Research Article - Journal of Biochemistry and Biotechnology (2022) Volume 5, Issue 5
Effect of Xylaria nigripes, Panax notoginseng, Cuscuta chinensis on GABA, GABA transaminase, glutamate decarboxylase, amyloid beta, and amyloid beta aggregation levels in PC12 neuron cells.
Su-Fen Huang1, Han-Wen Yang1, Kamesh Venkatakrishnan2, Tsu-Chung Chang1*, Wen-Liang Chang31Department of Biochemistry, National Defense Medical Center, No.161, Sec. 6, Minquan E. Rd, Neihu Dist, Taipei, Taiwan
2Department of Nutrition, Chung Shan Medical University, Taichung, Taiwan
3School of Pharmacy, National Defense Medical Center, No.161, Sec. 6, Minquan E. Rd, Neihu Dist, Taipei, Taiwan
- *Corresponding Author:
- Tsu-Chung Chang
Department of Biochemistry
National Defense Medical Center
No.161, Sec. 6, Minquan E. Rd
Neihu Dist, Taipei, Taiwan
E-mail: tcchang@mail.ndmctsgh.edu.tw
Received: 22-Aug-2022, Manuscript No. AABB-22-72556; Editor assigned: 24-Aug-2022, PreQC No. AABB -22-72556(PQ); Reviewed: 06-Sep-2022, QC No AABB-22-72556; Revised: 10-Sep-2022, Manuscript No. AABB-22-72556(R); Published: 17-Sep-2022, DOI:10.35841/aabb-5.5.121
Citation: Huang SF, Yang HW, Venkatakrishnan K, et al. Effect of Xylaria nigripes, Panax notoginseng, Cuscuta chinensis on GABA, GABA transaminase, Glutamate decarboxylase, Amyloid beta, and Amyloid beta aggregation levels in PC12 neuron cells. J Biochem Biotech 2022;5(5):121
Abstract
The purpose of this study is to determine the effect of three different botanical compositions, Xylaira Nigripes extract (XE), Xylaira Nigripes + Panax notoginseng extract (XPE), and Xylaira Nigripes+Panax notoginseng+Cuscuta chinensis extract (XPCE) on γ-Aminobutyric acid (GABA) content, GABA transaminase (GABA-T), Glutamate decarboxylase (GAD), cells viability, and Amyloid-β protein (Aβ) aggregation levels in PC12 neuron cell. For this study, different concentrations (40, 60, 80, and 120 μg/mL) of the three compositions were used and all the experiments were performed in triplicate. All three compositions could significantly improve (p<0.001) GABA production and also increase the activity of glutamate GAD along with decreased GABA-T activity in PC12 cells in a dose-dependent fashion. Moreover, all three compositions showed potent neuroprotective properties via increasing PC12 cell viability as well as significantly attenuating (p<0.001) the Aβ-induced injury and Aβ aggregation in a dose-dependent manner. Overall, all the three compositions exhibited strong neuroprotective activity by improving GABA release through elevated GAD activity with decreased GABA-T activity. Also, all three compositions enhance the proliferation (cell viability) of PC 12 cells and significantly lowers the Aβ aggregation. Out of the three compositions, XPCE (p<0.001) showed the best holistic neuroprotective and GABA releasing properties than XE and XPE.
Keywords
GABA, GAD, GABA-T, neuroprotective, Aβ aggregation.
Introduction
γ- Amino butyric acid (GABA) is a non-proteinogenic amino acid that acts as an inhibitory neurotransmitter, which helps to block the neuro signals/message and thereby reduce stress, anxiety, and fear (calming effect). Hence GABA plays a crucial role in stabilizing mood [1]. Researchers are showing immense interest in GABA and its uptake to regulate various neuropsychological issues, which are related to hyper neuronal excitability and thus useful for treating autism, schizophrenia, Tourette’s syndrome, and other hyperactive neuronal disorders [2,3]. GABA is produced from glutamate/glutamic acid (in GABA-ergic neuron) with the help of GABA decarboxylase (GAD) enzyme and degraded/ broken down by GABA transaminase or aminotransferase (GABA-T) enzyme therefore to maintain a nominal level of GABA in the system; the above-mentioned enzymes play a pivotal role [4]. Moreover, studies have shown that GABA has a strong association with insomnia, hypertension, and Alzheimer’s disease as it balances the production of various neurotransmitters/neurotropic factors and hormones [5,6]. Recently, many scientists have shown interest in regulating GABA levels (nominal level) to maintain a strong healthy mental status in normal and various neurological disorders subjects using various traditional medicine, which has traditionally been used in regulating mood, sleep, and stress/ depression [5,7].
Xylaria nigripes is an edible fungus (WulinShen) belonging to the Xylariaceae family. Xylaria nigripes have been traditionally used for treating various ailments including insomnia, anxiety, and neurodegenerative disorders [8]. It is commonly grown on dead trees and near abandoned termite nests [9]. Recently many researchers have shown that Xylaria nigripes possess various biological functions like antioxidant, anti-inflammatory, immunomodulatory, and anti-depressive properties along with hepatoprotevtive and neuroprotective activities [10-12]. The above-mentioned activities are due to the presence of various phytocomponents including polyphenols, adenosine, triterpenoids, and polysaccharides [13,14]. An ample amount of evidence indicated that Xylaria nigripes would improve GABA production and thus plays a key role in reducing depression and insomnia [15,16]. Panax notoginseng (root) is a popular traditional Chinese medicine (Chinese Ginseng) rich in ginsenosides (saponins). Previous studies have shown that ginsenosides from Panax notoginseng have considerably improved memory (cognitive function) and learning ability along with anti-depression, antioxidant and anti-inflammatory properties and thus altering the amyloid genesis process [17,18]. Likewise, Zheng and his colleagues in his study confirmed that ginsenosides from Ginseng could enhance the production of acetylcholine and GABA as well as lower β-amyloid protein accumulation and thereby attenuates Aβ-amyloid protein-induced neuronal damage [19].
Cuscuta chinensis is traditional medicine and is rich in flavonoids, phenolic acids, and polysaccharides [20]. Previously, various scientists have proved that Cuscuta chinensis exhibits numerous pharmacological activities including antioxidant, anti-inflammatory, anti-depressant, anti-aging as well as renoprotective, hepatoprotective, and neuroprotective properties [20-22]. Zhen and others indicated that Cuscuta chinensis could considerably improve the survival rate of PC12 cells via lowering oxidative stress and thereby exhibiting neuroprotective properties [23]. Aforementioned that GABA plays a crucial role in regulating mood, anxiety, and fear. Hence, this study was designed to explore the synergistic effect of three compositions including fermented Xylaria nigripes extract (XE); XE and Panax notoginseng extract (XPE); XE, XPE, and Cuscuta chinensis extract (XPCE) at different concentrations on GABA content, GABA-related enzyme activities, β-amyloid protein aggregation in PC12 cells.
Materials and Methods
Samples
All the three compositions including XE, XPE, and XPCE extract were provided by NuLiv Sciences USA, Inc. (Brea, CA, USA). XE contains only fermented Xylaira Nigripes extract (rich in polysaccharides and flavonoids). XPE contains the XE and Panax notoginseng extract (rich in ginsenosides) extract. XPCE contains the XE, XPE, and XPCE extract (rich in polysaccharides and flavonoids).
Culturing of PC12 cells
The PC12 cell line was purchased from BCRC (Hsinchu, Taiwan). PC12 cells were grown under Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 5% fetal bovine serum (FBS), 10% horse serum, and 50 μg/L gentamycin in a humidified atmosphere of 5% CO2 at 37°C. Experiments were performed only when PC12 cells grown to ~80% confluence between passages 2-6. Before three compositions (XE, XPE, and XPCE) treatments at different concentrations (40-120 μg/mL), the cells were grown in FBS/horse serum and an antibiotic-free medium for 16 h. The protein content of PC12 cell lysate was estimated using the BCA protein assay kit (ab102536) provided by Abcam (Cambridge, UK).
GABA content
GABA contents in PC12 cells treated with different concentrations of the three compositions (XE, XPE, and XPCE) were assayed by BioVision Quick Detect-GABA ELISA kit (E4457) purchased from BioVision (MA, USA) according to the manufacturer’s protocol.
GABA related enzymes
GABA Transaminase (GABA-T) activity: GABA-T activity in PC12 cells was detected using a GABA-T assay kit (E-134) provided by Biomedical Research Service Center and Application (NY, USA) based on the supplier’s instruction.
GABA Decarboxylase (GAD) activity: GAD activity was assayed using BioVision Glutamate Decarboxylase Activity Assay Kit (K2021) purchased from BioVision (MA, USA) according to the manufacturer’s protocol.
PC12 cells viability with and without Aβ 1-42 peptide
PC12 cells were plated in 96 well plates at a density of 30,000 cells/well and incubated with each composition at different concentrations (40-120 μg/mL) for 24 h at 37°C. Add 10 μl of the CCK-8 solution (provided with a Cell counting kit) to each well of the plate. Incubate the plate for 24 h in the incubator and the absorbance at 450 nm was measured using a microplate reader. Also, the cell viability of PC12 cells with Aβ1–42 (cytotoxic) peptide was also measured similarly as mentioned above to confirm the neuroprotective activity of the three compositions. In brief, Aβ1–42 (10 μM) was diluted with serum-free culture medium and incubated with PC12 cells (30,000 cells/well) for 24 h and then treated with three different compositions for another 24 h. Then, the PC12 cell viability was determined by using the Cell Counting Kit-8 (CCK-8) as mentioned above.
Aβ protein Aggregation
The process of beta-amyloid protein aggregation was monitored using the ThT assay kit. ThT can assemble with misfolded amyloid proteins and emit fluorescence as a result of this union. Initially, Aβ1–42 (10 μM final concentration) peptide/ protein was mixed in equal volumes with each composition (40-120 μg/mL), and ThT (200 μM final concentration) was added on 96-well plates. The ThT fluorescence was measured using a multi-detector microplate reader fluorescence spectrophotometer set at 450 nm for excitation and 485 nm for emission wavelengths. Fluorescence emission data were recorded every 5 minutes over a 3 h period.
Statistical Analysis
All the experiments were performed in triplicate (n=3). Data are expressed as the mean ± standard deviation (SD). All statistical analysis was conducted using SPSS software, version 17 (SPSS, IBM, NY, USA). All the values were evaluated using one-way ANOVA, followed by Duncan’s multi-comparison test to compare the difference between the three compositions at different concentrations. Differences were deemed significant when the probability values were less than 0.05 (p<0.05).
Results
Evaluation of GABA content in PC12 cells after treatment with the three compositions
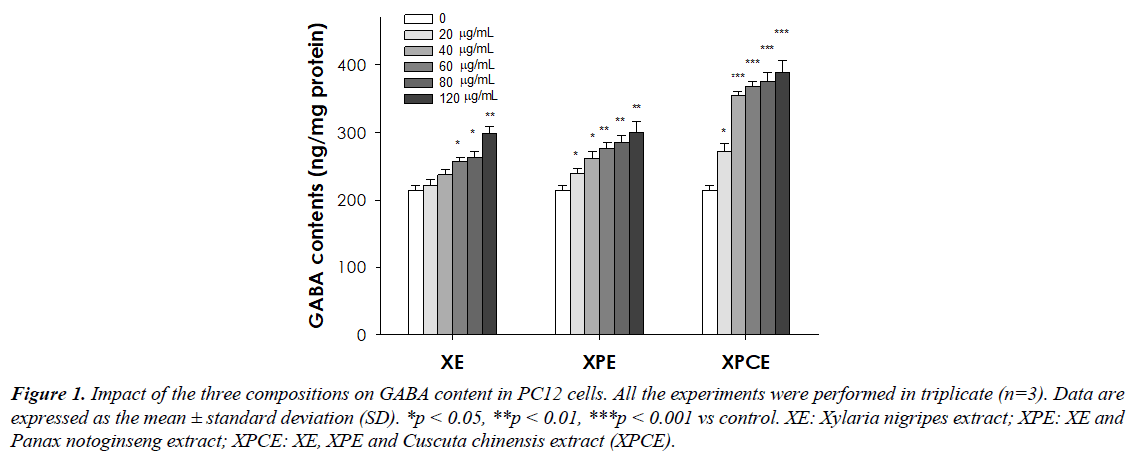
Figure 1 represents the GABA content (ng/mg protein) and Table 1 represents the relative GABA content (%) in PC12 after treatment with the three compositions. GABA content was considerably increased in PC12 cells, which were treated with the three compositions. Particularly, the 120 μg/mL concentration of XE (138.1%), XPE (139.4%), and XPCE (180.8%) showed the highest GABA levels than other concentrations. However, XPCE compositions rich in polysaccharides, flavonoids, and saponins exhibited the best GABA enhancing property (increase GABA synthesis) as compared to XE and XPE.
| Dose (µg/mL) (µg/mL) |
Control | Relative GABA contents (%) in PC-12 cells | ||
|---|---|---|---|---|
| XE | XPE | XPCE | ||
| 20 | 100.0 ± 3.2 | 103.0 ± 3.9 | 111.5 ± 3.1* | 126.9 ± 4.8* |
| 40 | 110.5 ± .4.0 | 121.9 ± .4.9* | 165.2 ± 2.6*** | |
| 60 | 120.0 ± 2.9* | 128.5 ± 3.9** | 171.4 ± 3.4*** | |
| 80 | 122.2 ± 4.7* | 132.9 ± 4.6** | 174.2 ± 6.6*** | |
| 120 | 138.1 ± 4.3** | 139.4 ± 7.5** | 180.8 ± 7.9*** | |
Table 1. Impact of the three compositions on GABA content (%) in PC12 cells. Data are expressed as the mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
GABA Transaminase (GABA-T) activity in PC12 cells after treatment with the three compositions
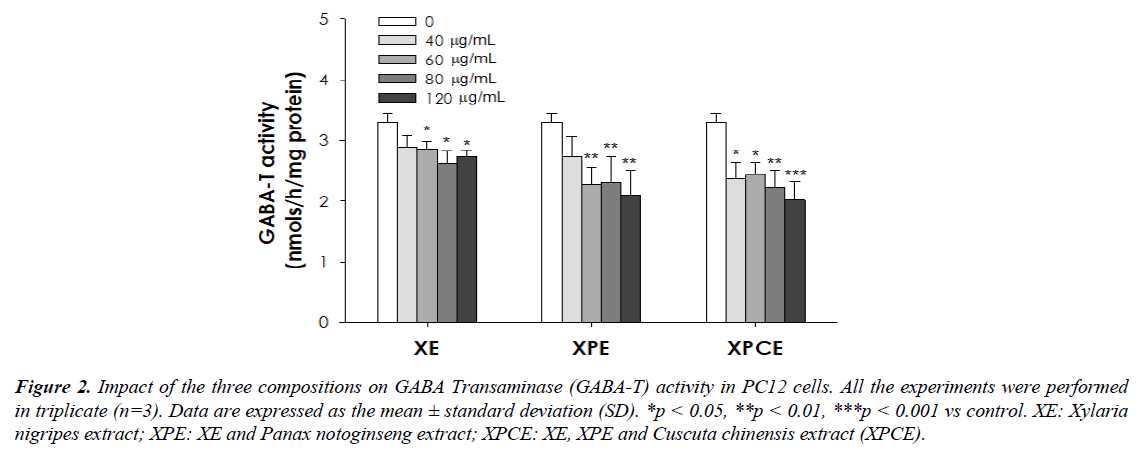
The GABA-T activity in PC12 cells after treatment with the three compositions and control were shown in Table 2 (%) and Figure 2. The relative activity of GABA-T was considerably suppressed by all the three compositions (XE/XPE/XPCE) in PC12 cells in a dose-dependent manner than the control group. Here also, XPCE (120 μg/mL; 60.2%) displayed the highest (p<0.001) GABA-T inhibitory activity and subsequently improved GABA content.
| Dose (µg/mL) | Control | GABA-T activity (%) in PC-12 cells | ||
|---|---|---|---|---|
| XE | XPE | XPCE | ||
| 40 | 100.0 ± 4.4 | 87.5 ± .5.9 | 82.6 ± .10.3 | 71.8 ± .7.8* |
| 60 | 86.6 ± 4.0* | 68.8 ± 8.8** | 73.9 ± .6.1* | |
| 80 | 79.5 ± 6.0* | 69.7 ± 13.2** | 67.2 ± 8.7** | |
| 120 | 82.9 ± 3.3* | 63.4 ± 12.7** | 60.2 ± 9.4*** | |
Table 2. Impact of the three compositions on GABA Transaminase (GABA-T) activity (%) in PC12 cells. Data are expressed as the mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
GABA Decarboxylase (GAD) activity in PC12 cells after treatment with the three compositions
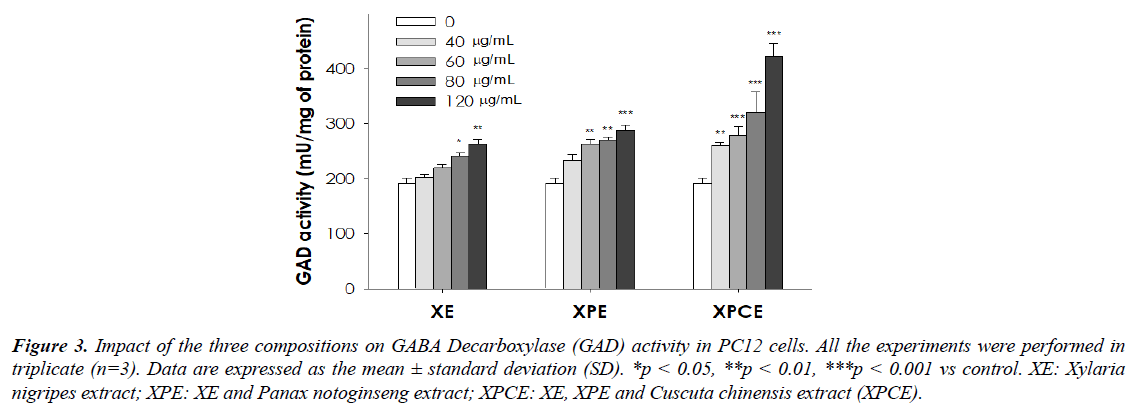
As shown in Table 3 (%) and Figure 3, the GAD activity in PC12 cells were significantly increased after treatment with XE (p<0.05; p<0.01), XPE (p<0.01; p<0.001), and XPCE (p<0.001) with different concentration. XPCE displayed greater GAD enhancing activity in a dose-dependent fashion than XE and XPE.
| Dose (µg/mL) |
Control | Relative GAD activity (%) in PC-12 cells | ||
|---|---|---|---|---|
| XE | XPE | XPCE | ||
| 40 | 100.0 ± 5.3 | 105.6 ± 3.5 | 121.9 ± 5.7 | 136.3 ± 2.6** |
| 60 | 114.7 ± 3.1 | 137.8 ± 4.1** | 145.6 ± 8.4*** | |
| 80 | 126.3 ± 2.6* | 141.3 ± 2.6** | 167.6 ± 19.4*** | |
| 120 | 134.6 ± 4.0** | 150.4 ± 5.3*** | 220.8 ± 12.3*** | |
Table 3. Impact of the three compositions on GABA Decarboxylase (GAD) activity (%) in PC12 cells. Data are expressed as the mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
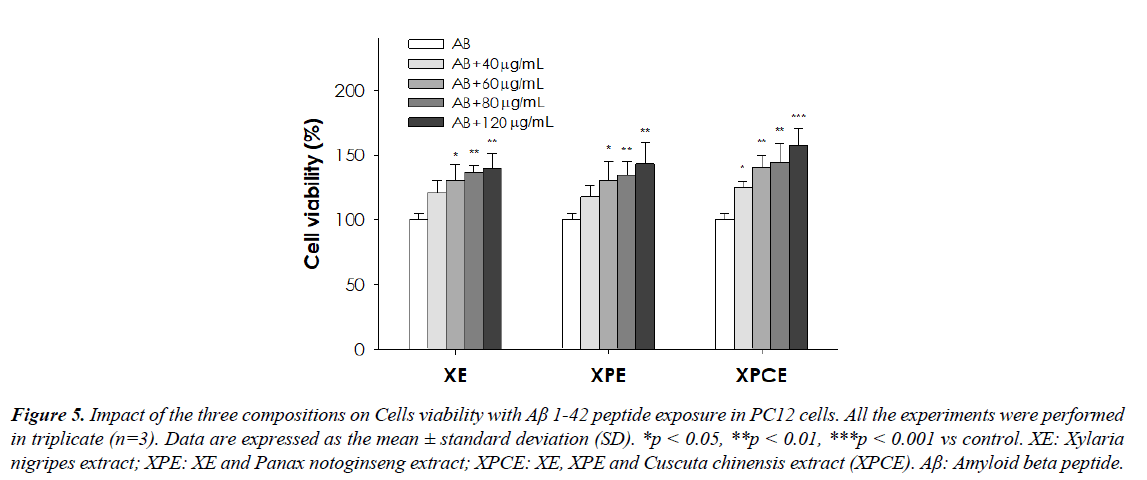
Cells viability with and without Aβ 1-42 peptide in PC12 cells after treatment with the three compositions
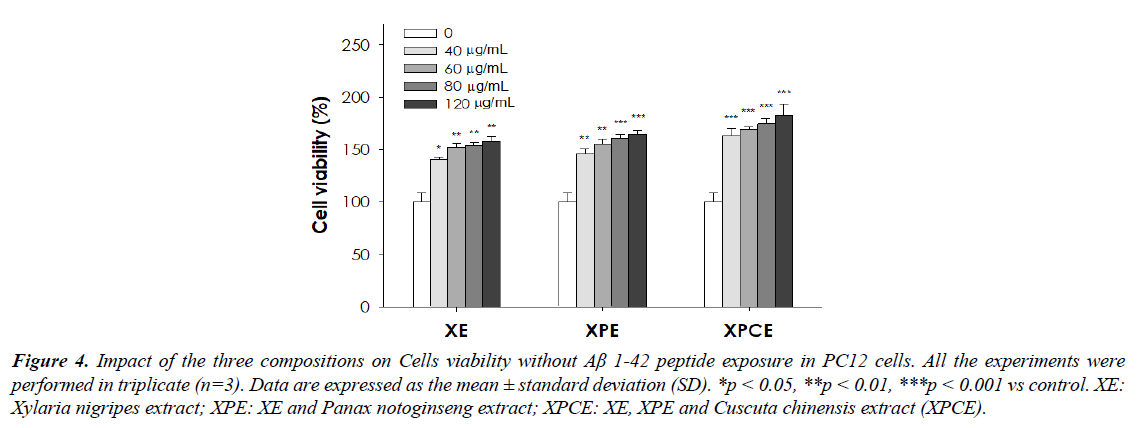
Initial analysis indicated the cell viability of PC12 cells was significantly increased when treated with the three compositions for 24 h. The result showed that the three compositions had no cytotoxic activity against the PC-12 cell (Table 4 and Figure 4). XPCE composition exhibited a higher proliferative effect (p<0.001) on the PC-12 cells than XE and XPE compositions. However, the cell viability of PC12 cells exposed to Aβ1-42 peptides for 24 h, followed by pretreatment with the three compositions has shown a considerable increase in a dose-dependent manner (Table 5 and Figure 5). In addition, XPCE (120 μg/mL) displayed the highest proliferative and cytoprotective activity (p<0.001) as compared to other compositions (XE and XPE).
| Dose (µg/mL) |
Control | Relative cell viability (%) under normal conditions in PC12 cells | ||
|---|---|---|---|---|
| XE | XPE | XPCE | ||
| 40 | 100.0 ± 8.5 | 140.3 ± 3.0* | 146.1 ± 4.6** | 164.0 ± 4.6*** |
| 60 | 152.6 ± 3.3** | 155.5 ± 4.8** | 169.6 ± 2.6*** | |
| 80 | 154.0 ± 2.9** | 160.9 ± 4.2*** | 174.5 ± 4.7*** | |
| 120 | 157.7 ± 4.8** | 165.1 ± 3.6*** | 182.4 ± 10.9*** | |
Table 4. Impact of the three compositions on Cells viability without Aβ 1-42 peptide (normal condition) exposure in PC12 cells. Data are expressed as the mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
| Dose (µg/mL) |
Control | Relative cell viability (%) under Aβ1-42 exposed condition in PC12 cells | ||
|---|---|---|---|---|
| XE | XPE | XPCE | ||
| 40 | 100.0 ± 4.7 | 121.1 ± 9.5 | 117.8 ± 8.4 | 124.9 ± 5.0* |
| 60 | 130.7 ± 12.3* | 130.2 ± 15.2* | 140.5 ± 9.5** | |
| 80 | 137.0 ± 5.5** | 134.6 ± 10.8** | 146.6 ± 14.5** | |
| 120 | 139.5 ± 11.9** | 143.3 ± 15.9** | 157.3 ± 13.1*** | |
Table 5. Impact of the three compositions on Cells viability with Aβ 1-42 peptide exposure in PC12 cells. Data are expressed as the mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
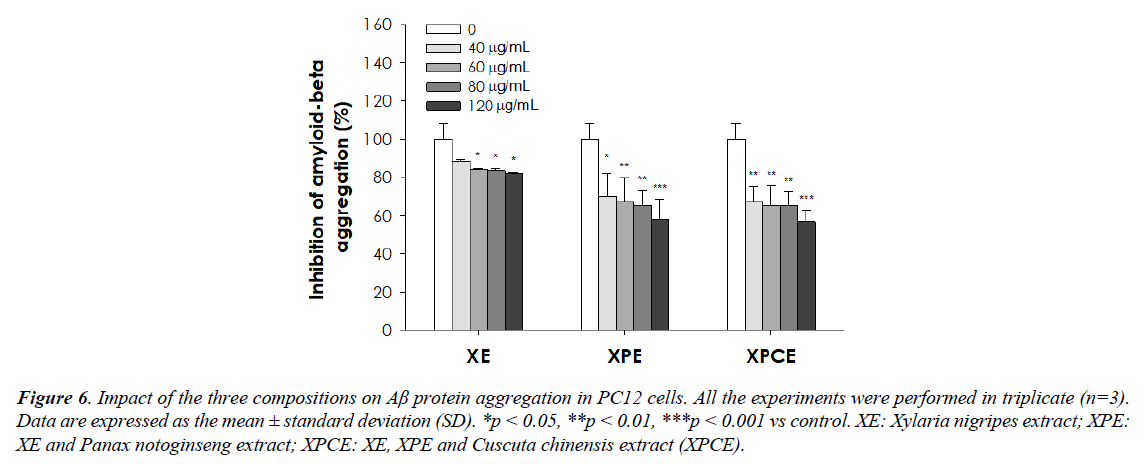
Aβ protein Aggregation in PC12 cells after treatment with the three compositions
The PC12 cells exposed with Aβ1-42 peptides and followed by the addition of the three compositions showed potent inhibitory activity of beta-amyloid protein aggregation and subsequent neuronal injury/damage. All three compositions showed good anti-Aβ1-42 peptides aggregation properties (Figure 6). Nevertheless, XPCE exhibited the highest (p<0.001) inhibitory activity of beta-amyloid protein aggregation in a dose-dependent fashion as compared with XE and XPE compositions. Table 6 shows the percentage change in inhibition of Aβ protein aggregation (%). Based on the above data, the author confirmed that XPCE composition showed better neuroprotective activity than XE and XPE compositions.
| Dose (µg/mL) |
Control | Inhibition of amyloid-beta aggregation (%) in PC12 cells | ||
|---|---|---|---|---|
| XE | XPE | XPCE | ||
| 40 | 100.0 ± 8.3 | 88.4 ± 1.0 | 70.3 ± 11.9* | 67.2 ± 8.1** |
| 60 | 84.2 ± 0.3* | 67.0 ± 12.7** | 65.3 ± 10.8** | |
| 80 | 83.4 ± 1.0* | 65.4 ± 7.4** | 65.1 ± 7.5** | |
| 120 | 82.2 ± 0.2* | 57.8 ± 10.8*** | 56.9 ± 5.8*** | |
Table 6. Impact of the three compositions on inhibition of Aβ protein aggregation (%) in PC12 cells. Data are expressed as the mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
Discussion
This is the very first study conducted to check the combinations of different traditional medicine (XE, XPE, and XPCE) on the GABA system and Aβ peptides aggregation. Since, various researchers have hinted that many Chinese traditional medicines like Xylaria nigripes, Panax notoginseng, and Cuscuta chinensis could positively maintain GABA levels and also lower Aβ peptides deposition in neuronal cells to exhibit potent neuroprotective activity [11,20,21]. Hence, this novel combination of traditional medicines (XE, XPE, and XPCE) was used to check the holistic effect on GABA production and lower beta-amyloid protein production and its aggregation. GABA plays a major role in maintaining a healthy mental status, as it regulates various neurotransmitters/neurotropic factors and hormones production and is thus useful in treating insomnia and depression [5,6] as well as various neuropsychological issues especially autism, schizophrenia, and other hyperactive neuronal disorders [1,2].
GABA shunt system play a crucial role in maintaining proper balance in GABA levels (metabolism of GABA) using various GABA metabolizing enzymes including GAD and GABA-T [2]. In the present study, all three composition treatments effectively improved GABA production and hence the GABA content was considerably improved. The author hypothesizes that the rich content of flavonoids, saponins, and polysaccharides in the three compositions might influence the GABA synthesis. Previously, many studies have proved that (Xylaria nigripes, Panax notoginseng, and Cuscuta) would trigger GABA production and thus plays a key role in reducing depression and insomnia [15,16,24,25]. To confirm the above statement, the author would like the check the activity of two major enzymes like GAD and GABA-T, which are involved in the GABA shunt system. The activities of GAD and GABA-T were considerably improved and suppressed respectively by all three compositions (XE, XPE, and XPCE) in PC12 cells in a dose-dependent manner. As the concentration of XE, XPE and XPCE increased the activity of GAD and GABA-T were significantly altered. However, the 120 μg/mL of XPCE composition showed the highest GABA synthesizing property (elevated GABA content), via increasing the activation of GAD and lowering the activity of GABA-T. Wu and others, also indicated that Xylaria nigripe can increase the permeability of glutamate and thus enhance the activity of GAD, which in turn regulate the activity of GABA-T and thus maintain the nominal levels of GABA [16].
To cross-check the cytoprotective and proliferative activity the author decided to evaluate the PC12 Cell's viability with or without Aβ1-42 peptide (cytotoxin). The PC12 cells after treatment with three compositions showed potent cytoprotective activity by increasing the PC12 cells count (proliferative activity) after 24 h of exposure to the Aβ1-42 peptide. The above statement proved that three compositions in particular XPCE displayed good cytoprotective and proliferative properties due to the presence of saponins and flavonoids. Likewise, Divate and his colleagues proved that the GABA-like property of Xylaria helps to protect the cells from H2O2-induced cytotoxicity [13]. Also, ginsenosides (saponins) are reported to improve PC12 cell proliferation and could effectively prevent MMP-induced cytotoxicity [26]. As mentioned previously that Aβ peptide/protein is a neurotoxin, which is the major contributor to various neurodegenerative disorders especially Alzheimer’s disease [6]. To assess the neuroprotective property of three compositions, the PC12 cells were exposed to Aβ peptide/protein and the amount of Aβ peptide/protein aggregation/deposition was measured. The present data indicated that PC12 cells exposed to Aβ peptide/ protein and followed by the addition of three compositions showed significant inhibitory activity of beta-amyloid protein aggregation/deposition. Thus the three compositions (XE, XPE, and XPCE) showcase its potent neuroprotective activity. In addition, XPCE composition showed the highest inhibitory activity of beta-amyloid protein aggregation and thus proving its better cytoprotective and neuroprotective properties than other compositions (XE, XPE). Since XPCE composition is rich in flavonoids, polysaccharides, and ginsenosides which are previously reported to lower oxidative stress (suppress free radical production) as well as inhibit Aβ protein misfolded/ aggregation (altering various mitochondrial proteins), thus possibly lowering the onset of AD [19,27,28].
Conclusion
All three compositions (XE, XPE, and XPCE) displayed better glutamate uptake and GABA release through elevated glutamate decarboxylase activity with decreased GABA transaminase activity. Also, all three compositions showed potent neuroprotective activity by improving the cell viability of PC12 cells and significantly lowering the Aβ aggregation. However, XPCE showed the best neuroprotective and GABA releasing properties than XE and XPE owing to high contents of various phytocomponents like polysaccharides, flavonoids, and saponins (holistic activity). The above data clearly showed that all three compositions improve GABA levels and exhibit strong neuroprotective activity making these novel compositions (XE, XPE, and XPCE) a potent candidate for treating insomnia, depression, and various neurological conditions (AD). In the future author would like to conduct an animal and clinical trial to cross-check the above-mentioned beneficial properties of all three compositions.
Authors Contribution
Author’s contributions for the present study as follows- SFH, HWY and WLC are involved in designing and conceptualization for this study. KV and TCC conducted statistical analysis. SFH, HWY and TCC were responsible for conducting this cell line study. KV and WLC have drafted this manuscript. WLC is the primary responsibility for final content.
Acknowledgements
Author would like to thank NuLiv Sciences USA, Inc. (Brea, CA, USA) for providing all the three compositions including XE, XPE, and XPCE extract.
Conflict of Interest
No conflict of interest to disclose.
References
- Sarasa SB, Mahendran R, Muthusamy G, et al. A brief review on the non-protein amino acid, gamma-amino butyric acid (GABA): its production and role in microbes. Curr Microbiol. 2020;77(4):534-44.
- Sarawagi A, Soni ND, Patel AB. Glutamate and GABA homeostasis and neurometabolism in major depressive disorder. Front Psychiatry. 2021;12:637863.
- Kendell SF, Krystal JH, Sanacora G. GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opinion Therapeutic Targets. 2005;9(1):153-68.
- Ngo DH, Vo TS. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules. 2019;24(15):2678.
- Yu L, Han X, Cen S, et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol Res. 2020;233:126409.
- Solas M, Puerta E, J Ramirez M. Treatment options in alzheimer s disease: the GABA story. Curr Pharm Des. 2015;21(34):4960-71.
- Byun JI, Shin YY, Chung SE, et al. Safety and efficacy of gamma-aminobutyric acid from fermented rice germ in patients with insomnia symptoms: a randomized, double-blind trial. J Clin Neurol. 2018;14(3):291-5.
- Zhou H, Zhao Y, Peng W, et al. Efficacy and safety of Wuling capsule for insomnia disorder: A systematic review and meta-analysis of randomized controlled trials. Sleep Med. 2022;24.
- Hung MC, Tsai CC, Hsu TH, et al. Biological activities of the polysaccharides produced from different sources of Xylaria nigripes (Ascomycetes), a Chinese medicinal fungus. Int J Med Mushrooms. 201517(2).
- Peng WF, Wang X, Hong Z, et al. The anti-depression effect of Xylaria nigripes in patients with epilepsy: A multicenter randomized double-blind study. Seizure. 2015;29:26-33.
- Song A, Ko HJ, Lai MN, et al. Protective effects of Wu-Ling-Shen (Xylaria nigripes) on carbon tetrachloride-induced hepatotoxicity in mice. Immunopharmacol Immunotoxicol. 2011 Sep 1;33(3):454-60.
- Ko HJ, Song A, Lai MN, Ng LT. Immunomodulatory properties of Xylaria nigripes in peritoneal macrophage cells of Balb/c mice. J Ethnopharmacol. 2011;138(3):762-8.
- Divate RD, Wang PM, Wang CC, et al. Protective effect of medicinal fungus Xylaria nigripes mycelia extracts against hydrogen peroxide-induced apoptosis in PC12 cells. Int J Immunopathol Pharmacol. 2017;30(1):105-12.
- Ma YP, Mao DB, Geng LJ, et al. Production optimization, molecular characterization and biological activities of exopolysaccharides from Xylaria nigripes. Chemical and Biochemical Engineering Quarterly. 2013;27(2):177-84.
- Zhao Z, Li Y, Chen H, et al. Xylaria nigripes mitigates spatial memory impairment induced by rapid eye movement sleep deprivation. Int J Clin Exp Med. 2014;7(2):356.
- Wu D, Xie ZX, Di P. Clinical application and efficacy of Wuling capsule. J China Prescript Drug. 2012;10:43-5.
- Lin J, Gao S, Wang T, et al. Ginsenoside Rb1 improves learning and memory ability through its anti-inflammatory effect in Aβ1-40 induced Alzheimer’s disease of rats. American J Translational Res. 2019;11(5):2955.
- Chen C, Zhang H, Xu H, et al. Ginsenoside Rb1 ameliorates cisplatin-induced learning and memory impairments. J Ginseng Res. 2019 Oct 1;43(4):499-507.
- Zheng Y, Gao X, Chen Q, et al. Effect of Ginsenosides on Prevention of Alzheimer’s Disease. InIOP Conference Series: Materials Science and Engineering 2019;612(2):022004.
- Donnapee S, Li J, Yang X, et al. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J Ethnopharmacol. 2014;157:292-308.
- Lin MK, Lee MS, Huang HC, et al. Cuscuta chinensis and C. campestris attenuate scopolamine-induced memory deficit and oxidative damage in mice. Molecules. 2018;23(12):3060.
- Mokhtarifar N, Sharif B, Naderi N, et al. Evaluation of anti-depressant effects of Cuscuta chinensis in experimental models. Res Pharm Sci. 2012;7(5):826.
- Zhen GH, Jiang B, Bao YM, et al. The protect effect of flavonoids from Cuscuta chinensis in PC12 cells from damage induced by H2O2. Zhong yao cai= Zhongyaocai= J Chinese Med Mat. 2006;29(10):1051-5.
- Forouzanfar F, Vahedi MM, Aghaei A, et al. Hydroalcoholic extract of Cuscuta epithymum enhances pentobarbitalinduced sleep: possible involvement of GABAergic system. Curr Drug Discov Technol. 2020;17(3):332-7.
- Zheng M, Xin Y, Li Y, et al. Ginsenosides: a potential neuroprotective agent. BioMed Res Int. 2018;8;2018.
- Hashimoto R, Yu J, Koizumi H, et al. Ginsenoside Rb1 prevents MPP+-induced apoptosis in PC12 cells by stimulating estrogen receptors with consequent activation of ERK1/2, Akt and inhibition of SAPK/JNK, p38 MAPK. Evid Based Complement Alternative Med. 2012;1;2012.
- Choi SM, Kim BC, Cho YH, et al. Effects of flavonoid compounds on β-amyloid-peptide-induced neuronal death in cultured mouse cortical neurons. Chonnam Med J. 2014;50(2):45-51.
- Shim JS, Song MY, Yim SV, et al. Global analysis of ginsenoside Rg1 protective effects in β-amyloid-treated neuronal cells. J Ginseng Res. 2017;41(4):566-71.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref