- Biomedical Research (2014) Volume 25, Issue 3
Effects of genistein on male sprague dawley rats reproductive development.
Nurul Iftitah Musameh, Siti Rosmani Md Zin, Normadiah M. Kassim*Department of Anatomy, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia
- *Corresponding Author:
- Normadiah M. Kassim
Department of Anatomy
Faculty of Medicine, University of Malaya
50603Kuala Lumpur
Malaysia
Accepted May 08 2014
Abstract
Genistein (Gen), is commonly consumed phytoestrogen among Asian and known to exert weak estrogenic effects. To account for potential reproductive effects in male rats, Control, Gen1, Gen10, Gen100 mg/kg body weight and Estradiol were administered to gestational day 10 (GD10) female Sprague Dawley for 5 weeks. At postnatal day, P50, the rats were sacrificed. Blood was taken and reproductive tissues were processed. At birth, body weight (BW) and anogenital distance (AGD) in Gen10 and Gen100 decreased significantly from Control. Throughout experiment, BW and AGD of Gen10 decreased significantly. Preputial separation (PPS) was significantly longer in Gen100 and one rat from Gen10 exhibited unilateral testis descent at P50. Testicular weight and serum testosterone level were reduced in a dose-dependent manner. Histopathological analysis of the seminiferous tubules in Gen1 group is comparable to the Control group. However, the seminiferous tubules of the Gen10 and Gen100 groups showed evidence of overstimulated spermatogenesis. From the immunohistochemical (IHC) analysis, there was higher staining intensity indicating increased expression of 3β-hydroxysteroid dehydrogenase (3β-HSD) and Connexin43 (Cx43) in Gen10 and Gen100 groups compared to Control. Thus, administration of genistein during the critical period of early development could cause antiestrogenic or/and estrogenic influence on the development and functions of the male reproductive system.
Keywords
Soy, testis, estrogens , testosterone, nutrition
Introduction
Genistein is a plant isoflavone with polyphenolic compounds derived from a common class of phytoestrogens. Phytoestrogens are classified into groups according to their chemical structures. The greatest estrogenic activity is found in flavones, flavonols, flavonones, lignans, chalcones and isoflavones [1]. These compounds share structural similarities to steroidal estrogens, hence, its binding to estrogen receptors therefore capable of exerting weak antiand/ or estrogenic effects mediated by ER-α and also ER-β through alternative signaling pathways [2-6]. Thus, it is known as an endocrine disrupting chemical (EDC) [4-6]. The relative affinity of phytoestrogens to the ER-β is higher than ER-α [7-9]. Isoflavone was first found to increase plasma concentrations of endogenous estrogens associated with infertility in ewes [10]. Genistein and daidzein form the most predominant isoflavones in soybean which contribute to the most important dietary source of phytoestrogens for mammals [11]. In human, exposure to genistein is mainly from consumption of soy-based food products such as soymilk, tempeh, tofu, miso, soy flour and soy sauce [12]. It was reported that Asian people consumed 1.5 mg genistein or other isoflavones daily higher than most Europeans and North Americans [13,14].
Many EDCs in the environment are identified as environmental estrogens. These environmental estrogens may endanger fetuses as they are at the stage of highly susceptible to minor endocrine disturbances that may give rise to developmental abnormalities including testicular dysfunction [15] and thus lead to infertility.
The morphological alteration is associated with lack of gap junction protein, Connexin43 (Cx43) which controls cell growth and differentiation of the germ cells [16-20]. Thus, Cx43 expression can be used as an indicator for the well-being of the intercellular communication in the seminiferous epithelium where it was normally expressed in rat testis [21], and was down-regulated in mice testis with disrupted spermatogenesis [22-24]. Failure of spermatogenesis is also associated with low testosterone levels due to disturbance in testicular steroidogenesis which depends on 3β-hydroxysteroid dehydrogenase (3β- HSD) enzyme for the synthesis and secretions of testosterone [25]. This is in agreement with analysis of contralateral testis of men with testis anomaly due to immature Sertoli cells is lacking in germ cells and Leydig cells (3β-HSD positive) hyperplasia [26,27].
For centuries, soy-based products have become the preferred alternative diet among vegetarians. Besides, soy-based supplements have a growing popularity because it is claimed to have many health benefits. Thus, the public is at risk of being exposed to high genistein content in soy. Therefore, it is necessary to investigate the potential adverse effects of genistein on human health especially the effects on developing fetuses from maternal exposure.
The present study aimed to investigate the safe dose of genistein from soymilk on early development of the male reproductive system. We hypothesized that genistein exposure during the critical period of perinatal development can have detrimental effects on early development of the male reproductive system that may cause disruption of the hormonal functions leading to infertility.
Materials and Methods
Chemicals
Genistein of at least 99 % purity were obtained from Indofine (Indofine Chemical Company, Hillsborough, New Jersey, USA),17-β Estradiol was obtained from Sigma (Sigma Aldrich Chemical, St. Louis, MO, USA). A testosterone enzyme-linked immunosorbent assay (ELISA) kit was purchased from IBL International GMBH (Germany). For immunohistochemical (IHC) study, specific goat polyclonal antibodies against steroidogenic enzyme 3β-HSD and Cx43 were used with the aid of ImmunoCruzTM goat ABC staining system (Santa Cruz, CA, USA).
Animals
A total of 10 time-mated (gestational day, GD10) female Sprague Dawley (SD) rats weighing 203.5 ± 4.87 g were obtained from Faculty of Medicine Animal Facilities, University of Malaya.This study has been approved by the Animal Care and Use Committee (ACUC) of University of Malaya. Animals were housed in individual cages in an air-conditioned room at room temperature of 25 ± 1 °C with a 12 h light: 12 h dark period. Rats were given free access to standard rat chow (RainTree, Australia) and water ad libitum in glass bottles. All experiments were carried out according to the institutionally approved protocols according to University of Malaya Guidelines for the care and use of laboratory animals (Ethic Number ANA/01/10/2007/0810/NMK(R))
Treatment scheme and dosing
The presence of vaginal plug was designated as gestational day 1 of pregnancy (GD1). Pregnant dams were divided into five groups and received the following treatment: a) Control: received the vehicle only (Tween-80 (1:9, v/v)) (Sigma Aldrich, St. Louis, MO, USA), b) Gen1: received genistein 1 mg/kg BW, c) Gen10: received genistein 10 mg/kg BW, d) Gen100: received genistein 100 mg/kg BW, and e) Estradiol group: received 7.5 μg/kg BW of 17-β Estradiol. Rats were treated daily with genistein dissolved in Tween-80 using oral gavage tube from GD10 to GD21.
Dams were allowed to litter spontaneously and the day after birth was designated as postnatal day 1 (P1). Only male pups were chosen for this study. Male pups continued to receive genistein treatment by subcutaneous injection until P21. Body weight and anogenital distance of pups were recorded weekly till P21. Testis descent were monitored. Study has been carried out without treatment from P22 until P50 by observing the evidence of testis descent and sign of puberty. Testis descent in rat normally occurs on P21 while the onset of puberty is when they attain preputial separation (PPS) which normally occurs between P40-P45 according to Korenbrot et. al. [28].
Necropsy and sample collection
At P50, rats were fasted overnight prior to sacrifice and were sedated followed by intraperitoneal injection of chloral hydrate (0.1 ml/100 g of BW). Blood samples (5 ml) were obtained from transcardiac puncture, centrifuged at 1000 rpm for 5 minutes, and serum was stored at -20 °C until assayed for testosterone. Gross morphology of the external genitalia was examined. Testes were weighed and fixed in 10 % formalin fixative before being processed.
Histological analysis and microscopy
Testes were processed using an automated tissue processor (Thermo Scientific) and embedded in paraffin (ParaPlast Plus, USA). Tissue sections of 5 μm thickness were mounted onto labeled glass slides and stained with haematoxylin and eosin (H&E). Diameters of 100 seminiferous tubules in transverse section per testes were analyzed under light microscope (Olympus CH-B145-2) and representative areas were measured using NISElements Software (NIS-Elements Advanced Reasearch, Nikon, Japan). Briefly, the round or approximately round seminiferous tubules (the shortest to the longest axis ratio greater than 0.8) were chosen based on the previous study [29].
Serum testosterone level in male pups
Serum testosterone level was measured using an ELISA kit reader (IBL International GMBH, Germany) according to the manufacturer’s guideline.
Immunohistochemical localization of Cx43 and 3β-HSD
Paraffin sections of 5 μm were cut and placed onto polysine-treated glass slides. Sections were dewaxed and rehydrated through decreasing concentration of ethanol solutions. The sections were then autoclaved at 30 °C for 15 min in 10 mM in citrate buffer (pH 6.0) for inactivation of endogenous peroxidase activity with 0.1 % H2O2 in methanol (room temperature, 30 min), and were pre-incubated with normal donkey serum in phosphate buffer saline (PBS) for 1 h to block nonspecific reaction. Following that, the sections were incubated overnight with goat polyclonal antibody against 3β-HSD and Cx43 respectively, at 4 °C (Santa Cruz, CA, USA; at dilution of 1:100), followed by three rinses in PBS. After that, the sections were incubated with secondary antibody for 1 h followed by the ImmunoCruzTM goat ABC staining system. Finally, the sections were counterstained with Mayer’s haematoxylin. For the negative control slides, normal donkey serum was used instead of primary antibody.
Qualitative analysis of 3β-HSD and Cx43 staining expression were carried out under a light microscope (Olympus CH-B145-2). Representative areas were photographed with a Nikon Eclipse 80i upright microscope equipped with a digital color camera controller (DS-5Mc-U2).
Statistical analysis
All data were tested for normality followed by Levene static test for homogeneity of variances. All the parameters were compared using One-way ANOVA and Tukey post hoc test for the determination of differences among the groups. The significant difference between the treatment group was considered at P<0.05. We performed the calculations using PASW18 student’s program for ANOVA
Results
Body weight
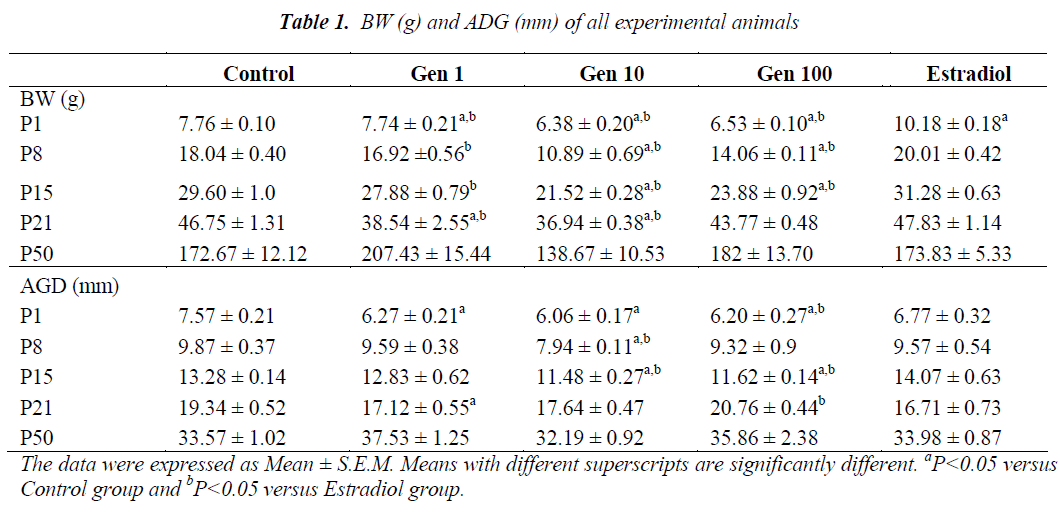
Exposure of male rats to genistein during prenatal life from GD10 to GD21 continued through neonatal life from P1 to P21 has resulted in a significant decrease in the mean BW of Gen10 and Gen100 groups compared to Control and Estradiol groups. A significant reduction of the mean BW was observed at birth and during subsequent weeks in all genistein-treated rats compared to the those of the Control and Estradiol (P1 to P21). However , the mean BW was normalized in the final week (P50) with no significant difference observed (Table 1).
Anogenital distance
At P1, the AGD of male rats were significantly shorter in all genistein-treated rats compared to those of the Control and Estradiol rats. After a week (P8) only the AGD of Gen10 rats were significantly shorter than those of the Control and Estradiol group, while at P15, it was significantly shorter in Gen10 and Gen100. During weaning (at P21), the AGD of Gen1 rats were significantly shorter compared to the Control while the AGD of Gen100 rats were longer compared to the Estradiol group. There was no significant difference of AGD in rats during adulthood (at P50) (Table 1).
Preputial separation (PPS) and testes descent
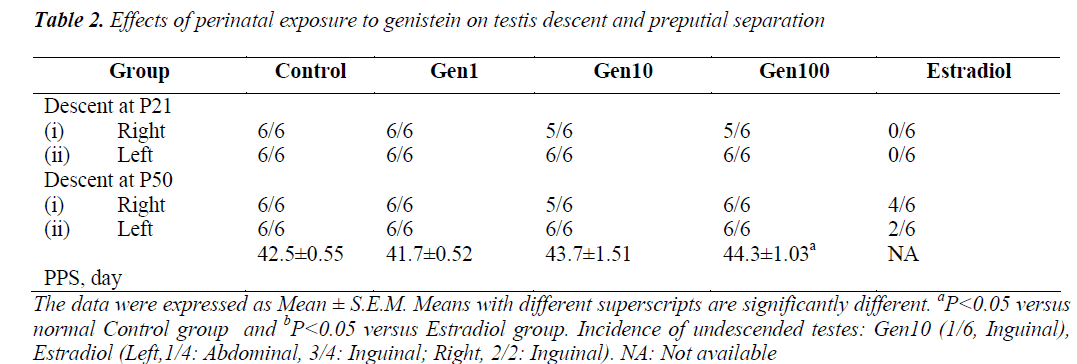
The mean day of PPS of rats in the Gen1 group was slightly earlier compared to the Control group while those of the Gen10 and Gen100 were delayed. However, only the mean PPS of the Gen100 group was significantly delayed compared to the Control group, while no rat in the Estradiol group exhibited PPS (Table 2).
As for influence of phytoestrogens on testis descent, it was noted that rats in Gen1 group exhibited normal testis descent similar to the Control group (Table 2). However at P50, one rat from Gen10 group exhibited unilateral undescended testis, while those of the Estradiol group, 4/ 6 testes on the right side and only 2/ 6 testes on the left side descended into the scrotum.
Effects of genistein on male rats reproductive development.
Testicular weight and seminiferous tubule measurement
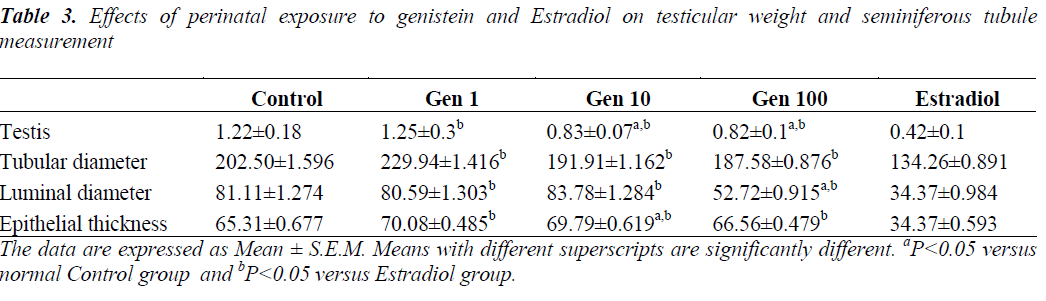
The mean testis weight in the Control group was 1.22±0.18 g while that of the Estradiol group was 0.42±0.1 g. In the experimental groups, the mean testis weight in Gen1 group was comparable with the Control group, while rats in the Gen10 and Gen100 groups exhibited significantly reduced testis weight compared to the Control rats but not as low as that of the Estradiol group (Table 3).
Quantification of morphological changes showed that the diameters of seminiferous tubules were smaller in Gen10 and Gen100 groups compared to Control. However, it was not significant. The spermatogenic cells were found normal without cell loss evidenced by increased epithelial thickness in genistein-treated groups (not significant) compared to Control. The population of Sertoli cells were indistinguishable between genisten-treated and Control animals. The interstitial tubular space contained many Leydig cells compared to Control. However, we did not quantify the number of Sertoli and Leydig cells in this study.
Serum testosterone
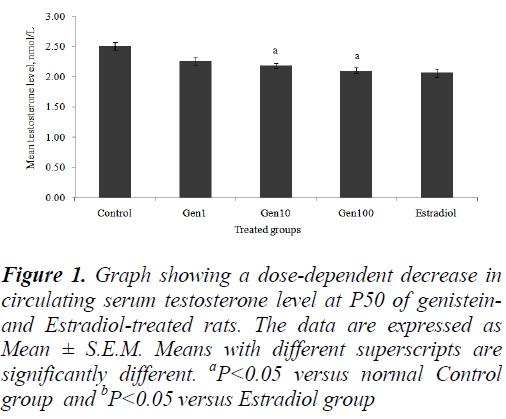
The mean serum testosterone levels of all the treatment groups decreased in a dose-dependent manner compared to the Control group. However, only the Gen10 and Gen100 groups showed a significant decrease testosterone levels compared to the Control (Fig. 1).
Figure 1: Graph showing a dose-dependent decrease in circulating serum testosterone level at P50 of genisteinand Estradiol-treated rats. The data are expressed as Mean ± S.E.M. Means with different superscripts are significantly different. aP<0.05 versus normal Control group and bP<0.05 versus Estradiol group
Microscopic evaluation of the testes
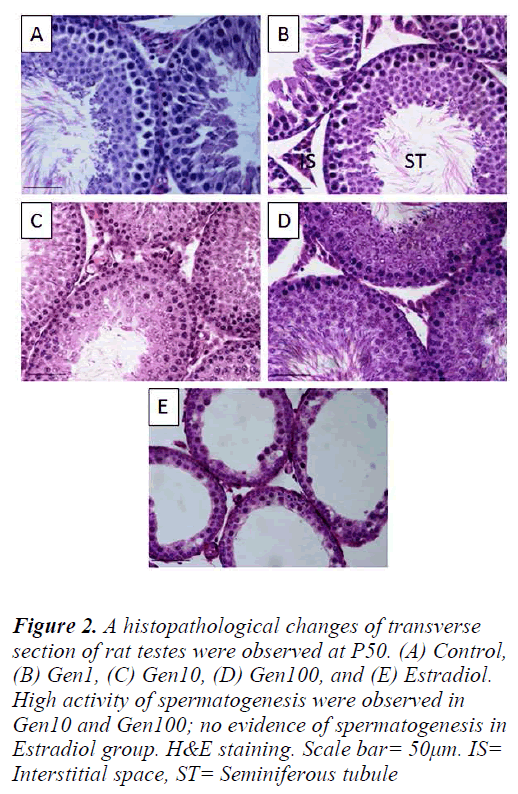
The Control testes comprised of seminiferous tubules closely arranged exhibiting the various stages of normal spermatogenesis with normal Sertoli cells and germ cells. The testes of Gen1 rats also exhibited normal spermatogenesis at various stages comparable to those of the Control testes. There were no apparent changes in the cells of interstitial spaces including Leydig cells. In contrast, the testes of Gen10 and Gen100 rats exhibited an overstimulated spermatogenesis with increased in germ cell population thus the germinal epithelium appeared thicker than that of the Control testes and some of the tubular lumina were completely filled with sperm tails. In the Estradiol group, the seminiferous tubular diameters as well as their epithelial height were very much reduced. There was no evidence of spermatogenesis and the number of Leydig cells in the intertubular spaces was also diminished (Fig. 2).
Figure 2: A histopathological changes of transverse section of rat testes were observed at P50. (A) Control, (B) Gen1, (C) Gen10, (D) Gen100, and (E) Estradiol. High activity of spermatogenesis were observed in Gen10 and Gen100; no evidence of spermatogenesis in Estradiol group. H&E staining. Scale bar= 50μm. IS= Interstitial space, ST= Seminiferous tubule
Immunohistochemical detection of 3B-HSD and Cx43 expression in rat testes
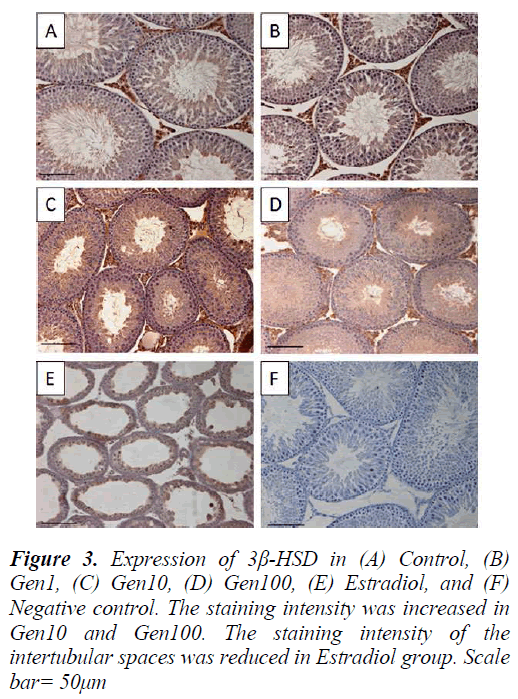
3β-HSD expression in the testis was localized in the Leydig cells with less intense staining in the seminiferous epithelium especially in the late stage spermatids in the control testis. The intensity of 3β-HSD expression in Gen1 was comparable to Control. The expression of 3β- HSD was more intense in Gen10 and Gen100 testes compared to Control and Gen 1, while there was almost no immunostaining of the intertubular spaces of the Estradiol testis. However, there were some staining of the seminiferous epithelium in the Estradiol testes (Fig. 3).
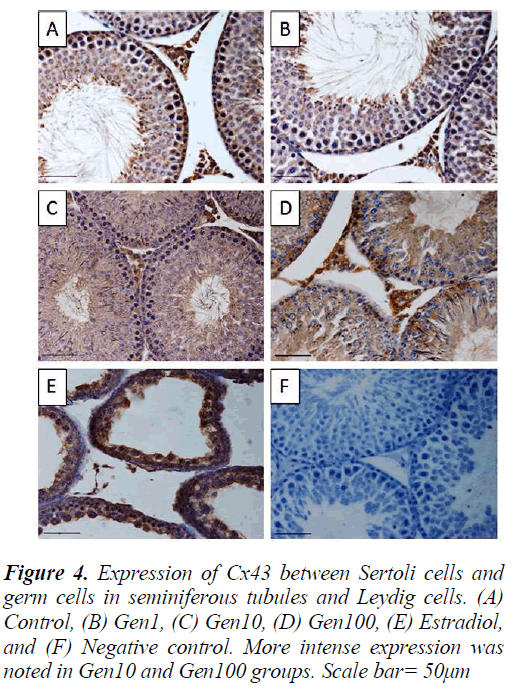
Similarly, the staining intensity for Cx43 was also localized in the Leydig cells as well as the seminiferous epithelium especially the spermatogonia and spermatocytes and late stage spermatids. There was no apparent difference in staining intensity between Gen1 compared to Control. Cx43 expression in Gen10 and Gen100 appeared more intense especially of the spermatogonia and spermatocytes. While in the Estradiol testes, the staining intensity was much less compared to Gen10 (Fig. 4).
Discussion
Estrogens are important in reproductive development and function. But, excess exposure to estrogens during critical period of development may exert detrimental consequences on development of the reproductive organs [30,31]. For centuries, soy has been widely consumed and its health benefit has been documented [32,33]. In the present study, we used low (Gen1), moderate (Gen10) and high (Gen100) doses of genistein to compare with the recommended level in the diet with genistein (6 to 9 mg/kg kg BW) [34]. Our study showed that perinatal exposure to genistein resulted in reduction in the mean BWof all genistein-treated rats at P21 except for the Gen100 rats where their mean BW increased at P21. It was hypothesized that fat deposition does not occur until the onset of suckling probably due to their habitual huddling with littermates in order to maintain body temperature [35,36]. The increased of mean BW in Gen100 rats at P21 is probably due to stimulation of adipogenesis at higher doses as reported by Heim et. al. [37] and might be the estrogenic effect of genistein. During adulthood (at P50), we noticed that the mean BW of all genistein-treated rats normalized and comparable to the Control.
Our study also showed that there was impairment of male reproductive system development as evidenced by reduction in testicular weight and AGD. In the present study, the AGD of Gen10 rats was significantly reduced from P1 to P21 but not significant at P50. This observation suggested that genistein interfered with the early development of external genitalia in males similar to the previous findings that showed endocrine disrupting chemicals intereference on the reproductive parameters that were identified as potential risk to the reproductive system development [38-40]. We also noted that all the AGD measurements of the rats normalized at P50. This observation has lead us to hypothesized that the development of the male external genitalia proceeds normally after weaning (at P21) once genistein treatment is discontinued.
Studies have shown that factors capable of interfering sexual development can also affect the development of the external genitalia thus, is commonly assessed by determining the anogenital distance [41,42]. In the present study, the reduction of AGD in genistein-treated rats could be due to reduction in androgen production. Since 17-β Estradiol is a potent estrogenic compound, it was used in our study to test the estrogenic activity in vivo as was employed by Lee et. al. [43]. Similarly, PPS is also used as an indicator for male puberty which also depends on androgens. Our findings showed that PPS was significantly delayed in Gen100 rats compared to the Control. This is in contrast to previous findings, which showed no significant effect on PPS of rats treated perinatally with genistein at 50 μg/d [44].
In rats, the testis descent is accomplished by P28 [45,46]. Our findings showed that testis descent was disturbed in some of the Gen10 and Gen100 rats and in all Estradiol rats at P50. This might be due to the chemical property of phytoestrogen, which acts as anti-androgen that interfered with testis descent in these groups or it might just be a transient effect. These findings can be correlated with the significant dose-dependent decrease of serum testosterone level in these groups and in studies using isoflavones in different doses [47-50]. However, this notion is not in agreement with the findings by Piotrowska and partners [51] who reported no significant difference in serum testosterone levels, but found significant reduction in testicular testosterone levels in rats treated with genistein.
Hormonal imbalance in experimental rats from perinatal exposure to genistein may also cause detrimental effects on the morphology of the testis in adulthood. The present study, showed that diet containing Gen10 and Gen100 effectively stimulate spermatogenesis in rat testes as evidenced by a significant increase in germinal epithelial thickness. Activation of cell proliferation by genistein on fetal testis is also seen in previous study (52). Additionally, genistein also increases the proliferation rate of gonocytes in vitro isolated from neonatal rats (53). Interestingly, in another study using cytotoxic drug that caused damage to rat testes, genistein was reported to suppress the drug cytotoxicity, as well as testosterone levels but stimulated spermatogenesis in rats [54]. Therefore, from our study, we can deduce that suppression of testosterone level was caused by the increase expression of stem cell factors (SCFs) of the Sertoli cells that are essential for spermatogenesis[54,55]. Genistein action on target cells appears to be associated with its estrogenic activity in addition to its inhibition of the key steroidogenic enzymes by its influence on the estrogen receptors [56].
Genistein exposure during the perinatal period was shown to increase proliferative activity of Leydig cells but exerted an opposite effect on androgen concentration in pubertal male rats. Androgen is an autocrine regulator of Leydig cells and mainly responsible for androgen production [57]. Therefore, it is possible that genistein inhibits testosterone secretion by delaying Leydig cell differentiation, which contributes to the decrease androgen secretion. The present findings of reduced testosterone concentration contradicts with previous finding that showed the increment in serum steroid hormone production under the perinatal exposure [58]. Serum sex hormone concentration was paradoxically depend not only on steroidogenic capacity but also on the number of Leydig cells [59]. Albeit increased proliferation was observed under immunohistological study, we did not quantify Leydig cell numbers in the present study. The reduction of serum testosterone levels in this study could be attributed by the inhibition of the steroidogenic pathway, not due to direct effect of genistein on Leydig cell numbers as in previous study (60). Nevertheless, it is likely that the greater Leydig cell populations may result from the longer duration of perinatal exposure to isoflavones [61].
Previous studies showed that increased steroidogenic acute regulatory (StAR) protein levels with decreased LH stimulation and reduced StAR phosphorylation, which is critical for translocation of cytosolic cholesterol into mitochondria [58,62,63]. However, this is in contrast with general consensus that StAR protein increased in the presence of decreased testostosterone production. The increase in Leydig cell numbers in the present study could be a consequence of the compensated Leydig cell failure in the presence of supranormal LH serum level [64]. Although serum LH levels were not assayed in this study, decreased 3β-HSD expression has been attributed to reduced LH stimulation of Leydig cells as evidenced by hypogonadal testis and feminized mice testis [65]. Therefore, the markedly increased expression in 3β-HSD protein expression in Gen10 and Gen100 in the present study were probably due to homeostatic adjustments provoked by diminished LH stimulation of cholesterol availibility and/or utilization in Leydig cells.
Several studies have reported on the influence of isoflavones on testicular morphology and Leydig cell development. In one study, genistein was found to induce hyperplasia of Leydig cells in mice [43], and in another study with marmoset developed large testes and increased number of Leydig and Sertoli cells [66]. Lower concentrations of serum testosterone associated with an increased number of Leydig cells have been observed in neonatal marmosets fed with soy milk formula when compared with animals fed with cow milk formula [67]. It has also been reported that long-term dietary administration of genistein reduces serum levels of testosterone (50) and also suppresses both basal and LH-stimulated androgen production by rooster Leydig cells in vitro [68]. The present results confirmed that genistein is capable of regulating Leydig cells function and support the direct action of genistein on Leydig cells as suggested previously (58) as well as incubation with isoflavones induced proliferative activity and supressed steroidogenic capacity in the Leydig cells [61].
The seminiferous epithelial cells are adjoined by different types of gap junctions that are linked with each other via their common adaptors or signaling processes [69,70]. Gap junction protein, Cx43, participate in germ cell development; any alteration to it can lead to loss of germ cells in either males or females [71-73]. In patients with low testosterone level (eg; carcinoma-in-situ or testicular seminoma), the Cx43 expression was reported to be down regulated indicating reduction of this gap junction protein [74-76]. Suprisingly, based on our findings, there was high expression of Cx43 in Gen10 and Gen100 testes. Thus, we hypothesized that there is no effect of weakening the signaling pathways following high dose genistein treatment and the germinal cells in the seminiferous epithelium were intact with no sign of cell loss observed.
To date, the effects of genistein to reproductive health are still unclear. Although many studies reported some deleterious effects of isoflavone exposure to infants at different doses and routes, the results are still inconsistent. It is worthwhile to highlight the agents that may induce toxicity on critical stages of development of the reproductive system as well as its possibility on interfering with the later stages of development.
Conclusion
Perinatal exposure to genistein could compromise the development of the male reproductive system in rats, as evidenced by the reduction in body weight, anogenital distance, delayed preputial separation, and overstimulation of spermatogenesis and other reproductive parameters. However, there is no evidence that genistein at the recommended dosage can produce the similar effects in human. Future studies with longer duration of genistein exposure is necessary to better account for its effects and possible mechanisms as an endocrine disruptor to the reproductive system of men. Thus, despite the indiscriminate recommendation on the use of soy and its derivatives, the results of this study show that genistein is not totally free from undesirable effects.
Acknowledgement
This work was supported under grant number: RG203- 10HTM. The authors also would like to thank Department of Anatomy, Faculty of Medicine, University Malaya, Kuala Lumpur for the facilities provided in the conduct of the research.
References
- Lethaby AE, Brown J, Marjoribanks J, Kronenberg F, Roberts H, Eden J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev 2013(12): CD001395.
- Duncan AM, Phipps WR, Kurzer MS. Phytoestrogens.Best Pract Res Clin Endocrinol Metab 2003; 17: 253- 271.
- Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr 2003; 133(956-964).
- Rowland I, Faughnan M, Hoey L, Wahala K, Williamson G, Cassidy A. Bioavailability of phyto- oestrogens. British Journal of Nutrition 2003; 89(1): 45-58.
- Zung A, Reifen R, Zohar K, Zvi Z. Phytoestrogens: the pediatric perspective. J Pediatr Gastroenterol Nutr 2001; 33(2): 112-118.
- Setchell KDR, Brown NM, Eva Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 2002; 132(12): 3577-3584.
- Kuiper G, Enmark E, Pelto-Huikko M, et al. Cloning a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 1996; 93: 5925-5930.
- Miksicek RJ. Interaction of naturally occurring no ste- roidal oestrogens with expressed recombinant human oestrogen receptor. J Steroid Biochem Mol Biol 1994; 49: 153-160.
- Collins B, McLaughlin J, Arnold S. The anti-oestroge- nic activities of phytochemicals with human oestrogen receptors expressed in yeast. Steroids 1997; 62: 365-372.
- Bennetts HW, J. UE, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J 1946; 22(1): 2-12.
- Nagata C, Takatsuka N, Kurisu Y, Shimizu H. Decrea- sed serum total cholesterol concentration is associated with high intake of soy products in Japanese men and women. J Nutr 1998; 128(2): 209-213.
- Cederroth CR, Zimmermann C, Nef S. Review: Soy, phytoestrogens and their impact on reproductive health.Molecular and Cellular Endocrinology 2012; 355: 192-200.
- Coward L, Neil CB, Setchell KDR, Barnes S. Genistein, daidzein, and their β-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets J Agric Food Chem 1993; 41(11): 1961-1967.
- Nagata C, Inaba S, Kawakami. N., Kakizoe T, Shimizu H. Inverse association of soy product intake with serum androgen and estrogen concentrations in Japanese men. Nutr Cancer 2000; 36(1): 14-18.
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect 1996; 104(4): 741-803.
- Brehm R, Zeiler M, Rüttinger C, Herde K, Kibschull M, Winterhager E, et al. A Sertoli cell-specific knock- out of connexin 43 prevents initiation of spermatogene- sis. Am J Pathol 2007; 171(1): 19-31.
- Sridharan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, et al. Proliferation of adult Sertoli cells following conditional knockout of the gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod 2007; 76(5): 804-812.
- Vinken M, Ceelen L, Vanhaecke T, Vera Rogiers V. Inhibition of gap junctional intercellular communica- tion by toxic metals. Chem Res Toxicol 2010; 23(12): 1862-1867.
- Batias C, Defamie N, Lablack A, Thepot D, Fenichel P, Segretain D, et al. Modified expression of testicular gap-junction connexin 43 during normal spermatogene- tic cycle and in altered spermatogenesis. Cell Tissue Res 1999; 298: 113-121.
- Kumar NM, Gilula NB. The gap junction communica- tionn channel. Cell 1996; 84: 381-388.
- Risley MS, Tan IP, Roy C, Sáez JC. Cell-, age- and stage-dependent distribution of connexin 43 gap junctions in testes. J Cell Sci 1992; 103(1): 81-96.
- Roscoe WA, Barr KJ, Mhawi AA, Pomerants DK, Kidder GM. Failure of spermatogenesis in mice lacking connexin43. Biol Reprod 2001; 65: 829-838.
- Defamie N, Berthaut I, Mograbi B, Chevallier D, Dadoune JP, Fénichel P, et al. Impaired gap junction connexin43 in Sertoli cells of patients with secretory azoospermia: a marker of undifferentiated Sertoli Cells. Lab Invest 2003; 83(3): 449-456.
- Matsuo Y, Nomata K, Eguchi J, Aoki D, Hayashi T, Hishikawa Y, et al. Immunohistochemical analysis of connexin 43 expression in infertile human testes. Acta Histochem Cytochem 2007; 40(3): 69-75.
- Sun J, Zhong L, Zhu Y, Liu G. Research on the isolation of mouse Leydig cells using differential digestion with a low concentration of collogenase. J Reprod Dev 2011; 57(3): 433-436.
- Berthelsen J, Skakkebaek NE. Gonadal function in men with testis cancer. Fertil Steril 1983; 39: 68-75.
- Hoei-Hansen CE, Holm M, Rajpert-De M. E., Skakkebaek NE. Histological evidence of testicular dysgenesis in contralateral biopsies from 218 patients with testicular germ cell cancer. J Pathol 2003; 200(3): 370-374.
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod 1977; 17(2): 298-303.
- Lei ZM, Mishra S, Ponnuru P, Li X, Yang ZW, Rao CV. Testicular Phenotype in Luteinizing Hormone Receptor Knockout Animals and the Effect of Testoste- rone Replacement Therapy. Biol Reprod 2004; 71: 1605-1613.
- Sharpe RM, Fisher JS, Macpherson S, Marchetti N. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of rat to dibutyl phthalate. Hum Reprod 2003; 18(7): 1383-1394.
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008; 89(2): 33-38.
- Borgwardt K, Bonifatius S, Gardemann A. Acidic peptides enhanced genistein-dependent inhibition of human platelet aggregation: potential protective effect of digestible peptides plus genistein against atheroscle- rosis. Nutr Res 2008; 28(8): 523-531.
- Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr 2008; 138: 1244-1249.
- Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy- based infant formula. The Lancet 1997; 350(9070): 23-27.
- Cannon B, Connoley E, Obregon M-J, Nedergaard J. Perinatal activation of brown adipose tissue The Endocrine Control of the Fetus 1988: 306-320.
- Spray CM, Widdowson EM. The effect of growth and development on the composition of mammals. Br J Nutr 1950; 4: 332-353.
- Heim M, Frank O, Kampmann G, Sochocky N, Pennimpede T, Fuchs P, et al. The phytoestrogen geni- stein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 2004; 145(2): 848-859.
- Doerge DR, Churchwell MI, Chang HC, Newbold RR, Delclos KB. Placental transfer of the soy isoflavone genistein following dietary and gavage administration to Sprague-Dawley rats. Reprod Toxicol 2001; 15(2): 105-110.
- Levy JR, Faber KA, et al. The effects of prenatal exposure the phytoestrogen genistein on sexual differe- ntiation in rats. Proceedings of the Society of Experi- mental Biology and Medicine 1995; 208: 60-66.
- Boberg J, Mandrup KR, Jacobsen PR, Isling LK, Hadrup N, Berthelsen L, et al. Endocrine disrupting effects in rats perinatally exposed to a dietary relevant mixture of phytoestrogens. Reprod Toxicol 2013; 40: 41-51.
- de Zegher F, Devlieger H, Eeckels R. Fetal growth: boys before girls. Horm Res 1999; 51(5): 258-259.
- Keisler LW, Saal FSV, Keisler DH, Walker SE. Hormonal manipulation of the prenatal environement alters reproductive morphology and increase longevity in autoimmune Nzb/W mice. Biol Reprod 1991; 44(4): 707-716.
- Lee BJ, Jung EY, et al. Effects of exposure to genistein during pubertal development on the reproductive system of male mice. Journal of Reproduction and Development 2004; 50(4): 399-409.
- Roberts D, Veeramachaneni DNR, et al. Effects of chronic dietary exposure to genistein, a phytoestrogen, during various stages of development on reproductive hormones and spermatogenesis in rats. Endocrine 2000; 13(3): 281-286.
- Kim KS, Torres CR, Yucel S, Raimondo K, Cunha GR, Baskin LS. Induction of hypospadias in a murine model by maternal exposure to synthetic estrogens. Environ Res 2004; 94(3): 267-275.
- Yang J, Nakagawa H, Tsuta K, Tsubura A. Influence of perinatal genistein exposure on the development of MNU-induced mammary carcinoma in female Sprague-Dawley rats. Cancer Lett 2000; 149(1): 171-179.
- Delclos KB, Bucci TJ, Lomax LG, Latendresse JR, Warbritton A, Weis CC, et al. Effects of dietary genistein exposure during development on male and female CD (Sprague-Dawley) rats. Reprod Toxicol 2001; 15(6): 647-663.
- Wisniewski AB, Cernetich A, Cernetichb A, Gearhartc JP, Kleinb SL. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiology and behavior 2005; 84(2): 327- 334.
- Wisniewski AB, Klein SL, Lakshmanan Y, Gearhart JP. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J Urol 2003; 169(4): 1582-1586.
- Weber KS, Setchell KDR, Stocco DM, Lephart ED. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5α- reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol 2001; 170(3): 591-599.
- Piotrowska K, Baranowska-Bosiacka I, Marchlewicz M, Gutowska I, Noceń I, Zawiślak M, et al. Changes in male reproductive system and mineral metabolism induced by soy isoflavones administered to rats from prenatal life until sexual maturity. Nutrition 2011; 27(3): 372-379.
- Montani C, Penza M, Jeremic M, Biasiotto G, La Sala G, De Felici M, et al. Genistein is an efficient estrogen in the whole-body throughout mouse development. Toxicol Sci 2008; 103(1): 57-67.
- Thuillier R, Mazer M, Manku G, Boisvert A, Wang Y, Culty M. Interdependence of platelet-derived growth factor and estrogen-signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol Reprod 2010; 82(5): 825-836.
- Chi H, Chun K, Son H, Kim J, Kim G, Roh S. Effect of genistein administration on the recovery of spermato- genesis in the busulfan-treated rat testis. Clin Exp Reprod Med 2013; 40(2): 60-66.
- Udagawa K, Ogawa T, Watanabe T, Yumura Y, Takeda M, Hosaka M. GnRH analog, leuprorelin acetate, promotes regeneration of rat spermatogenesis after severe chemical damage. Int J Urol 2001; 8(11): 615-622.
- Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 1996; 17(2): 271-275.
- O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ. Failure of normal adult Leydig cell development in androgen-receptordeficient mice. J Cell Sci 2002; 115 (17): 3491-3496.
- Sherrill JD, Sparks M, Dennis J, Mansour M, Kemppai- nen BW, Bartol FF, et al. Developmental exposures of male rats to soy isoflavones impact Leydig cell differen- tiation Biol Reprod 2010; 83(3): 488-501
- Benton L, Shan LX, Hardy MP. Differentiation of adult Leydig cells. J Steroid Biochem Mol Biol 1995; 53(1- 6): 61-68.
- Lehraiki A, Chamaillard C, Krust A, R. H, Levacher C. Genistein impairs early testosterone production in fetal mouse testis via estrogen receptor alpha. Toxicol in Vitro. 2011; 25(8): 1542-1547.
- Napier ID, Simon L, Perry D, Cooke PS, Stocco DM, Sepehr E, et al. Testicular Development in Male Rats Is Sensitive to a Soy-Based Diet in the Neonatal Period. Biol Reprod 2014; 90(2): 40.
- Hancock KD, Coleman ES, Tao YX, Morrison EE, Braden TD, Kemppainen BW, et al. Genistein decreases androgen biosynthesis in rat Leydig cells by interference with luteinizing hormone-dependent signaling. Toxicol Lett 2009; 184(3): 169-175.
- Svechnikov K, Spatafora C, Svechnikova I, Tringali C, S¨oder O. Effects of resveratrol analogs on steroido- genesis and mitochondrial function in rat Leydig cells in vitro. J Appl Toxicol. 2009; 29(8): 673-680.
- de Kretser DM. Editorial: Is spermatogenic damage associated with Leydig cell dysfunction? J Clin Endo-crinol Metab. 2004; 89(7): 3158-3160.
- Couse JF, Yates MM, Walker VR, Korach KS. Charac- terization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol 2003; 17(6): 1039-1053.
- Tan KA, Walker M, Morris K, Greig I, Mason JI, Sharpe RM. Infant feeding with soy formula milk: effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Human Reprod 2006; 21(4): 896-904.
- Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, et al. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Human Reprod 2002; 17(7): 1692-703.
- Opałka M, Kamin´ ska B, Ciereszko R, Dusza L. Genistein affects testosterone secretion by Leydig cells in roosters (Gallus gallus domesticus). Reproductive Biology 2004; 4(2): 185-193.
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 2002; 82(4): 825-874.
- Lee NPY, Cheng CY. Mini-review: Adaptors, junction dynamics, and spermatogenesis. Biol Reprod 2004; 71(2): 392-404.
- Juneja SC, Barr KJ, Enders GC, Kidder GM. Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod 1999; 60(5): 1263-1270.
- Plum A, Hallas G, Magin T, Dombrowski F, Hagen- dorff A, Schumacher B, et al. Unique and shared functions of different connexins in mice. Curr Biol 2000; 10(18): 1083-1091.
- Askert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 2001; 233(2): 258-270.
- Brehm R, Marks A, Rey R, Kliesch S, Bergmann M, Steger K. Altered expression of connexins 26 and 43 in Sertoli cells in seminiferous tubules infiltrated with carcinoma-in-situ or seminoma. J Pathol 2002; 197(5): 647-653.
- Okada K, Katagiri T, Tsunoda T, Mizutani Y, Suzuki Y, Kamada M, et al. Analysis of gene-expression profiles in testicular seminomas using a genome-wide cDNA microarray. Int J Oncol 2003; 23(6): 1615-1635.
- Yan HH, Mruk DD, Lee WM, , Cheng CY. Blood- testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetcis of protein endocytosis and recycling in Sertoli cells. FASEB J 2008; 22(6): 1945-1959.