Research Article - Current Pediatric Research (2021) Volume 25, Issue 4
Effectiveness of Nutritional Supplement in Growth and Development of Children aged 2-12 years.
Jasjit Singh Bhasin1, Vaman Khadilkar2*, IPS Kochar3, Uday Pai4, Gautam Mittal5, Prashant Sankar6
1 Department of Pediatrics, BLK Super Specialty Hospital, New Delhi, India
2 Department of Pediatrics, Jehangir Hospital, Pune, India
3 Department of Pediatrics, Indraprastha Apollo Hospital, New Delhi, India
4 Department of Pediatrics, Pai’s Clinic, Mumbai, India
5 Department of Pediatrics, Khalsa college colony, Patiala, India
6 Department of Pediatrics, Porur Child Health Clinic, Chennai, India
- Corresponding Author:
- Dr. Vaman Khadilkar
Department of Pediatrics Jahangir Hospital Pune, India
Tel: +91- 99205 90710
E-mail: vamankhadilkar@gmail.com
Accepted date: April 15, 2021
Abstract
Objective: Observational study to determine the effect of 6 months consumption of nutritional supplement on growth and development of children aged 2-12 years. Background: Young children in India suffer from some of the highest levels of stunting, underweight, and wasting observed in any country in the world. The levels of over-nutrition are also on a rise. Prevention of child malnutrition require diets providing adequate energy and essential nutrients to promote catch-up growth, strengthen resistance to infection, and support normal mental, physical and metabolic development.
Methods: This is an observational study, where an oral nutritional supplement is given to 776 children, aged 2-12 years, for 6 months along with normal diet. Anthropometric parameters (height, weight and BMI) are assessed at baseline, 3 and 6 months. The z score for height, weight and BMI is used to analyze the results using Khadilkar growth chart, Indian Pediatrics 2009.
Results: A total of 763 subjects are included in the analysis. Each child is grouped according to age bracket (2-3 yrs; 4-6 yrs; 7-9 yrs; 10-12 yrs). After consumption of nutritional supplement for 6 months, z-scores for height, weight and BMI are shown improvement in almost all age groups, compared to baseline. The improvement is significant in weight and BMI z-score. The standard deviation scores from expected increase in mean of weight, height and BMI is well within the permissible range. No adverse event is observed.
Conclusion: This study showed that 6 months intake of nutritional supplement by children provided a significant improvement in anthropometric parameters, with no adverse event.
Keywords
Nutritional supplements, Body mass index, Weight, Children 2-12 years.
Introduction
India, a developing country, faces several health challenges in terms of maternal and fetal growth, nutritional inequalities as well as disease management.
Around 27% of Indian population (26% in urban areas and 28% in rural areas) has been determined to be below the poverty line, defined as the expenditure needed to obtain, on an average, 2400 Kcal per capita per day in the rural areas and 2100 Kcal in urban areas. Although the country has witnessed some great economic growth rate since few decades, poverty and under nutrition continue to top the chart of the issues that need immediate attention [1].
Poverty and nutritional problems often coexist and results in poor child growth, increased micronutrient deficiencies, increased susceptibility to diseases and hampered physical and mental development.
Long term malnutrition in early age also leads to stunting, wasting, mortality and morbidity. India is home to 31% and 42% of the world's children who are stunted and underweight respectively, while many others are affected by micronutrient deficiencies [2]. The Indian Academy of Pediatrics (IAP) recommends use of IAP growth for monitoring height and weight and determining the need of intervention as appropriate [3].
The Academy of Nutrition and Dietetics (the Academy) and the American Society for Parenteral and Enteral Nutrition (ASPEN), recommend use of standardized set of diagnostic indicators to identify and document pediatric malnutrition/ under nutrition in routine clinical practice.
The recommended indicators include z scores for weight-for- height/length, body mass index-for-age, or length/height-for- age or mid-upper arm circumference when a single data point is available [4]. Such a standardize approach would pave a way for establishing recommendations as well as determining the interventions needed for correction of nutritional deficiencies.
Nutritional supplements have been recognized as the most suitable method of improving growth and physical health of children in developing countries, they are also crucial for early development including cognition [5].
A meta-analysis including 29814 children from 20 developing countries suggested that nutritional supplementation could improve children’s cognitive development (d 0.08, 95% CI 0.03-0.13).
Fortified food is also a suitable public health approach to increasing vitamin intakes [6]. Based on the available evidence, with an objective to provide adequate energy and essential nutrients and promote catch-up growth, strengthen resistance to infection, support normal mental, physical and metabolic development, we determined the effect of 6 months consumption of nutritional supplement on growth and development of children aged 2-12 years.
Methods
This was observational study, to assess the effectiveness and safety of an oral nutritional supplement in children aged 2 to 12 years. The study was conducted according to the ethical guidelines. All approvals were obtained before start of the study.
All children were between 2-12 years and had regular eating habits. Data from the children were grouped based on their age group as 2-3 years, 4-6 years, 7-9 years and 10-12 years. Children with concomitant systemic infection or clinically significant diseases were excluded from the study.
Children with stomach infection, infestations and suspected liver disorders were also not included in the study. In addition, children who were previously on a nutritional supplement, or have been taking multivitamins, calcium supplements in addition to the diet had to undergo a period of washout before enrolling in the study.
The Oral Nutritional Supplement (ONS) was given (30 gm in 100 mL water or milk, twice daily) to children for 6 months along with normal diet and they were asked to follow-up at regular intervals. ONS in this study (Groviva®) is a signature child nutrition supplement with 38 key nutrients- including Certi5TM- dual protein, dietary fiber, DHA, probiotics and calcium which has been formulated for Indian children as per ICMR guidelines.
Anthropometric parameters (height, weight and Body Mass Index (BMI)) were assessed at baseline, 3 and 6 months. The z-score (gives you an idea of how far from the mean a data point is) for height, weight and BMI was used to analyze the results using Khadilkar growth chart 2009. A z-score Safety was assessed by monitoring of adverse events. The p value <0.05 was considered statistically significant.
Results
The data obtained from studies conducted at 145 clinics across 116 cities in India from 1st March 2018 to 30th October 2018. A total of 776 children were enrolled and received the nutritional supplement (Figure 1).
The compliance observed was 100% with zero dropouts. The demographics and baseline characteristics are presented in Table 1.
| Variables | All Children (N=707) |
|---|---|
| Age (Yrs) | 5.2 |
| Male | 451 |
| Female | 312 |
| Height (cm) | 100.3 |
| Weight (kg) | 14.9 |
| BMI* | 14.5 |
Table 1. Baseline demographics.
The median age was 5.2 ± 0.4 years (range 02-12) and the gender distribution includes 451 males and 256 females. None of the children enrolled had wasting (z score ≥ -2), however the children in the 10-12 years age group showed stunted growth (z score ≥ -2); however most of them were underweight (z score ≤ -1.88).
After consumption of nutritional supplement, changes were observed as early as 3 months. The changes in height, weight and BMI at both 3 and 6 months are presented in Table 2. A substantial increase in 3 parameters, among all age groups was observed at both 3 months and 6 months due to consumption of ONS. In the children aged 2-3 years, a statistically significant (p<0.05) weight increase from 11.6 to 12.9 and 13.8 kg was observed from baseline to 3 months and 6 months respectively, which also led to increase in corresponding BMI values. In age group of 8-10 years at both 3 months and 6 months significant increase (p<0.05) in weight with corresponding BMI was observed. Similar increase in age group 11-12 years was seen at 6 months consumption.
| Age | Parameter | Baseline | 3 months | 6 months |
|---|---|---|---|---|
| 2-4 yrs | Height (cm) | 89.8 | 91.2 | 92 |
| Weight (kg) | 11.6 | 12.9 | 13.8 | |
| BMI | 14.4 | 16.1 | 18.1 | |
| 5-7 yrs | Height (cm) | 104.4 | 105.7 | 107.6 |
| Weight (kg) | 16.0 | 16.6 | 17.9 | |
| BMI | 14.6 | 14.9 | 15.5 | |
| 8-10 yrs | Height (cm) | 119.7 | 121.4 | 121.8 |
| Weight (kg) | 20.8 | 22 | 23.4 | |
| BMI | 14.5 | 14.9 | 15.5 | |
| 11-12 yrs | Height (cm) | 131.9 | 133.0 | 135.6 |
| Weight (kg) | 27.9 | 29.1 | 30.6 | |
| BMI | 15.9 | 16.3 | 16.4 |
Table 2. Change in height, weight & bmi from baseline to month 3 and 6.
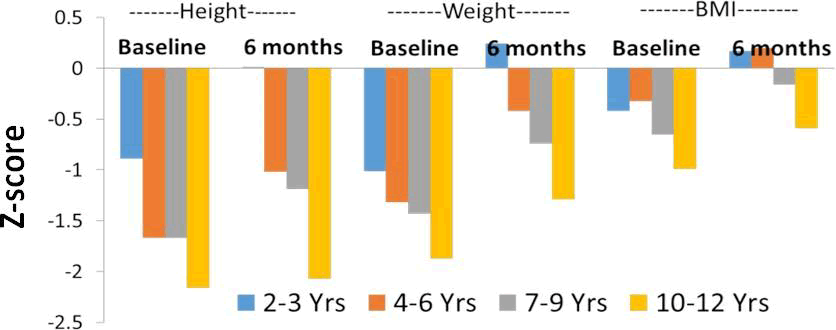
The data was also presented in form of Z-score. In the children aged 2-3 years, the z scores for height, weight and BMI improved from -0.89, -1.01 and -0.42 respectively at baseline to -0.46, -0.33 and -0.06 at Month 3. Similar results were noted for age group 4-6 (Baseline: height, -1.67, weight, -1.32, BMI, -0.32 to Month 3: height, -1.73, weight, -0.87, BMI, -0.05); age 7-9 years (Baseline: height, -1.67, weight, -1.43, BMI, -0.65 to Month 3: height, -1.46, weight, -1.11, BMI, -0.43) and age 10-12 years (Baseline: height, -2.16, weight, -1.87, BMI, -0.99 to Month 3: height, -1.91, weight, -1.6, BMI, -0.83). After consumption of nutritional supplement for 6 months, substantial improvements were observed in z-scores for height, weight and BMI compared to baseline in almost all age groups (Figure 2). The improvements were significant in weight, height, and BMI z-score. The standard deviation scores from expected increase in mean of weight, height and BMI were well within the permissible range. No adverse events were observed.
Discussion
Long-term malnutrition is a concern not only to the individual but also the nation as it leads diet related disorders, improper growth, increased morbidity, reduced work capacity as well as untimely death in few cases. This results in increased healthcare loss, great economic burden and is detrimental to the development of a country like India. The National Family Health Survey 4 (NFHS-4) showed that at birth 31.9% children present with signs of wasting, and 17.7% less than five years of age still continue to have the problem. Moreover around <1% children in almost all the States suffer from both severe wasting and severe stunting, increasing the risk of mortality in them [7]. Micronutrient deficiencies are also common in India, and high prevalence of anemia (14%-88%) and low dietary iron intakes (30%-50% of the Recommended Dietary Allowance (RDA)) has been observed in school children. Furthermore, around 44%-66% of children even from affluent backgrounds suffer from vitamin A, B2, B6, B12, and C deficiencies [8]. to combat this challenge, proper nutrition is a mandate.
In the present study keeping in view the nutritional needs of growing children, the nutritional supplement provided was scientifically formulated with 38 key nutrients to support cognitive function, natural immunity, normal growth and development, gut health, bone health and decrease fatigue in children between 2-12 years. The changes in height, weight and BMI were visible as early as 3 months and substantial after 6 months of use. This is in line with the reports of the European Food Safety Authority (EFSA) which noted that young child formulae and supplement consumption was the shortest way to cover the EFSA nutrient requirements of UK children [9]. Previous studies reported positive association between specific nutrient intake such as protein, DHA, dietary fibers or calcium with linear growth and development, suggesting that intake of certain nutrients may be specifically important to promote growth [10]. Nutritional supplements not only aid in improving the health status of normal children but are also useful for children with Attention-Deficit Hyperactivity Disorder (ADHD), autism etc [11,12].
The results of our trial support the results of the study which determined the 1-year effectiveness and safety of nutritional supplementation with the study formula on linear growth and weight gain in short and lean prepubertal children. Throughout the entire year height continued to improve, with a total gain of 0.19 ± 0.14 SD. The study concluded that 1 year of a nutritional supplement was effective in promoting the linear growth of short and lean prepubertal children, with no change in body mass index status [13].
Some limitations of this current 6-month study include absence of a comparator, restricting the enrolled children to healthy individuals and limited assessment parameters. The present study however paves a way for further recognizing the nutritional needs of children in India and also identifying suitable interventions that can be recommended to correct malnutrition/under nutrition. The Government should also play a crucial role in eradicating nutrition related problems by providing easy access to the supplements. Further studies are required to assess the clinical effectively and safety of such nutritional supplements in a larger group of population with or without disease conditions.
Conclusion
In 6 months of observational study, young children of age 2-12 year supplemented with ONS as nutritional supplement to their diet, led to significant increase in height, weight and BMI. No adverse effects were observed across the study.
Key Message
A proper nutrient supplementation is required for growing children and various guidelines from American and European bodies support. This study adds the Indian perspective of food supplement to this group of children.
References
- Varadharajan KS, Thomas T, Kurpad AV. Poverty and the state of nutrition in India. Asia Pac J Clin Nutr 2013; 22(3): 326-39.
- Sankar R, T. van den Briel. Prospects for better nutrition in India. Asia Pac J Clin Nutr. 2014; 23(Suppl 1): S1-3.
- Khadilkar V, Yadav S, Agrawal KK, et al. Revised IAP growth charts for height, weight and body mass index for 5 to 18-year-old Indian children. Indian Pediatr. 2015; 52(1): 47-55.
- White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: indicators recommended for the identification and documentation of pediatric malnutrition (under nutrition). Nutr Clin Pract 2015; 30(1): 147-61.
- Ip P, Ho FKW, Rao N, et al. Impact of nutritional supplements on cognitive development of children in developing countries: A meta-analysis. Sci Rep 2017; 7(1): 10611.
- Hennessy A, Browne F, Kiely M, et al. The role of fortified foods and nutritional supplements in increasing vitamin D intake in Irish preschool children. Eur J Nutr 2017; 56(3): 1219-31.
- Hemalatha R, Radhakrishna KV, Kumar BN. Under nutrition in children & critical windows of opportunity in Indian context. Indian J Med Res 2018; 148(5): 612-20.
- Muthayya S, Thankachan P, Hirve S, et al. Iron fortification of whole wheat flour reduces iron deficiency and iron deficiency anemia and increases body iron stores in Indian school-aged children. J Nutr 2012; 142(11): 1997-2003.
- Vieux F, Brouzes CMC, Maillot M, et al. Role of young child formulae and supplements to ensure nutritional adequacy in UK. young children. Nutrients 2016; 8(9).
- Lange KW, Hauser J, Lange KM, et al. The role of nutritional supplements in the treatment of ADHD: What the evidence says. Curr Psychiatry Rep 2017; 19(2): 8.
- Sathe N, Jeffrey C. Andrews, Melissa L. et al. Nutritional and dietary interventions for autism spectrum disorder: A systematic review. Pediatrics 2017; 139(6).
- Gurlek Gokcebay D, Emir S, Bayhan T, et al. Assessment of nutritional status in children with cancer and effectiveness of oral nutritional supplements. Pediatr Hematol Oncol 2015; 32(6): 423-32.
- Yackobovitch-Gavan M, Lebenthal Y, Lazar L, et al. Effect of nutritional supplementation on growth in short and lean prepubertal children after 1 year of intervention. J Pediatr 2016; 179(e1): 154-9.