Review Article - Journal of Environmental Waste Management and Recycling (2021) Volume 4, Issue 1
Environment and water treatment
N Ben Tahar*, G Otmanine, A. Saoud, M Nabiev
Faculty of Science, M’hamard Bougara university, Boumerdes
- Corresponding Author:
- N Ben Tahar
Faculty of Science M’hamard Bougara university
Algeria
E-mail: nbentahardz@yahoo.fr
Accepted Date: December 10, 2020
Citation: Tahar NB, Otmanine G, Saoud A, Nabiev M. Environment and water treatment. Environ Waste Management Recycling. 2021;4(1):1-3.
Abstract
it is often said that water is not necessary to life ... "Water is simply life" In this world 75% of surface is covered by water. These figures are impressive, but compared to the amazing photographs that come from space, they have little effect. They reveal a beautiful blue planet, bathing in water, partly hidden in a veil of clouds. Water is also critical in manufacturing processes in any industry. Water use in a production unit has been greatly reduced by cutting waste and by technological advances. The restrictions go beyond just saving water they require for recycling in the circuits and fire service and water for extra cooling. In most processes, the water can act either as reactive as a solvent or as an agent used to carry cold or heat. In each specific case of use it is essential to know the water quality. Indeed the natural water that is to say the water in the rough may contain harmful substances which could have adverse consequences on equipment or chemical reactions. So we must recognize all these substances to adjust their content in the water before assigning it to a particular purpose. The legislation also imposes constraints on the content of water discharged into carbon compounds and suspended solids. There are also standard on nitrogen excretion.
Keywords
Water, pollutants, environment
Introduction
The wastewater treatment has become essential to modern societies. Indeed, the development of human activity is inevitably accompanied by increased production of emissions. Water resources are not inexhaustible. Degradation as a result of discharges of polluted water can not only seriously damage the environment but also entail risks of shortage [1]. Our country's resources are already insufficient water. The deterioration of their quality as an aggravating the shortage. Therefore we must clean the wastewater minimize pollution of our meagre reserves [2]. The wastewater is done taking into account several parameters such as:
• The quality of the receiving environment and water uses
• Type network (separation unit)
• Pollution. Depending on the type of pollution different types of processes can be implemented
• Terrain features-namely the location, topography and available area
• The problem of operation and reliability of facilities
According to the classical treatment, the wastewater treatment requires a series of steps involving very different processes. Thus the urban wastewater can be treated by processes to varying degrees of treatment depending on the level de quality required by the receiving environment and subsequent uses of the water [1-3]. The goal of treatment is to separate water from undesirable substances to the receiving environment, these substances raw or processed should be disposed of as satisfactory for the environment [4]. The treatment process may include in principle several steps:
• Pre-treatments
• Primary treatment
• Secondary treatment
• Complementary therapies
The aim of our work is to replace certain transactions by processing on the processed and activated bentonite. This type of treatment might be beneficial because some steps would be dropped altogether. It is synthesized adsorbents whose performances are close to those of industrial adsorbents and that we could deal adequately with certain effluent domestic wastewater, industrial water or sea A number of samples been taken following the standards [5]. To address these samples, we prepared four types of adsorbents from local bentonite. The clay object of this study was taken at a place called "Azaghar" located in the region Boghni.
Materials and Methods
Preparation of adsorbents.
Obtaining adsorbents is made from bentonite [6] and according to a procedure described below:
• Crushing of bentonite
• Separation of particles by sieving
• Dissolved in distilled water to 2.5% ethanol
• Agitation for 8 hours at 90°C
• Filtration
• Drying in the open air for 12 hours
• Drying in the oven to 120°C for 12 hours
Bentonite obtained is analysed by fluorescence and results are on the following Table 1.
| Adsorbents | Fluore`scence |
|---|---|
| SiO2 | 53.87 |
| Al2O3 | 18.11 |
| Fe2O3 | 6.67 |
| MgO | 2.06 |
| CaO | 3.22 |
| Na2O | 0.5 |
| K2O | 2.67 |
| TiO2 | 0.78 |
| P2O5 | 0.18 |
| MnO | 0.03 |
| Cr2O3 | 0.12 |
| SO3 | 2.46 |
| LOI | 8.5 |
Table 1: Fluorescence analysis of obtained bentonite.
Bentonite pre-treated and will provide support for the preparation of a set of four adsorbents differentiated by their chemical composition [7]. These adsorbents were prepared by the following procedure:
• The pre-treated bentonite is mixed with other ingredients such as silica gel, alumina, ammonium chloride and chromium oxide in a liter of distilled water 2.5% ethyl alcohol
• The mixture was stirred for 8 hours at 90°C. The resulting solution is filtered
• The cake obtained on the filter is transformed into small rods of length 0.8 cm or 1 cm
• The adsorbent thus obtained is dried in the open air for 12 hours; this drying continues
• In oven for another 12 hours
• This is followed by its calcination at 800°C for 12 hours
• In this way four adsorbents have been developed and analysis presented in the Table 2 below, where you can see and their composition in wt %.
| Adsorbents | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| SiO2 | 39.63 | 49.58 | 41.28 | 40.51 |
| Al2O3 | 13.67 | 16.87 | 12.83 | 10.34 |
| Fe2O3 | 6.82 | 4.03 | 2.51 | 2.47 |
| CaO | 3.87 | 2.87 | 1.97 | 1.76 |
| MgO | 4.36 | 2.66 | 1.66 | 1.63 |
| SO3 | 2.46 | 1.48 | 0.92 | 0.91 |
| K2O | 2.67 | 1.59 | 0.99 | 0.97 |
| Na2O | 0.5 | 1.9 | 0.17 | 0.15 |
| Cr2O3 | 0.03 | 0.02 | 0.67 | 4.77 |
| L.O.I | 13.56 | 8.29 | 8.88 | 7.81 |
Table 2: Analysis of four adsorbents by calcination at 800°C for 12 hours.
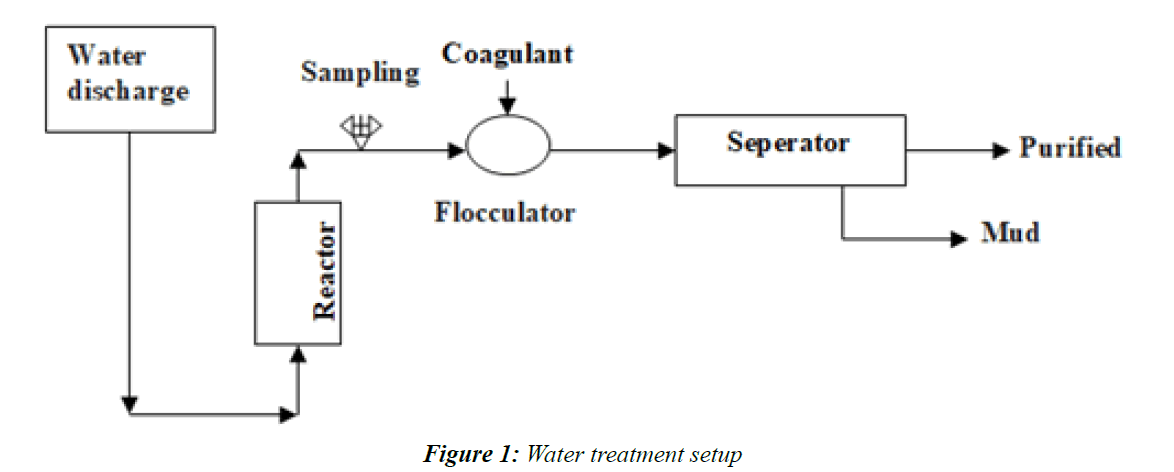
The aim of the present work is to show how a wastewater treatment using adsorbents can replace certain stages of the conventional treatment of wastewater. So we designed a simple but effective setup for this kind of treatment [8], she includes a football power that can be a funnel, a reactor at radial injection power plant and upwards. This facility can be supplemented in the case of wastewater loaded solids suspension of a flocculants and a decanter the wiring Figure 1 is presented below after.
The wastewater treatment unit producing milk and cheese Boudouaou, east of Boumerdes was done to aid and adsorbents prepared [9]. Three types of samples were collected and analysed before and after treatment as shown in Table 3:
| Date | 23 -04-2019 | 3/5/2019 | 4/5/2019 | |||
|---|---|---|---|---|---|---|
| Time | 09h 30 | 09h00 | 09h30 | |||
| Sample | Before | After | Before | After | Before | After |
| Temp, °C | 29 | 29 | 20 | 20 | 21 | 20 |
| Temp, sample °C | 20 | 18 | 26 | 20.5 | 21 | 20 |
| pH | 7.46 | 8.6 | 6.08 | 7.94 | 7.86 | 7.92 |
| Conductivity ms/cm | 2.97 | 2.7 | 3.79 | 2.93 | 2.6 | 2.89 |
| Turbidity(FTU) | 239 | 10 | 461 | 29 | 100 | 27 |
| NO2(mg/l) | 5 | 2 | 5.75 | 1 | 12 | 9 |
| NO3(mg/l) | 0.6 | 1.1 | 4.1 | 1.3 | 4 | 1.2 |
| NH4(mg/l) | 0.44 | 0.12 | 0.76 | 0.43 | 0.18 | 0.05 |
| PO4(mg/l) | 1.6 | 5.3 | 4.5 | 6.15 | 1.09 | 2.08 |
| TSS(mg/l) | 0.12 | 0.009 | 1.81 | 0.06 | 0.01 | 0.006 |
| VSS (mg/l) | 0.11 | 0.008 | 0.12 | 0.01 | 0.1 | 0 |
| MM% | 8.33 | 11.11 | 6.43 | 23.33 | 18.89 | 100 |
| BOD5(mg/l) | 3.4 | 316 | 16.9 | 13 | 2 | 15.5 |
| COD (mg/l) | 384 | 54 | 1650 | 84 | 249 | 95 |
| MW | 270.66 | 20.66 | 760.66 | 39.26 | 171 | 42 |
Table 3: Results of analysis of samples.
In order to evaluate the effectiveness of our method several physicochemical parameters [9] were reviewed:
1. Temperature measurement (T°C): The temperature has practically no influence on the process. Like any process of adsorption is favoured by low temperature, so the room temperature should be quite the process.
2. Measurement of pH: According to the measurement of pH average values vary between 6.5 and 8.5, and then there is compliance with the standards recommended.
3. Conductivity measurement: The conductivity is expressed in (ms/cm). According to the results obtained values of the conductivity depend on the presence of a high quantity of dissolved minerals.
4. Measurement of nitrates and nitrites: Nitrites can be found in waters, but generally at very low doses, they come either from oxidation of ammonia or a reduction of nitrate is an intermediate species of nitrogen compounds. Nitrite is rapidly oxidized to nitrate; the presence of nitrates indicates an ancient and distant pollution. In the middle oxygen of nitrite interest lies not only in their role as farmland fertilizer since it is essentially in this form that plants absorb nitrogen [10]. Normally the content of NO3 exceeds NO2 content, the latter is unstable, it is oxidized by NO3 in the next cycle.
5. Nitrogen ammonia NH4+: The habit has been taken to designate by the word ammonia forms ionized (NH4) and non-ionized (NH3), ammonia nitrogen is often found in water. This shows that the low concentrations obtained during analysis and usually reduces a process of degradation of organic matter is an index of pollution present. We must make determinations of ammonia nitrogen to avoid toxicity and plan the application rate of sludge on agricultural land so as to give the soil more nutrient.
6. Phosphorus PO4: Phosphorus is essential to the metabolism of micro-organisms, there are many forms: in aqueous solution, ortho phosphorus, organic phosphorus. It applies to reduce its concentration as it contributes to algal proliferation that leads to eutrophication [10]. The concentration of phosphorus in these waters should not exceed the specified value which is 0.1 mg/l, cons by our result shows the PO4 content of which exceeds this standard, this is because of the existence of a significant amount detergents.
7. Suspended solids TSS: The TSS is suspended solids, which indicate levels of pollution effluent. According to the results obtained, the values of TSS were below 30 mg/l, we can note that concentrations of TSS are complying with standards. PM-Materials (MM) and volatile suspended solids (VSS): The MM represent the mineral, the VSS are volatile suspended. It is the volatile matter determined after calcinations at 550°C, the amount weighed after ignition is the MM. The levels of MM must be below 50%. The analysis conducted gave values in MM still below 50% except in the output sample taken on 04-05-2005 because there's an absence of volatile matter in suspension [1-4]. So the MM values obtained are consistent with the standards.
8. Measurement of COD (chemical oxygen demand): The DCO is a way to measure the degree of pollution of wastewater. This method can include most of oxidizable organic compounds found in a wastewater sample.
Conclusion
From local materials, it is possible to prepare adsorbents to purify wastewater. This alternative treatment can be economical since it can replace the expensive treatment ponds to achieve and expensive to manage. It is also indicate that these basins occupy large areas of land. It would be useful to apply this method to purify seawater
References
- Gilbert N. Environment: the disappearing nutrient. Nat News. 2009;461:716-18.
- Pimentel D, Houser J, Preiss E, et al. Water resources: agriculture, the environment, and society. BioSci. 1997;47:97-106.
- Wuithier P. Petroleum, refining and chemical engineering. Paris. 2nd ed. 1972.
- Frank NK. Water manual. Paris:Technique & documentation. Lavoisier. 1st ed. 1984.
- https://www.afrik21.africa/en/category/water-environment/
- Kabeel AE, Sathyamurthy R, Manokar AM, et al. Experimental investigation of a hybrid setup for distilled water and power production. Desalination Water Treat. 2019;162:30-6.
- Mandevere B, Steven J. Household solid waste management: how effective are the strategies used in Harare Zimbabwe?.J Environ Waste Mngmt Recyl. 2018;2:16-22.
- Ali I. Water treatment by adsorption columns: evaluation at ground level. Sep Purif Rev. 2014;43:175-205.
- Ahmad T, Ahmad K, Alam M. Sustainable management of water treatment sludge through 3 ‘R’concept. J Clean Prod. 2016;124:1-3.
- Park PC, Cheung JP, Zhu XR, et al. Statistical assessment of proton treatment plans under setup and range uncertainties. Intl Radiat Oncol Bio Phy. 2013;86:1007-13.