Review Article - Journal of Biomedical Imaging and Bioengineering (2020) Volume 4, Issue 3
EFFICACIOUSNESS OF SUBSTRATE-INTERGRATED OF MICROELECTRODES (SIAMs) IN NEUROSCIENCE
A. Mohamed Sikkander*, Sangeeta R.Mishra
Department of Chemistry, Velammal Engineering College, Chennai-INDIA
Corresponding Author:
Accepted date: November 10, 2020
Citation: Sikkander M, Mishra R S. Efficaciousness of Substrate-Intergrated of Microelectrodes (siams) in Neuroscience.J Biomed Imag Bioeng 2020; 4(3): 1-5.
Abstract
Micro- and nanotechnologies have been newly combined together, opening new routes for the use of substrate-integrated arrays of microelectrodes (SIAMs) in Neuroscience. Perilously, MEAs offer extensive flexibility in culture preparation, experimental design, and high-through put approaches when compared to predictable electrophysiological techniques. SIAMs have been pioneered in the 70 s, where near the beginning microfabrication techniques were used to obtain devices to be electrochemically united to neurons in vitro and in vivo. The make use of of SIAMs has been established for both recording of bioelectric signals as well as for electrical stimulation of neurons, and shown to profitably enable a non-invasively monitoring of the activity of cultured networks as well as target CNS regions in vivo. Preferably, all materials used in SIAMs fabrication should have far above the ground biocompatibility, excellent electrical properties for a high signal-to-noise ratio of signal detection, transparency for the direct cell analysis, while being lucrative. However, the nearly everyone crucial and desirable feature is the close proximity and cherished mechanical contact between neurons and devices. This is known to lead to dramatic improvements in the electrical coupling between the device and the neurons, defined as the ratio stuck between the maximal signals detected by the device and the maximal transmembrane budding of an excitable cell. The key advantage of SIAM technology is the possibility to increase the spatial decree of conventional electrophysiological techniques, enabling the recordings of simultaneous extracellular signals at spatially distinct sites in a highdensity arrangement.
Keywords
Microelectronic, Network dynamics, Scaffolds, Nanotechnology, Nanoscale, Electropolymerisation.
Introduction
One of the very newest technological achievements of microelectronic integration in the field is represented by the Neuropixel probe, where an incredibly high density of ~1,000 active electrical contacts are crowded in a single shank device, suitable for in vivo implants(Nicholas A Steinmetz 2018). However, supplementary conventional devices, such as the commercial MED64 system, have been expansively used and presented in the literature. These have lower microelectrode counts, e.g., arranged in 8 × 8 layouts of 64 microelectrodes, where each unreceptive metal contact is self-possessed of platinum black, gold, and nickel, microfabricated on a glass substrate, and connected via indium tinoxide (ITO) strip conductors to exterior contacts.
Research methodology:
As an option to calcium- and voltage-sensitive dyes imaging, SIAMs have been successfully used to investigate network dynamics on in cooperation dissociated cultures and acute brain explants. For instance, performing SIAM recordings on hippocampal brain slices could associate peripheral persistent nociception to changes in chronological and spatial plasticity of synaptic associations and of function within the hippocampal formation (Ed Bullmore 2019). In one more study, cultured organotypic slice co-cultures of the rat spinal cords on top of SIAMs and characterized the spatial and sequential patterns of their spontaneous activity and the degree of synchronization sandwiched between the two slices.
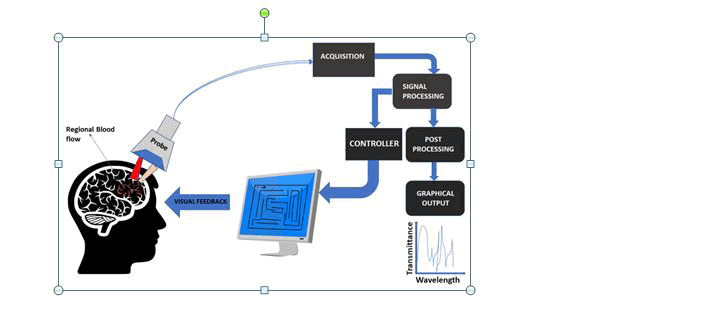
The current trend in higher density electrode arrays on SIAM devices has led to the accomplishment of novel nanotechnologybased approaches to enhance SIAM performance for neuronal footage and stimulus and new features have been developed to facilitate precise cell expansion and regulation on novel nanoenhanced arrays(Fig:1).
In exacting, nanoscale grooves urbanized through photolithographic techniques have been successful in enhancing neuronal interaction with array substrates (Tania Betancourt 2006). For example, in a revise of axonal outgrowth on nano-imprinted patterns, developed nanoscale grooves with depths of 300 nm and varying widths of 100–400 nm on polymethylmethacrylate (PMMA)- enclosed silicon chips. They monitored the expansion of mouse sympathetic and sensory ganglia cultured in medium containing 25 ng/ml of nerve growth aspect to stimulate axonal outgrowth. Using immunocytochemistry and scanning electron microscopy, they found that axons displayed contact regulation on the patterned surfaces but grew preferentially on crumple boundaries and elevations in the patterns rather than in grooves.
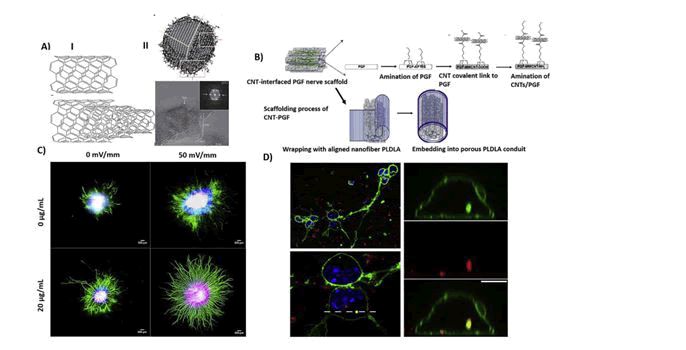
During the most recent few years, the discovery of new nanomaterials allowed the construction of nano-modified SIAMs, in order to get better cell adhesion properties and cell-to-substrate SIAM. For instance, employed conductive Carbon Nanotubes (CNTs) films, entrenched in a polymeric support, to fabricate flexible SIAMs aimed at neuronal recording, and stimulation (Fig: 2).
The CNT-electrodes displayed an extremely huge capacitance and low electrical impedance, enabling extremely efficient neuronal recording, and stimulation as established in chick retinas (Lilach Bareket-Keren 2012). It has obtainable novel electrode arrays composed of cell-appealing CNT islands of microelectrodes coated by a layer of dense and entwined CNTs. They showed that the CNTs islands strongly paying attention and anchored dissociated primary rat neurons to pre-defined locations and enabled the formation of stable sub-networks on electrically active recording sites. They concluded that CNT-encrusted electrodes were wellsuited to assist interfacing between electrically active biological cells and conformist electronic systems. In another study, Wang et al. presented a SIAM neural interface employing perpendicularly aligned multi-walled CNT pillars as micro- electrodes. They established the efficiency of their platform using hippocampal sliced cultures grown on the CNTs-modified SIAM device and exposed superior charge injection limits than compared to standard platinum electrodes. Likewise it’s tested vertically aligned carbon nanofiber electrode arrays for their probable to record electrophysiological activity and reported the stimulation and extracellular recording of spur-of-the-moment and evoked electrical activity in organotypic hippocampal slice cultures(Wei Gong 2016). Their results recommended the potential use of such a platform in civilizing electrophysiological studies of neuronal populations by enabling multimodal recordings at far above the ground spatial resolutions.
Result and Discussion:
SIAMs have also been investigated as far above the ground quality, chronic in vivo neural interfaces and the nano-modification techniques have found increasing use for civilizing implantable SIAM devices.The use of CNTs covalently fond of to either aminefunctionalized gold electrodes or electropolymerized by way of the conductive polymer polypyrrole to the electrode surface. In cooperation strategies were used for in vivo study of the rat motor cortex and the monkey visual cortex and showed, reduced impedance and noise, enabling concurrent measurements of local meadow potentials and spike activity from the same electrode site (Nicolas A. Alba 2015). Likewise,developed a transparent, carbon-layered microelectrode array made of graphene. They well-established such a device in the rat cortex and, in addition to direct optogenetic stimulation and fluorescence imaging at the microelectrode sites, they be able to record neural signals with the similar quality of the platinum-based MEAs, with equivalent longitudinal tissue responses. Graphene was also used to build supple MEAs. These showed how their Graphene microelectrodes (GMEAs), madeup in a dense array on a stretchy polyimide substrate, displayed outstanding robustness, and low-noise recordings when combined to rat-derived acute heart tissue and cardiac muscle cells.
3D Scaffolds
An additional important recent application of nanotechnology is that of tissue-engineering scaffolds. These are three-dimensional synthetic nanostructures used as substrates to boundary biological cells or tissues in vitro and in vivo . Owing to their characteristic 3D structure, the ease of exterior functionalization, and to the assortment of forms and materials, ranging from CNTs to biomaterials and hydrogels, scaffolds became extremely successful in a vast range of fields, from regenerative medicine, to biomedical applications to neuroscience necessary, and applied research (Maria P. Nikolova 2019). In tissue engineering, for instance, biomimetic scaffolds are budding as a possible treatment after neural tissue degradation or injury, as hydrogel scaffolds were shown to endorse axonal renaissance after a peripheral nerve lesion. In fact, hydrogel scaffolds could soon become a viable substitute to conventional drug-release systems. For the duration of the gelification process, it is probable to incorporate different types of molecules or biological cells into the gel structure, facilitated by the far above the ground quantity of water that enables the uptake and diffusion of soluble molecules.
In recent times, with the development of self-assembling peptide nanofiber scaffolds (SAPNS), a new-fangled defensive, therapeutic strategy for intracerebral hemorrhage (ICH) has emerged (Jiasong Guo 2009). One study evaluated ICH-related brain injury and functional revival by observing the effects of hematoma aspiration and intrastriatal administration of RADA16-I. Intracerebral rescue of SAPNS into the hemorrhagic lesion of a rat model of ICH replaced the hematoma and abridged acute brain injury. With SAPNS execution as a biocompatible material in hemorrhagic brain cavities, the formation of brain cavities was abridged, and an improvement in recovery of sensorimotor function was also pragmatic. The local delivery of SAPNS as a treatment for ICHconnected brain injury may allow better repair of ICH brain damage and enhanced recovery rates.
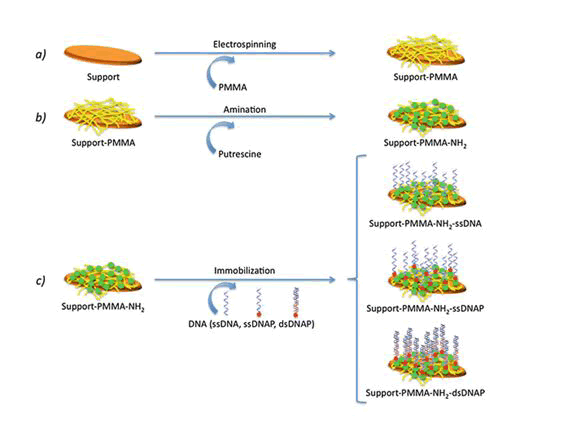
When the patterning of a substrate is required, electrospinning and microcontact printing are the most extensively employed technique ( Shi J 2009). Electrospinning is a fabrication technique(Fig:3) that uses electric charges to outward appearance fine fibers from polymers solution, as demonstrated with in cooperation synthetic [i.e., polycaprolactone, poly(glycolic acid)] and innate polymers, such as collagen.
For the period of the process, a polymer solution is passed through a tip and subjected to far above the ground voltage that charges the conductive liquid (Rajan Sharma Bhattarai 2019). Liquid droplets are then lingering beyond the expected shape by electrostatic repulsion, into a resulting surface known as the Taylor's cone. By the side of a critical point in space, the liquid erupts in a stream. As the jet dries, charge migrates to the surface of the fiber and the mode of current gush changes from conductive to convective. The jet is then stretched out by whipping, caused by electrostatic repulsion, and captured on an electrically stranded collector surface. Affidavit of the stream onto the edge of a rotating disk produces allied nanofibers.
Microcontact printing, on the other hand, is extremely accessible and produces substrates with controlled patterning (András Perl 2009). For the duration of a first step, a “hard stamp” based on a Si wafer is fabricated by resources of a printed photolithographic mask containing the pattern of interest. Then, a squashy stamp is made from poly-dimethylsiloxane (PDMS) from the mask. The PDMS may be then enriched with proteins, growth factors, and supplementary molecules.
As mentioned beyond, electrospinning is more often than not used to produce Nanofibers with a diameter lower than 1,000 nm and mainly in use in wound healing and tissue repair (Jiajia Xue 2019). Nanofibers have been applied in a variety of scaffolds and interfaced with many different biological samples, as electrospun meshes in general comprise of non-woven fibers with diameters in the range of hundreds of nanometers, and extremely interconnected pores that are tens of micrometers in diameter. The colossal surface area–volume ratio of such fibrous meshes also ensures plentiful area for cell attachment, allowing an elevated density of cells to be cultured than with smooth, two-dimensional surfaces. Electrospun poly(ε-caprolactone) (PCL) scaffolds have been given away to be biocompatible, as they well-integrated in the caudate putamen of the adult rat brain, with no confirmation of microglial encapsulation after 60 days in vivo, with visible neuronal processes piercing into the scaffold as another evidence of a triumphant neuronal-scaffold integration.
In order to get hold of sustained release of drugs in situ, scaffolds in the form of nanofibers were found to take hold of potential as implants for neurological therapies, such as Parkinson's disease (Matteo Bordoni 2020). Initial attempts for site-specific deliverance of dopamine to minimize its peripheral side effects were focused on scheming and biometric simulation of a prototype nano-enabled scaffold device (NESD) comprising of an alginate scaffold entrenched with dopamine-loaded cellulose acetate phthalate (CAP) nanoparticles. The device was entrenched in the parenchyma of the frontal lobe of rats and was found to transport dopamine over 30 days with 10-fold more dopamine in CNS as compared to systemic absorption.
Hydrogel scaffolds, beyond tissue regeneration, have been also established as optimal substrates for neuronal growth (Fig: 4).
The urbanized scaffolds of poly (2-hydroxyethyl methacrylate) (pHEMA) with changeable architectures on which primary hippocampal neurons were grown (Jennifer N. Hanson Shepherd 2011). It showed that neuronal organization was strappingly reflecting the scaffold spatial organization, proving that neurons were able to flimsily sense mechanical spatial cues and rearrange their network organization accordingly. As an alternative developed a microfluidic PDMS-based device, fictitious with different agarose/alginate parallel layers and thus resembling the cortex neuronal layers. In such layers, dissociated cortical neurons could appropriately grow and extend their neurites across adjacent layers. As the agarose/alginate hydrogel could potentially be inhabited by discrete cell types and by drug compounds, this scaffold represents a constructive tool to address questions on neural networks progress and for drug testing experiments.
In another work, it was revealed that laminin-functionalized nanofibers in 3D hyaluronic acid hydrogels enabled a noteworthy alignment of neuronal neurites along the nanofibers, while appreciably increasing the distance over which neurites could extend. Another example of the potential of these substrates as an most favorable tool for investigating neuronal ensembles has been given, who confirmed that three-dimensional collagen-based scaffolds promoted the demarcation of neural stem cell (NSC) into mature neurons previous than what achieved in neurospheres refined in suspension. The similar kind of cells were shown to grow not only on hydrogel-made scaffolds, but also on permeable scaffolds of graphene: in this case, it was seen that not only the scaffold supported the NSC growth, maintenance the cells at an active proliferation, but also that cells recognized a good electrical contact with the graphene foam, as talented to electrically encourage the cells through the scaffold itself.
The grouping of NSCs with nanofiber scaffolds exposed also great potential in regenerating axons through the formation of a growthsupportive microenvironment, and is well (apadimitriou L 2020). Poly(lactic-co-glycolic acid) (PLGA) scaffolds containing pores intended for axonal guidance, and an fundamental layer seeded with NSCs, were entrenched in rats with spinal cord hemisection lesions. Implantation of this scaffold led to axonal renaissance and a functional recovery which was better to that seen in rats implanted with the scaffold or NSCs alone.
The three-dimensional geometry importantly impacts neuronal network activity, while being reminiscent of the effectual brain tissue architecture. It has been in fact demonstrated that neuronal ensembles were much more strappingly synchronized and active if interfaced to a three-dimensional scaffold. This has been shown by using dissimilar types of scaffold materials, ranging from CNTs to the use of various types of polymer fibers. It was further confirmed how grafting a scaffold containing Human induced Neuronal (iN) cells into the mouse striatum for 3 weeks led to a far above the ground percentage of viable cells one order-of-magnitude greater than that the grafting of inaccessible cells.
Conclusion:
A gifted route is the combination of MEAs with other modalities. Out-of-the-way from electrical recording and stimulation, brain activity mapping and manipulation at cellular decree have also been done by means of optical methods, e.g., fluorescent calcium indicators, genetic markers, optogenetics, two-photon microscopy, etc. Analogous to extracellular recordings, the presence of numerous molecules and compartments in the brain with different optical properties render optical recording and analysis demanding. It is of awareness to pinpoint the advantages and constraints of both electrophysiological and optical methods to determine how they can complement each other. One more example is the use of optogenetics to manipulate the activity of specific cellular subpopulations. By means of MEAs to measure the response of the cortical circuit at multiple locations during optogenetic treatment, it is possible to study the functional roles of dissimilar classes of neurons. In addition, other technologies that can enhance MEA experiments are microfluidics for proscribed delivery of drugs, chemical sensing to study the biochemistry concerned in neuronal function, and measurement of metabolic processes. The involvedness of the data obtained from all the above mentioned and highly developed measurement schemes necessitates the application of systems biology techniques for analysis. Computational methods such as multi-scale modeling can coalesce recordings from dissimilar modalities at dissimilar time and/or spatial scales into a topological model of a system, e.g., cortical circuit. Establishment to end multi-scale modeling, the overall neuronal network activity can be understood, while also having the capability to zoom in to single neurons and even in an exact part of a neuron to learn the details of the biochemical and electrical reactions involved.
References
- Nicholas A Steinmetz, Christof Koch, Kenneth D Harris, and Matteo Carandini,(2018) Curr Opin Neurobiol. Jun; 50: 92–100.doi: 10.1016/j.conb.2018.01.009
- Ed Bullmore(2019) BMC Neurosci. 2019; 20(Suppl 1): 56.Published online 2019 Nov 14. doi: 10.1186/s12868-019-0538-0
- Tania Betancourt and Lisa Brannon-Peppas(2006) Int J Nanomedicine. 2006 Dec; 1(4): 483–495.Published online 2006 Dec. doi: 10.2147/nano.2006.1.4.483
- Lilach Bareket-Keren and Yael Hanein(2012)Front Neural Circuits. ; 6: 122.Published online 2013 Jan 9. doi: 10.3389/fncir.2012.00122
- Wei Gong, Jure Senčar, Douglas J. Bakkum, David Jäckel, Marie Engelene J. Obien, Milos Radivojevic and Andreas R. Hierlemann(2016) Front. Neurosci., 22 November 2016 | https://doi.org/10.3389/fnins.2016.00537
- Nicolas A. Alba, Zhanhong J. Du, Kasey A. Catt, Takashi D. Y. Kozai, and X. Tracy Cui(2015) Biosensors (Basel). Dec; 5(4): 618–646.Published online 2015 Oct 13. doi: 10.3390/bios5040618
- Maria P. Nikolova and Murthy S. Chavali(2019) Bioact Mater. Dec; 4: 271–292.Published online 2019 Oct 25. doi: 10.1016/j.bioactmat.2019.10.005
- Jiasong Guo , Ka Kit Gilberto Leung, Huanxing Su, Qiuju Yuan, Li Wang, Tak-Ho Chu, Wenming Zhang, Jenny Kan Suen Pu, Gloria Kowk Po Ng, Wai Man Wong, Xiang Dai, Wutian Wu(2009) Nanomedicine Sep;5(3):345-51. doi: 10.1016/j.nano.2008.12.001. Epub 2009 Mar 4.
- Shi J., Li Wang., Yong Chen.,(2009) Langmuir 25(11):6015-8 DOI: 10.1021/la900811k
- Rajan Sharma Bhattarai ,Rinda Devi Bachu ,Sai H. S. Boddu andSarit Bhaduri (2019) Pharmaceutics ,11(1), 5; doi.org/10.3390/pharmaceutics11010005
- András Perl, David N. Reinhoudt, Jurriaan Huskens(2009) Advanced Materials 21(22):2257 - 2268 DOI: 10.1002/adma.200801864
- Jiajia Xue, Tong Wu,Yunqian Dai, and Younan Xia(2019) Chem Rev. Apr 24; 119(8): 5298–545415. doi: 10.1021/acs.chemrev.8b00593
- Matteo Bordoni, Eveljn Scarian, Federica Rey, Stella Gagliardi, Stephana Carelli, Orietta Pansarasa, and Cristina Cereda(2020) Int J Mol Sci. 2020 May; 21(9): 3243.doi: 10.3390/ijms21093243
- Jennifer N. Hanson Shepherd, Sara T. Parker, Robert F. Shepherd, Martha U. Gillette, Jennifer A. Lewis, and Ralph G. Nuzzo(2011) Adv Funct Mater. Jan 7; 21(1): 47–54.doi: 10.1002/adfm.201001746
- apadimitriou L.,Manganas P.,Ranella A.,Stratakis E., (2020) Materials Today BioVolume 6, March, doi.org/10.1016/j.mtbio.2020.100043