. p<0.05 was considered to be significant.
Results
Pharmacokinetic experiments
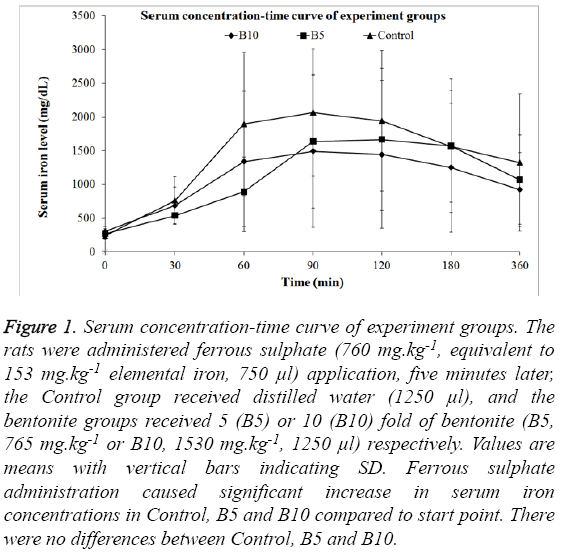
No significant differences regarding the basal iron concentrations between the groups were detected. Iron administration caused a significant increase in serum iron concentrations of rats within all groups compared to their basal levels (Control p<0.0001, B5 p=0.0003 and B10 p<0.0001 respectively). Although iron concentrations in control group continuously remained higher than B5 at all-time points except 180th minute and B10 at all-time points, no significant difference at any time point was detected. (Table 1, Figure 1). There was also no significant difference regarding AUC (0-360), Cmax and tmax between the groups (Table 2).
| Time (min) | Control (n=6)* | B5 (n=6)* | B10 (n=7)* |
|---|---|---|---|
| 0 | 237±54 | 268±76 | 303±80 |
| 30 | 764±350 | 538±128 | 687±273 |
| 60 | 1895±1064 | 892±518 | 1340±1042 |
| 90 | 2097±943 | 1640±988 | 1493±1126 |
| 120 | 1945±1043 | 1668±1054 | 1446±1095 |
| 180 | 1564±829 | 1572±990 | 1251±955 |
| 360 | 1325±1018 | 1070±662 | 925±544 |
*All values of iron is µg/dl.
Table 1: Serum iron concentrations of all groups.
| Control (n=6) | B5 (n=6) | B10 (n=7) | |
|---|---|---|---|
| AUC(0-360) (min µg/dl) | 539730 ± 290003 | 456045 ± 244368 | 408574 ± 277297 |
| Cmax (µg/dl) | 2157 ± 1056 | 1953 ± 1146 | 1560 ± 1117 |
| tmax (min) | 85 ± 12 | 105 ± 41 | 81 ± 23 |
Table 2: Pharmacokinetic evaluation.
Figure 1: Serum concentration-time curve of experiment groups. The rats were administered ferrous sulphate (760 mg.kg-1, equivalent to 153 mg.kg-1 elemental iron, 750 μl) application, five minutes later, the Control group received distilled water (1250 μl), and the bentonite groups received 5 (B5) or 10 (B10) fold of bentonite (B5, 765 mg.kg-1 or B10, 1530 mg.kg-1, 1250 μl) respectively. Values are means with vertical bars indicating SD. Ferrous sulphate administration caused significant increase in serum iron concentrations in Control, B5 and B10 compared to start point. There were no differences between Control, B5 and B10.
Survival experiment
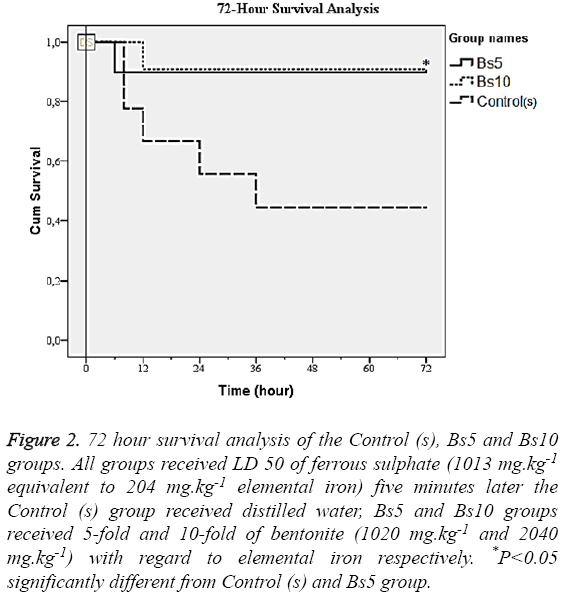
Bentonite administration significantly diminished the mortality rate at the 72nd hour in Bs10 (9.1%) compared to Bs5 (10%) and Control (s) (55.6%) (Figure 2). No significance was detected in mortality rates between Bs5 (10%) and the Control (s) (55.6%) groups.
Figure 2: 72 hour survival analysis of the Control (s), Bs5 and Bs10 groups. All groups received LD 50 of ferrous sulphate (1013 mg.kg-1 equivalent to 204 mg.kg-1 elemental iron) five minutes later the Control (s) group received distilled water, Bs5 and Bs10 groups received 5-fold and 10-fold of bentonite (1020 mg.kg-1 and 2040 mg.kg-1) with regard to elemental iron respectively. *P<0.05 significantly different from Control (s) and Bs5 group.
Discussion
This study aimed to test whether bentonite might affect the iron disposition and survival rates in a rat model of iron toxicity. Iron administration caused a significant increase in the serum iron concentrations in all groups. Although bentonite administration did not significantly affect iron disposition, it was significantly effective in preventing the mortality.
The intensity of toxic effects of iron was shown to differ between the sexes [14]. In our study, female rats were chosen to observe iron toxicity more dramatically, since oral administration of iron were shown to significantly lead to shorter survival times and higher iron levels and peaks in female rats than it is in the male rats.
Activated charcoal prevents the absorption of most ingested substances; however, its binding efficacy for ionic salts like lithium, iron and cyanide and small polar molecules like alcohol is low [15]. In vivo and clinical studies also demonstrated its ineffectiveness in adsorbing iron [16-18]. Ghafari et al. showed that oral deferoxamine administration significantly decreases the mean serum iron levels compared to charcoal and vitamin C administration [18].
Ponampalam et al, have suggested that bentonite might effectively adsorb lithium and has tested this hypothesis in vitro. The study demonstrated that bentonite, when applied in ratios of 5:1, 10:1 and 30:1decreased the recovery of lithium from deionized water by 2.5%, 12.89% and 20.55% respectively. Larger reduction has been detected in simulated gastric fluid, in which bentonite caused a 24.98%, 41.66% and 48.09% decrease in recovered lithium when applied in ratios of 5:1, 10:1 and 30:1 respectively [10]. In paraquat poisoning, the use of bentonite as an adsorbent was also suggested [9]. These data, although limited, suggest bentonite might be an alternative adsorbent for the compounds which were not effectively adsorbed by activated charcoal.
In our study, iron administration led to a significant increase in serum iron concentrations at the 30th minute in Control, B5 and B10 respectively. This increase remained stable throughout the entire experiment. Serum iron concentrations between Control, B5 and B10 groups at 30th, 60th, 90th, 120th, 180th and 360th minutes showed no significant differences. Bentonite caused a slight, but not significant decrease in AUC (0-360) and Cmax in B5 and B10 compared to control, respectively. High variations in serum iron concentrations, which were also reported by the previous studies, might be suggested as one of the reasons for this insignificance [19-21].
In our study, bentonite caused a significantly decreased the mortality rate at the 72nd hour in Bs10 (9.1%) compared to Bs5 (10%) and Control (s) (55.6 %) groups. One explanation for this effect might be the possible protective action of bentonite on the Gastrointestinal System (GIS) mucosa since less haemorrhage was observed in the autopsies of bentonite groups in both the pharmacokinetic and survival experiments. However, this was not a systematically conducted observation and remains to be further studied.
In conclusion, bentonite administration significantly decreased the mortality rates in rat model of iron toxicity. This effect may be attributed a possible protective effect of bentonite on GIS mucosa rather than decreasing systemic bioavailability of iron since no significant differences were detected in iron disposition between groups. Further studies in this field, should aim at investigating other compounds for which activated charcoal lacks efficacy as an adsorbent (e.g. lithium), since high variations in iron levels may complicate pharmacokinetic calculations and have the potential to mask significances.
Limitations
Lack of liver and/or histopathology assessment was one of the most important limitations of our study; however, severe intestinal haemorrhage was observed in autopsies of the Control (s) group and in rats with lower survival rates. Additionally, biochemical parameters or molecular parameters of iron toxicity were not investigated since the primary focus was the evaluation of iron pharmacokinetics and survival rates.
Acknowledgements
The authors are grateful to Amcol Minerals Europe Ltd./Amcol Mineral Madencilik A.S. Istanbul-Turkey for supplying us bentonite as a gift. The authors would also like to thank Sevinc Tombul for the professional care of animals during housing and experiments.
Conflict of Interest
The authors declare that they have no conflicts of interest in the research.
References
- Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH. 2007 Annual Report of the American Association of Poison Control Centers National Poison Data System (NPDS): 25th annual report. ClinToxicol (Phila) 2008; 46: 927-1057.
- Bronstein AC, Spyker DA, Cantilena LR, Jr., Green JL, Rumack BH, Giffin SL. 2008 Annual Report of the American Association of Poison Control Centers National Poison Data System (NPDS): 26th annual report. ClinToxicol (Phila). 2009; 47: 911-1084.
- Manoguerra AS. Iron. Poisoning and drug overdose. New York: Lange Medical Books/McGraw-Hill (5th edn.) 2007; 230-232.
- Breitbart R, Abu-Kishk I, Kozer E, Ben-Assa E, Goldstein LH. Intraperitoneal N-acetylcysteine for acute iron intoxication in rats. Drug ChemToxicol 2011; 34: 429-432.
- Klasco Re. Bentonite. POISINDEX® SystemThomson Reuters, Greenwood Village, Colorado 2010; 1432010.
- Eren E. Removal of lead ions by Unye (Turkey) bentonite in iron and magnesium oxide-coated forms. J Hazard Mater 2009; 165: 63-70.
- Jaynes WF, Zartman RE. Aflatoxin toxicity reduction in feed by enhanced binding to surface-modified clay additives. Toxins (Basel) 2011; 3: 551-565.
- DAuria M, Emanuele L, Racioppi R, Velluzzi V. Photodegradation of crude oil on a solid support. J ChromatogrSci 2009; 47: 263-271.
- Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remião F, Bastos ML. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008; 38: 13-71.
- Ponampalam R, Otten EJ. In vitro adsorption of lithium by bentonite. Singapore Med J 2002; 43: 086-089.
- Berkovitch M, Livne A, Lushkov G, Segal M, Talmor C. The efficacy of oral deferiprone in acute iron poisoning. Am J Emerg Med 2000; 18: 36-40.
- Iron. Package insert. Abbott Lab Inc AP IL 2011.
- Eskelinen S, Haikonen M, Raisanen S. Ferene-S as the chromogen for serum iron determinations. Scand J Clin Lab Invest 1983; 43: 453-455.
- Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers National Poison Data System (NPDS): 31st annual report. ClinToxicol (Phila) 2014; 52: 1032-1283.
- Kearney TE. Charcoal activated. Poisoning and drug overdose. New York: Lange Medical Books/McGraw-Hill (5th edn.) 2007; 432-433.
- Gades NM, Chyka PA, Butler AY, Virgous CK, Mandrell TD. Activated charcoal and the absorption of ferrous sulfate in rats. Vet Hum Toxicol 2003; 45: 183-187.
- Gomez HF, McClafferty HH, Flory D, Brent J, Dart RC. Prevention of gastrointestinal iron absorption by chelation from an orally administered premixed deferoxamine/charcoal slurry. Ann Emerg Med. 1997;30:587-592.
- Ghafari R, Gharehdaghi J, Solhi H. Comparison of deferoxamine, activated charcoal, and vitamin c in ?changing the serum level of fe in iron overloaded rats. Iranian J Toxicol 2014;7:940-943.
- Berkovitch M, Livne A, Lushkov G, Barr J, Tauber T, Eshel G. Acute iron intoxication: significant differences between sexes. Vet Hum Toxicol 1997;39:265-267.
- Barr J, Berkovitch M, Tavori I, Kariv N, Schejter A. Acute iron intoxication: the efficacy of deferiprone and sodium biocarbonate in the prevention of iron absorption from the digestive tract. Vet Hum Toxicol 1999; 41: 308-311.
- Abu-Kishk I, Kozer E, Goldstein LH, Weinbaum S, Bar-Haim A. Oral N-acetylcysteine has a deleterious effect in acute iron intoxication in rats. Am J Emerg Med 2010; 28: 8-12.