Research Article - Biomedical Research (2017) Volume 28, Issue 7
Effects of testosterone replacement therapy on glucose and lipid metabolism in middle-aged and elderly high-fat-fed male rats
Ting-Yu Wen and Dong-Mei Kang*Department of Senile Endocrinology, Anhui Provincial Hospital Affiliated to Anhui Medical University, Hefei, China
- *Corresponding Author:
- Dong-Mei Kang
Department of Senile Endocrinology
Anhui Provincial Hospital Affiliated to Anhui Medical University, China
Accepted date: December 5, 2016
Abstract
This study aims to observe the effects of testosterone replacement therapy on glucose and lipid metabolism in middle-aged and elderly high-fat-fed male rats. This study established a high-fat-fed rat model and supplemented the rats with different doses of testosterone after castration. The rats were divided into control, complete lack of testosterone, low testosterone, medium testosterone, and high testosterone groups. Peripheral blood testosterone, blood lipid, blood glucose, and insulin levels were measured and the livers stained. Total Cholesterol (TC) and Low-Density Lipoprotein Cholesterol (LDL-C) levels in the low testosterone and complete lack of testosterone groups were higher than those in the control group (P<0.05); TC and LDL-C in the high and medium testosterone groups were less than those in the complete lack of testosterone group (P<0.05). There were no significant differences in blood glucose and insulin levels between groups (P>0.05). The groups, in increasing order of degree of liver steatosis, were the high testosterone, control, medium testosterone, low testosterone, and complete lack of testosterone groups. Visible fibrosis was observed only in the complete lack of testosterone, low testosterone, and medium testosterone groups. Testosterone can regulate peripheral blood TC and LDLC levels in middle-aged and elderly male rats, and testosterone deficiency can aggravate liver ectopic fat deposition.
Keywords
Glucose metabolism disorder, Lipid metabolism disorder, Rat, Testosterone
Introduction
Glucose and lipid metabolism disorders are characterized by anomalous qualities and quantities of glucose and lipids in the blood and other tissues and organs, which are caused by congenital or acquired factors. Epidemiological studies have confirmed that they are risk factors for type 2 diabetes, hypertension, metabolic syndrome, ischemic stroke, coronary heart disease, and other diseases [1,2].
A previous study reported that testosterone levels in middleaged and elderly men decline with age [3], while the incidence of dyslipidaemia and diabetes increases with age. The study shows a correlation between male testosterone level and lipid metabolism, the level of testosterone in the patients with abnormal lipid metabolism was low, while hypotestosteronemia may cause abnormal lipid metabolism [4]. A large number of domestic studies have come to the same result that low testosterone levels in older men were associated with increased Total Cholesterol (TC) and Low-Density Lipoprotein Cholesterol (LDL-C) levels, as well as decreased High-Density Lipoprotein Cholesterol (HDL-C) levels. Administration of a small amount of testosterone to older men with lower testosterone levels, which can improve glucose and lipid metabolism and carotid artery intima-media thickness, offers cardiovascular benefits.
Testosterone levels in middle-aged and elderly men influence blood lipid metabolism. The current consensus is that the serum testosterone and HDL-C are positively correlated, while Total Cholesterol (TC), Triglyceride (TG), and LDL-C are negatively correlated with serum testosterone; this indicates that endogenous testosterone may be a favourable factor for lipid metabolism balance in men [5]. Decreased male testosterone is closely associated with metabolic syndrome [6]; the androgen has a protective effect against type 2 diabetes mellitus and may be clinically useful to improve elderly diabetes and metabolic syndrome. The current study developed and tested different concentrations of testosterone doses in a high-fat–fed rat model to evaluate the effects of Testosterone Replacement Therapy (TRT) on glucose and lipid metabolism in middle-aged and elderly male rats fed a high-fat diet after castration.
Materials and Methods
Animals and chemicals
The study population included 50 healthy, 10 month-old male Sprague Dawley rats, weighing 380-767 g obtained from the Anhui Medical University Laboratory Animal Center. Animal experiment license number: scxk (Wan) 2011-002. They were initially fed clean grade animal feed, housed three per cage at a temperature and humidity of 22 ± 2°C and 55% ± 5%, respectively, with a 12 h/12 h light-dark cycle. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Anhui Medical University. Commercial testosterone and insulin Enzyme Linked Immunosorbent Assay (ELISA) kits for rats were obtained from Shanghai Yuan Ye Biological Technology Co., Ltd. (Shanghai, China); materials for testosterone propionate injection were purchased from Tianjin Jin Yao Amino Acid Co., Ltd. (Tianjin, China); crystallized chloral hydrate from Jiangsu Meng De electroplating chemicals Co., Ltd. (Zhengjiang, China); medical corn oil injection from Shijiazhuang North China Pharmaceutical Company (Shijiazhuang, China); basic feed (barley powder 20%, flour 10%, corn flour 16%, bran 16%, yeast 1%, fish meal 10%, bone meal 5%, salt 2%, dehydrated vegetable 20%) and highfat feed (cholesterol 2%, egg yolk powder 5%, bile acid sodium 0.2%, fat 10%, standard feed 82.8%) provided by the Anhui Medical University Laboratory Animal Center (Hefei, China).
Establishment of a high-fat-fed rat model and treatment groups
The high-fat-fed rat model was established after providing all surviving rats (n=47) a high-fat feed for six weeks. Of these, 41 rats were castrated randomly and six rats were falsecastrated as a control group. The rats were fed a high-fat diet after 12 days, and castrated rats alive after 24 days were randomly divided into four testosterone dosage groups: high testosterone (n=7, subcutaneous injection of 25 mg/kg testosterone propionate every three days), medium testosterone (n=6, subcutaneous injection of 5 mg/kg testosterone propionate every three days), low testosterone (n=5, subcutaneous injection of 1 mg/kg testosterone propionate every three days), and complete lack of testosterone (n=6). The control group was injected with the corresponding volume of medical corn oil. All groups were dosed for eight weeks.
Specimen collection and preservation
Before and after the high-fat feeding, the rats were fasted 12 hours overnight, weighed, and 2 ml venous blood was collected from the orbital venous plexus. Rats were fasted 12 hours overnight before sacrifice, weighed, and anesthesia was performed by intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g). The abdominal cavity was opened, and, after taking blood from the abdominal aorta, the rats were sacrificed by cervical dislocation. The liver was removed from each rat; after saline flushing, the tissue was placed in a 10% formaldehyde solution used for dyeing.
Biochemical index determination
The fasting blood serum was separated by centrifugation, and serum TC, TG, HDL-C, LDL-C, and Fasting Blood Glucose (FBG) levels were determined by enzymatic coupling colorimetry using Japanese Hitachi 7600-020 automatic biochemical analyser and supporting reagents; T and Fasting insulin (Fins) levels were evaluated by ELISA (according to the operation instructions of rat-specific kit).
Morphological observation
The 10% formaldehyde-fixed livers were subjected to conventional paraffin embedding, 5 micron thick serial sectioning, staining, and microscopic observation. JEDA 801D morphology image analysis system (Version 6.0) was used for oil red semi-quantitative detection, in which the red dye was used to calculate the percentage of neutral fat particles.
Statistical analysis
SPSS Statistics for Windows, version 17.0 was used to perform all statistical analyses. Measurement data were expressed as means ± standard deviation (x̄ ± s). Independent samples t-tests were used for comparisons between the two groups, while Analysis of Variance (ANOVA) and Least Significant Difference (LSD) were used for comparison between groups and between multiple sets of two, respectively. P<0.05 was considered statistically significant.
Results
General information
Three rats died during the high-fat feeding phase; the surviving rats gain weight steadily (total weight increase of 2.7% compared to weights before starting the high-fat diet). During testosterone supplementation, three rats in the complete lack of testosterone group died one rat each in the low and medium testosterone groups died, and four rats in the high-testosterone group died; the remaining rats had good appetite, mental state, and hair gloss and gained weight steadily. The control and high testosterone groups were more active, and their fur was visibly denser. Activity levels were lower in the complete lack of testosterone, low testosterone, and middle testosterone groups.
Blood lipid, FBG, and insulin Fins after high-fat diet
After consuming a high-fat diet for six weeks, the serum levels of TC, TG, and LDL-C were higher than that before starting the high-fat diet (all P<0.01). The HDL-C levels were lower than that before the high-fat diet (P<0.01). After consuming the high-fat diet for six weeks, the FBG levels were higher than before starting the high-fat diet (P<0.01), while Fins levels were lower than those before the high-fat diet (P<0.01) (Table 1).
| T | TC | TG | HDL-C | LDL-C | FBG | FINS | Weight | |
|---|---|---|---|---|---|---|---|---|
| High-fat building before | 235.10 ± 28.77 | 2.43 ± 0.47 | 0.44 ± 0.35 | 1.31 ± 0.28 | 0.60 ± 0.17 | 6.01 ± 0.82 | 0.28 ± 0.02 | 546.09 ± 86.25 |
| High-fat building after | 171.94 ± 16.95* | 3.40 ± 0.65* | 1.12 ± 0.71* | 0.91 ± 0.26* | 2.15 ± 0.62* | 6.80 ± 0.91* | 0.22 ± 0.01* | 561.44 ± 85.35 |
Table 1. Blood lipids, blood glucose and insulin level changes of rats before and after high-fat building (͞x ± s, n=47).
Testosterone, blood lipid, FBG and Fins levels after testosterone supplementation
The testosterone levels of the medium testosterone and control groups were similar (P>0.05), while the level in the high testosterone group was higher than that of the control group (P<0.05). The testosterone levels in the complete lack of testosterone and low group were lower than that of the control group (all P<0.05).
Compared with the control group, there were no statistically significant differences in the TC, TG, HDL-C, and LDL-C levels in the high and middle testosterone groups (P>0.05). TC and LDL-C levels in the low testosterone and complete lack of testosterone groups were elevated (P<0.05), while the TG and HDL-C levels did not differ significantly (P>0.05). The TC and LDL-C levels in the high and medium testosterone groups were lower than that of the complete lack of testosterone group (all P<0.05), while the TG and HDL-C levels did not differ significantly (P>0.05). FBG and insulin levels did not differ significantly between groups (P>0.05, Table 2).
| Groups | T | TC | TG | HDL-C | LDL-C | FBG | FINS |
|---|---|---|---|---|---|---|---|
| High testosterone dose (n=3) | 722.83 ± 30.37* | 2.11 ± 0.88# | 0.34 ± 0.23 | 0.65 ± 0.08 | 1.04 ± 0.22# | 10.20 ± 4.32 | 13.91 ± 1.47 |
| Middle testosterone dose (n=5) | 141.14 ± 18.79 | 4.17 ± 0.28# | 0.65 ± 0.24 | 0.77 ± 0.22 | 2.86 ± 0.81# | 9.41 ± 3.12 | 14.65 ± 3.18 |
| Low testosterone dose (n=4) | 34.25 ± 7.01* | 6.03 ± 1.00* | 0.46 ± 0.16 | 0.68 ± 0.06 | 4.41 ± 1.15* | 5.33 ± 0.01 | 16.86 ± 2.02 |
| Complete lack of testosterone (n=3) | 18.57 ± 6.22* | 8.7 ± 2.38* | 0.74 ± 0.83 | 0.83 ± 0.39 | 5.54 ± 1.20* | 11.7 ± 0.11 | 14.05 ± 1.31 |
| Control (n=5) | 160.04 ± 12.15 | 2.98 ± 0.40 | 0.53 ± 0.04 | 0.74 ± 0.16 | 1.93 ± 0.45 | 9.72 ± 1.62 | 13.68 ± 3.14 |
Table 2. Peripheral blood testosterone concentrations in the changes of blood lipid, blood glucose, insulin (͞x ± s, n=20).
Haematoxylin and eosin staining
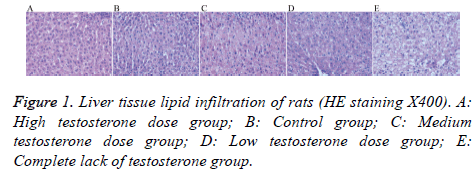
Liver cells in the high testosterone group showed mild steatosis; while those of the control group showed mild-tomoderate steatosis, with a mild increase in the volume of the hepatic lobule and a mild-to-moderate disorder of the structure cells. The liver cells in the medium testosterone group showed moderate steatosis, mild volume increase in the lobular liver cells, and structure cells arranged in moderate disorder. Liver cells in the low testosterone group showed moderately severe steatosis, increased lobular volume, and moderately severe disorder arrangement of structure within the cell. Finally, liver cells in the complete lack of testosterone group showed severe steatosis, increased lobular volume within liver cells, and structure cells arranged in severe disorder (Figure 1).
Oil red staining
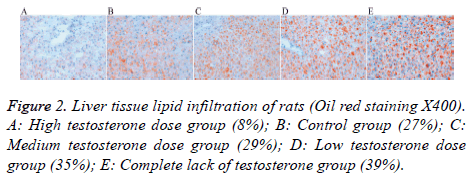
Lipid drops gradually increased in the medium testosterone (29%), low testosterone (35%), and complete lack of testosterone (39%) groups; these levels were higher than that of the control group (27%). There were fewer lipid droplets in the high testosterone group (8%) (Figure 2).
Masson’s stain
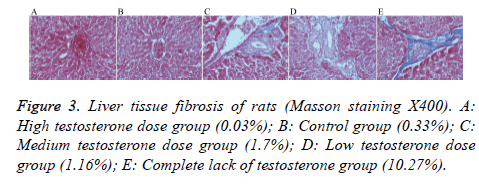
After staining, there was no obvious blue collagen fiber in the high testosterone group (0.03%), the control group had only a small amount of blue collagen fiber (0.33%), and the medium testosterone group showed slightly bluer collagen fiber (1.70%), with an incomplete fiber interval. The low testosterone group also showed more blue collagen fiber (1.16%) than the high testosterone group, with an incomplete fiber interval. The group with complete lack of testosterone had the most obvious hyperplasia of blue collagen fibers (10.27%), there is fibrous cord formation outside the central vein and is connected to the adjacent central vein or to the fibrous cord of the portal region, form a complete fiber interval (Figure 3).
Discussion
The results of the current study further verified clinical research results by administering different doses of testosterone after castration in order to simulate testosterone concentrations in middle-aged and old males. The effects of different concentrations of testosterone on peripheral blood sugar, fat metabolism, liver tissue lipid infiltration and fibrosis were studied, and we observed morphological changes that were not observed in human studies. The results in the current study were comparable to those of early clinical studies.
The results showed that testosterone could affect peripheral blood TC and LDL-C levels; high levels of testosterone can decrease TC and LDL-C levels, while low levels of testosterone can increase TC and LDL-C levels. Our preliminary clinical study also observed that increasing age and decreasing serum testosterone levels in elderly men is negatively related to TC and TG levels and negatively correlated with carotid intima-media thickness [7]. A domestic large cross-sectional study in senile men showed significantly negative correlations in total testosterone, TC, and LDL-C, and significant positive relationships with HDL-C levels [8]. Studies in Japan and Germany have reported similar findings [9,10]. Low testosterone levels may cause adverse effect on the lipid distribution, as well as high TC, TG, and LDL-C and low HDL-C levels [11,12]; however, other studies have reported no correlation between the two [13,14].
Testosterone Replacement Therapy (TRT) can improve lipid metabolism disorders [15], decrease TC and LDL-C levels, and increase HDL-C levels. TRT has been used to treat metabolic disorders and cardiovascular disease [16,17]. Corona found that TRT could reduce TG levels and increase HDL-C levels [18] in patients with metabolic syndrome and type 2 diabetes; it may also improve central obesity and improved control of blood sugar [19]. We previously reported on low testosterone in middle-aged and elderly men, observing that physiological doses of TRT improved glucose and lipid metabolism [20].
The results of this study also show that testosterone did not significantly influence blood sugar and insulin levels and that a lack of testosterone may increase rat liver ectopic fat deposition. Liver fibrosis was observed, but there were no significant differences between groups. This observation may be related to confounding factors of the high-fat diet and the relatively short observation time. High endogenous androgens in older men are reportedly associated with insulin resistance and metabolic syndrome [21]. Testosterone levels also tend to be lower in male patients with abnormal glucose tolerance and increased incidence of gonadal hypofunction has been reported in male patients with type 2 diabetes [22]. Androgen treatment can improve insulin sensitivity in patients [23]. In order to better understand how testosterone influences glucose and lipid metabolism in rats, future studies will be needed that adjust for relevant confounding factors.
The current studies suggest that the possible mechanisms of testosterone affect lipid metabolism are the following: testosterone by aromatization of E2, indirectly increase LDL-C receptor activity, and promote LDL-C and its receptor binding, thereby reducing LDL-C. Testosterone increases the activity of lipoprotein lipase (LPL), the key enzyme in lipid metabolism, to promote the hydrolysis of TG in VLDL and CM, thereby lowering TC and elevating HDL-C. Testosterone may also be purified by Scavenger Receptor (SR-B1) and HL, hydrolysis of HDL surface phospholipids, SR-B1 uptakes HDL selectively, and thus play the role of anti-atherosclerosis. However, there are few studies on the molecular mechanism of testosterone involved in lipid metabolism. Future studies will also be needed to assess the molecular mechanism of the abnormal glucose and lipid metabolism caused by testosterone deficiency.
Conclusion
In summary, the results of this study demonstrate that testosterone can improve rat lipid metabolism, suggesting that TRT for low testosterone levels in middle-aged and elderly men may offer clinical benefits. TRT may also have a positive effect on the prevention and treatment of lipid metabolismrelated diseases.
Acknowledgement
Fund Project: Natural Science Foundation of Anhui Province (1608085MH206), Science and Technology Project of Anhui Province (12010402134).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ozden C, Ozdal OL, Urgancioglu G, Koyuncu H, Gokkaya S, Memis A. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol 2007; 51: 199-120.
- Laukkanen JA, Laaksonen DE, Niskanen L, Pukkala E, Hakkarainen A. Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev 2004; 13: 1646-1650.
- Chu LW, Tam S, Kung AW, Lo S, Fan S, Wong RL, Morley JE, Lam KS. Serum total and bioavailable testosterone levels, central obesity, and muscle strength changes with agingin healthy Chinese men. J Am Geriatr Soc 2008; 56: 1286-1291.
- Firtser S, Juonala M, Magnussen CG, Jula A, Loo BM, Marniemi J, Viikari JS, Toppari J, Perheentupa A, Hutri-Kähönen N, Raitakari OT. Relation of total and free testosterone and sex hormone-binding globulin with cardiovascular risk factors in men aged 24-45 years. Atherosclerosis 2012; 222: 257-262.
- Garcia-Cruz E, Piqueras M, Huguet J, Perez-Marquez M, Gosalbez D, Peri L, Izquierdo L, Luque P, Ribal MJ, Alcaraz A. Hypertension, dyslipidemia and overweight are related to lower testosterone levels in a cohort of men undergoing prostate biopsy. Int J Impot Res 2012; 24: 110-113.
- Ahsan T, Banu Z. Male partial hypogonadotrophic hypogonadism with gynaecomastia and metabolic syndrome. J Coll Physicians Surg Pak 2012; 22: 105-107.
- Kang DM, Shen G, Liu Y, Zhu X, Shen GD, Hu SL. Effects of testosterone supplementation on carotid intima-media thickness in middle-aged and elderly men. Chin J Geriatrics 2013; 32: 699-701.
- Zhang N, Zhang H, Zhang X, Zhang B, Wang F, Wang C, Zhao M, Yu C, Gao L, Zhao J, Guan Q. The relationship between endogenous testosterone and lipid profile in middle-aged and elderly Chinese men. Eur J Endocrinol 2014; 170: 487-494.
- Haring R, Baumeister SE, Volzke H, Dorr M, Felix SB, Kroemer HK, Nauck M, Wallaschofski H. Prospective association of low total testosterone concentrations with an adverse lipid profile and increased incident dyslipidemia. Eur J Cardiovasc Prev Rehabil 2011; 18: 86-96.
- Akishita M, Fukai S, Hashimoto M, Kameyama Y, Nomura K. Association of low testosterone with metabolic syndrome and its components in middle-aged Japanese men. Hypertens Res 2010; 33: 587-591.
- Soisson V, Brailly-Tabard S, Empana JP, Feart C, Ryan J, Bertrand M, Guiochon-Mantel A, Scarabin PY. Low plasma testosterone and elevated carotid intima-media thickness: importance of low-grade inflammation in elderly men. Atherosclerosis 2012; 223: 244-249.
- Tsujimura A, Yamamoto R, Okuda H, Yamamoto K, Fukuhara S, Yoshioka I, Kiuchi H, Takao T, Miyagawa Y, Nishida M, Yamauchi-Takihara K, Moriyama T, Nonomura N. Low serum free testosterone level is associated with carotid intima-media thickness in middle-aged Japanese men. Endocrine J 2012; 59: 809-815.
- Kabakci G, Yildirir A, Can I, Unsal I, Erbas B. Relationship between endogenous sex hormone levels, lipoproteins and coronary atherosclerosis in men undergoing coronary angiography. Cardiology 1999; 92: 221-225.
- Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men-a meta-analysis. Clin Endocrinol (Oxf) 2005; 63: 280-282.
- Haidar A, Yassin A, Saad F, Shabsigh R. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male 2007; 10: 189-196.
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes.an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2010; 95: 2009-2354.
- Kang HY. Beyond the male sex hormone: deciphering the metabolic and vascular actions of testosterone. J Endocrinol 2013; 217: C1-3.
- Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8: 272-283.
- Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab 2013; 27: 557-579.
- Liu Y, Kang DM, Zhu X, Shen GD, Shen G, Song QQ. Effects of testosterone supplementation on glucose and lipid metabolism in middle-aged and elderly men. Chin J Clin Healthcare 2013; 2: 161-164.
- Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab 2005; 90: 2618-2623.
- Morton A. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2005; 90: 1903.
- Maneschi E, Morelli A, Filippi S, Cellai I, Comeglio P, Mazzanti B, Mello T, Calcagno A, Sarchielli E, Vignozzi L, Saad F, Vettor R, Vannelli GB, Maggi M. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J Endocrinol 2012; 215: 347-362.