Research Article - Journal of Biochemistry and Biotechnology (2023) Volume 6, Issue 2
Effect of Astragalus membranaceus and Panax notoginseng extract on arginine absorption, intestinal permeability, microbiota population, immune activation, and appetite in human subjects with Ulcerative Colitis: A Pilot Study
Ching-Pin Lin1,2, Yao-Tsung Yeh3,4, Min-Hsi Chiu3,4, Ting-Yn Pan5, and You-Cheng Shen5,6*
1Department of Internal Medicine and Division of Gastroenterology, Chung Shan Medical University Hospital, Taichung 402, Taiwan
2Institute of Biochemistry, Microbiology, and Immunology, Chung Shan Medical University, Taichung 402, Taiwan
3Aging and Disease Prevention Research Center, Fooyin University, Kaohsiung City 831, Taiwan
4Department of Medical Laboratory Science and Biotechnology, Fooyin University, Kaohsiung City 831, Taiwan
5Department of Health Industry Technology Management, Chung Shan Medical University, Taichung 402, Taiwan
6Department of Nutrition, Chung Shan Medical University Hospital, Taichung 402, Taiwan
- Corresponding Author:
- You-Cheng Shen
Department of Health Industry Technology Management
Chung Shan Medical University, Taichung, Taiwan
E-mail: youcheng@csmu.edu.tw
Received: 01-Mar-2023, Manuscript No. AABB-23-90556; Editor assigned: 03-Mar-2023, PreQC No. AABB-23-90556(PQ); Reviewed: 17-Mar-2023, QC No AABB-23-90556; Revised: 22-Mar-2022, Manuscript No. AABB-23-90556(R); Published: 29-Mar-2023, DOI:10.35841/aabb-6.2.138
Citation: Ching-Pin Lin, Yao-Tsung Yeh, Min-Hsi Chiu, Ting-Yn Pan, and You-Cheng Shen. Effect of Astragalus membranaceus and Panax notoginseng extract on arginine absorption, intestinal permeability, microbiota population, immune activation, and appetite in human subjects with ulcerative colitis: A Pilot Study. J Biochem Biotech 2023;6(2):138
Abstract
To the best of our knowledge, there are no clinical trials conducted with Astragalus and Panax notoginseng extracts in Ulcerative Colitis (UC) patients. Saponin extracts of Astragalus or ginseng has potential to reduce inflammation and regulate gut microflora in animals. The purpose of this pilot study is to investigate whether a standardized Astragalus and Panax notoginseng extract (APS) supplementation could improve arginine absorption, intestinal permeability, microbiota population, immune cell count, and appetite in patients with UC. This trial was a randomized, double-blind, parallel study on patients with UC between the ages of 20 and 80. Patients took one capsule of APS (or placebo) before each breakfast and dinner daily during the trial. Blood and fecal tests were collected, including plasma arginine, biochemical indicators, indicators of
inflammation and appetite, and fecal microorganisms. Additionally, leaky gut test and colonic tissue examination were performed. A total of eight people completed the trial. After a 3-month trial supplementing with APS, fecal calprotectin decreased significantly by 60.9% (P=0.015) in the APS group. APS group's ratio of lactulose/mannitol decreased significantly by 40% (P=0.02), suggesting improved intestinal mucosal integrity. Furthermore, there was a significant 58% decrease in MPO (P=0.046) in the APS Astragalus, Panax notoginseng, IBD, Ulcerative Colitis, Inflammation.by 66.7%), and a significantly higher arginine absorption by 49.7% compared to the placebo group (P=0.008). The immune cell of peripheral blood was also assessed, displaying that the UC patients’ proportion of neutrophils and lymphocytes increased by 11.7% and 20.5%,
respectively, in the APS group. After the 3-month trial, the alpha diversity index of the placebo group had a downward trend, whereas the APS group had an upward trend (up by 60%). The pro-inflammatory cytokines IL-6, IL-17, and IL-1β were decreased by 70.1%, 66.9%, and 44.6%, respectively, in APS-supplemented UC patients. The results of gut microflora analysis by NGS showed Faecalibacterium prausnitzii, which is one of the major butyric acid-producing bacteria in the human intestine, increased by 420% in the APS group. Similarly, another probiotic Bifidobacterium adolescentis increased by 181% in the APS group. Furthermore, the total amount of appetite-increasing hormones Ghrelin increased by 48% in the APS group. In conclusion, APS supplementation showed improvement in arginine absorption, intestinal permeability, microbiota population, immune cell count, and appetite in patients with UC.
Keywords
Astragalus, Panax notoginseng, IBD, Ulcerative Colitis, Inflammation.
Introduction
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), is a chronic and relapsing inflammatory disorder of the gut. IBD usually is characterized by diarrhea, rectal bleeding, abdominal pain, fatigue, and weight loss. Both UC and CD severely compromise the quality of life of affected individuals, and IBD represents a major risk for cancer development since 20% of these patients can develop colitis-associated carcinogenesis. The diagnosis of IBD is based on clinical symptoms combined with results found in endoscopic and radiological examinations.
Calprotectin accounts for approximately 60% of total soluble proteins in the cytosol fraction of neutrophils. The amount of calprotectin in feces provides a noninvasive quantitative measure of neutrophil flux to the intestine [1]. Numerous studies have shown that fecal calprotectin concentration demonstrates a good correlation with intestinal inflammation [2, 3]. This simple, noninvasive, and less expensive quantitative fecal calprotectin test is the most widely used surrogate marker for monitoring intestinal inflammatory activity [4].
Arginine plays a key role in cellular metabolism, as it is involved in many important physiological functions, including protein synthesis, creatine biosynthesis, polyamine formation, and Nitric Oxide production [5, 6]. In addition, previous studies indicate that arginine promotes intestinal and colonic epithelial wound repair and enhances cell proliferation and restoration of intestinal epithelial cell integrity [7].
Immunological responses in the gastrointestinal (GI) tract to the altered gut microbiota, mucosal injury, and loss of intestinal epithelial cell function all contribute to a complex mechanism underlying IBD pathogenesis [8]. Recently, the role of gut microbiota in the pathophysiology of IBD has been highlighted. The most consistent observation is reduced bacterial diversity, a decrease of Firmicutes, and an increase of Proteobacteria in IBD. Notably, the Firmicutes/Bacteroidetes (F/B) ratio is widely accepted to have an important influence in maintaining normal intestinal homeostasis. Decreased F/B ratio is regarded as dysbiosis, whereby it is usually observed in IBD [9].
One of the common signs of IBD is loss of appetite. Growing evidence supports the role of gut microbiota in influencing host appetite and food intake via the microbiota-gut-brain axis. Notably, this decreased gut microbial diversity could play a key role in the associated dysregulation of appetite, metabolism, and food-related behaviors [10]. Each bacterial species within the gut aims to increase its fitness, habitat, and survival via specific fermentation of dietary nutrients and secretion of metabolites, many of which can influence host appetite and eating behavior by directly affecting nutrient sensing and appetite satiety-regulating systems. These include microbiota-produced neuroactive and short-chain fatty acids [11].
The primary hormone associated with appetite is Ghrelin, which increases appetite. Ghrelin, a 28-amino acid appetitestimulating peptide hormone predominantly produced by the stomach, is involved in controlling food intake and energy metabolism, influencing the endocrine pancreatic function and glucose and lipid metabolism [12]. In addition, two forms of Ghrelin, including acylated Ghrelin (A-Ghr) and nonacylated Ghrelin (NA-Ghr), have been described. Therefore the amount of A-Ghr and NA-Ghr is regarded as an appetite-promoting indicator.
The major components of Radix Astragali (Astragalus) are astragalosides; as well as saponins, flavonoids, and other compounds [14]. Astragalus is a traditional Chinese herb that has been widely used to treat various ailments, such as type 2 diabetes, kidney disease, and autoimmune diseases. Modern pharmacological studies and clinical practices indicate that Radix Astragali possesses various biological functions, such as immunomodulation, antioxidant, anti-inflammation and antitumor activities [15]. The major components of Panax notoginseng are ginsenosides; as well as alkaloids, volatile oils, flavonoids, polysaccharides, and dencichine. Panax notoginseng is one of the most widely used herbs in traditional and complementary medicine. Previous studies showed that ginsenosides increase intestinal probiotics [16], repair intestinal mucosal injury [17], and are anti-inflammatory [18] in animals.
To the best of our knowledge, no clinical trials have been conducted with a standardized Astragalus and Panax notoginseng extract (APS) in UC patients. Therefore, the current study aimed to investigate the role of APS in arginine absorption, intestinal permeability, microbiota population, immune cell count, and appetite in patients with UC.
Material and Methods
Samples and chemicals
APS is an equal mixture of dried extracts of Astragalus membranaceus (10:1 hydroethanolic extract) and Panax notoginseng (50:1 aqueous extract) roots blended with maltodextrin as an excipient; its production is compliant with current Good Manufacturing Practice. The final blend is beige to light yellow powder and is standardized to contain ≥1.5% saponins. The APS used in this study was provided by NuLiv Science USA, Inc. (Brea, CA, USA). The APS capsules consisted of 50 mg of the proprietary extract mixtures, and the placebo capsules contained maltodextrin.
Participants and Study design
Ethics
The protocol for this study received ethics approval from Chung Shan Medical University Hospital IRB (Protocol No. CS2-19061). This study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All participants signed the informed consent form before the trial.
Inclusion/Exclusion criteria
This study was conducted from Oct 2020 to Mar 2022 with 14 recruited individuals aged >20 years at Chung Shan Medical University (Taichung City, Taiwan). 9 patients were enrolled in these three months, double-blinded, randomized, placebo-controlled clinical trial. One participant was unable to cooperate with the inspection time due to the impact of COVID-19 during the trial and withdrew, and the remaining 8 participants completed the study. Participants were required to take one APS or placebo capsule before each breakfast and dinner every day during the study. In addition, the participants maintained their lifestyle and dietary habits (no caloric restrictions). The inclusion criteria for this study included participants aged >20 with ulcerative colitis (UC) who had voluntarily agree to participate in this study, had Fecal calprotectin >200 (μg/g), and a Mayo Score of 4~9 (Moderate). The exclusion criteria included participants that had chronic diseases (cardiovascular, kidney, or liver disease, cancer, uncontrolled diabetes mellitus, or a history of major medical or operation history). We excluded participants who smoked, consumed alcohol regularly, were pregnant or lactating, were participating in another human trial during the first 30 days of the study, or were taking any health products or drugs that might interfere with the research (health foods, amino acid supplements). A participant was considered to reach the clinical endpoint when any of the following conditions were met: completion of the protocol and required follow-up, the occurrence of an adverse event, loss of contact, non-compliance, use of medication, medical contraindication, and withdrawal of consent, death, or other reasons.
L-arginine absorption
Participants in the study took an APS or placebo (maltodextrin) capsule at 9 p.m. and began fasting for 12 hours, except for a small amount of water the night before the experiment. All participants had their first blood sample collected at 9 a.m. the next morning from the median cubital vein using the indwelling catheter method. Immediately after the first blood collection, all participants took APS/placebo capsule and 5 g of arginine with 250 mL of water. Additional blood samples were collected at 15, 30, 45, 60, 90, 120, 150, and 180 min for analysis of the plasma concentration of arginine. Blood amino acids were measured using reagents and liquid chromatography-tandem mass spectrometer API 4000 triple quadrupole mass spectrometer (AB SCIEX, MA, and USA)
Intestinal permeability analysis
Intestinal permeability analysis measures the amount of mannitol and lactulose in urine, two sugar molecules not metabolized in the human body. Urine collected overnight from all participants between going to bed and waking up, is used to calculate the proportion of lactulose and mannitol in the urine that is presented as percentages. Lactulose and mannitol were measured using reagents and liquid chromatographytandem mass spectrometer API 4000 triple quadrupole mass spectrometer (AB SCIEX, MA, USA), respectively.
Fecal Calprotectin measurement
Stool specimens were taken within two days after the endoscopic procedure. Quantum Blue Calprotectin (Quantum blue fCAL) rapid test was used for fecal calprotectin measurement (Quantum Blue Reader, Switzerland). Fecal calprotectin level is measured between 30-300 ug/g. Stool extract is diluted with buffering solution and centrifugated; the supernatant part is used for analysis.
Myeloperoxidase (MPO) activity
The colon biopsy specimen was taken from actively inflamed mucosa of the participants and homogenized. Myeloperoxidase (MPO) activity was conducted by MPO Activity Assay Kit (Elabscience Biotechnology Inc. USA).
Immunohistochemistry (Activity score)
To evaluate the severity of ulcerative colitis (UC) in colon biopsy specimen, the Nancy Histological Index Activity Score was utilized. A 5-level classification defines the Nancy index activity score: Grade 0 (absence of significant histological disease activity), Grade 1 (chronic inflammatory infiltrate), Grade 2 (mild active disease), and Grade3 (Moderately active disease), and Grade 4 (severely active disease).
Fecal DNA extraction
Fecal genomic DNA was extracted from the fecal samples using the QIAmp Fast DNA Stool Mini Kit (Qiagen, Germany) with the modified instructions. Briefly, the stool sample was centrifuged at 13,200 rpm for 5min to remove the storage buffer and then re-suspended and homozygote with the InhibitEX buffer. Add the proteinase K and ethanol to obtain the processed supernatant. Finally, the supernatant was washed with the QIAamp spin column and eluted with the elution buffer. The concentration was assessed by Nano Drop 2000 (Thermo Scientific, MA, USA) and then performed 10X dilution with elution buffer.
Next generation Sequencing (NGS) analysis
The gut microbiome library was constructed with the standard V3?V4 region of the 16S rRNA gene. PCR was amplified with the KAPA HiFi hotstart ready mix (Roche, ID, USA) and was purified with the AMPure XP magnetic beads (Beckman Coulter, USA). PCR amplification was assessed by Fragment Analyzer (Advanced Analytical Solutions, WV, and USA) and quantified by Qubit 3.0 Fluorometer. The library was sequenced on a MiSeq (Illumina, CA, USA) with paired?end reads (2 × 300 nt) and at least 100,000 reads for every sample.
White blood cell counts (Neutrophils and lymphocytes)
Blood samples were collected from all participants after completing an overnight fast. Peripheral complete blood cell count was determined using an automated cell counter (XN- 2000 Hematology Alpha Transportation System, Sysmex Corporation, Kobe, Japan).
Appetite parameters: Ghrelin Enzyme-linked immunosorbent assay (ELISA)
The serum samples were obtained and transferred into chilled tubes with 500 U/ml aprotinin. To quantify serum ghrelin levels, a commercial ghrelin enzyme-linked immunosorbent assay (ELISA) kit (Bertin Bioreagent, MB, France) was used according to the manufacturer's instructions.
Bioinformatics Analysis and Statistics
All experimental data are expressed as mean ± standard deviation (SD), and differences between the APS and placebo groups at different time intervals were analyzed using Student’s t-test. Also, the paired t test was used to compare the difference within the same group. The analysis was performed using SPSS software (IBM, version 18.0 for Windows, NY, USA). A p-value of less than 0.05 was deemed statistically significant. The raw paired?end reads were trimmed, and that passed the quality filters assigned to operational taxonomic units (OTU), which ≥97% similarity with the Green Gene Database (version 13.8).
OTU taxonomic (relative abundance), alpha diversity, beta diversity (PCoA), and heatmap were performed with the MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/ MicrobiomeAnalyst/upload/OtuUploadView.xhtml), CLC genomics workbench (Qiagen, Germany) and GraphPad Prism 8 (GraphPad Software, USA). The abundance bacteria analysis (LEfSe, Linear discriminant analysis Effect Size) and functional analysis (PICRUSt, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) were performed by Galaxy/Hutlab website (http://huttenhower.sph. harvard.edu/galaxy/).
Results
Demographic data of participants
A total of fourteen participants were recruited in this study, wherein five were excluded because they did not meet the inclusion criteria, and 9 were enrolled in the study. Excluding one participant who was unable to cooperate with the inspection time due to the impact of COVID-19 during the trial and withdrew, the data of 8 participants were analyzed. The cohort comprised four men and five women aged 25–70 years, with an average age of 43.4 years.
L-arginine absorption
Previous studies indicate more than half of Ulcerative Colitis patients experience malabsorption. Prior research has also indicated arginine promotes intestinal and colonic epithelial wound repair and enhances cell proliferation and restoration of intestinal epithelial cell integrity [7]. In the L-arginine absorption test (Table 1), the AUC of L-arginine in the APS group was significantly higher than the placebo group 49.7% (P=0.008). In addition, the Tmax time was 7.7% earlier than the placebo group, and the Cmax concentration was 21.7% higher than the placebo group. We speculate supplementing APS can help ameliorate inflammation in the intestine of the participants who were supplemented with APS.
| APS (N=5) | Placebo (N=3) | P-value | |
|---|---|---|---|
| AUC (min*μmol/L) | 12737.08± 1818.50 | 8509.23 ± 409.60 | 0.008 |
| Tmax (min) | 60.00± 20.49 | 65.0±18.7 | 0.285 |
| Cmax (μmol/L) | 230.0 ±7.5 | 189.0± 34.3 | 0.126 |
Value present as Mean ±SD. A p-value less than 0.05 were considered statistically significant.
Table 1. L-Arginine absorption analysis in UC patients after supplementation with APS or Placebo.
Lactulose/mannitol assay
Lactulose/mannitol ratio test is clinically used for assessing disorders of gut permeability [24]. Results of the Intestinal permeability analysis are shown in (Table 2). After three months of APS supplementation, the percentage of lactulose absorption decreased by 24.1% and the ratio of lactulose / mannitol was significantly decreased by 40% (P=0.02) in the APS group, suggesting improved intestinal mucosal integrity in the participants who were supplemented with APS. The ratio of lactulose/mannitol in the placebo group did not change significantly (Table 2).
| APS (N=5) | P-value | Placebo (N=3) | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0M | 1.5M | 3M | 1.5M/3M | 0M | 1.5M | 3M | 1.5M/3M | |
| Lactulose (%) | 0.54± 0.46 | 0.37±0.29 | 0.41±0.42 | 0.125/ 0.218 | 0.43±0.55 | 0.53±0.41 | 0.55±0.63 | 0.826/ 0.111 |
| Mannitol (%) | 10.11±3.69 | 12.37±4.60 | 12.78±6.59 | 0.176/ 0.469 | 11.73±3.27 | 11.60±3.04 | 11.17±4.26 | 0.423/ 0.25 |
| Lactulose/ Mannitol | 0.05±0.07 | 0.03±0.02 | 0.03±0.06 | 0.231/ 0.047 | 0.04±0.03 | 0.05±0.04 | 0.05±0.03 | 0.816/ 0.423 |
Values were expressed as Mean ±SD. A p-value less than 0.05 were considered statistically significant.
Table 2. Lactulose/mannitol assay
Mannitol was quickly absorbed and can be used as a marker of intestinal absorption; lactulose was not easily absorbed and can be used as a marker of intestinal mucosal integrity. Therefore, decreased mannitol absorption indicates intestinal malabsorption, which may lead to malnutrition. On the other hand, increased lactulose absorption indicates increased permeability in the small intestine. Therefore, increased lactulose/mannitol ratio indicates increased intestinal permeability, which increases the chance of the passage of larger molecules such as antigens, toxins, or microorganisms. These symptoms have been labelled the leaky gut syndrome.
Calprotectin assay
The inflammatory response, gut microbiota, mucosal injury, and loss of intestinal epithelial cell function all contribute to a complex mechanism underlying IBD pathogenesis. Calprotectin is a calcium-containing protein in neutrophils and macrophages found in feces when there is an active inflammation and is a marker of acute inflammatory cell activation [20]. Fecal calprotectin appears in copious quantities in feces of IBD patients. Therefore, the amount of calprotectin can be used as an indicator of intestinal inflammation[1]. Fecal calprotectin significantly decreased by 60.9% (P=0.015) after three months APS supplementation and there was no significant difference in the placebo group with only 10.9% decrease (Table 3).
| Fecal calprotectin (μg/g) | ||||
|---|---|---|---|---|
| 0M | 1.5M | 3M | P-value 1.5M/3M | |
| APS (N=5) | 704.5±548.0 | 237.1±221.9 | 275.5±324.8 | 0.105/0.015 |
| Placebo (N =3) | 1616.2±864.8 | 1383.0±1104.6 | 1440.6±697.0 | 0.450/0.599 |
Values were expressed as Mean ±S.D. A p-value less than 0.05 were considered statistically significant.
Table 3. The change of fecal calprotectin in UC patients.
MPO assay
Myeloperoxidase (MPO) is a peroxidase that mainly catalyzes the oxidative damage to tissue during inflammation and is regarded as a biomarker of IBD [19]. MPO significantly decreased by 58% (P=0.046) after three months APS supplementation, suggesting improved inflammatory response after APS supplementation. In contrast, there was no significant difference in the placebo group with only 7.0% decrease (Table 4).
| APS (N=5) | P-value | Placebo (N=3) | P-value | |||
|---|---|---|---|---|---|---|
| Initial | the end | Initial | the end | |||
| MPO (ng/mL) | 209.0±150.6 | 87.8±89.3 | 0.046 | 312.5±195.3 | 290.5±183.7 | 0.264 |
| Activity score | 2.4±1.5 | 0.8±1.8 | 0.078 | 2.7±0.6 | 2.0±1.0 | 0.184 |
MPO, Myeloperoxidase; Values were expressed as Mean ±S.D. A p-value less than 0.05 were considered statistically significant.
Table 4. MPO of colon biopsy specimen and Activity score
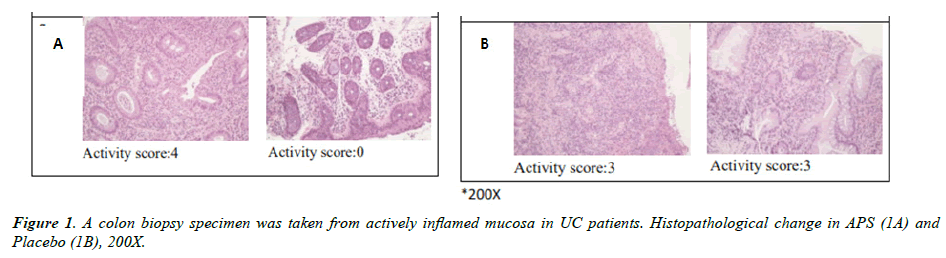
Immunohistochemistry analysis
The immunohistochemistry (IHC) analysis of colonoscopy biopsy was shown in Figure 1 (histology from APS and placebo groups were shown in Figure 1A and 1B, respectively). The results indicated there are many chronic inflammatory cells throughout the lamina propria, inflammatory cell infiltration was observed between the bottom of the crypt and the muscular mucosae, and ulcers were also included at the beginning of the study. Inflammatory response in injured tissue significantly improved after three-month APS supplementation while ulcers and chronic inflammation remained unchanged in the placebo group. The activity score decreased by 66.7% after three months APS supplementation while the activity score in the placebo group decreased by 25.9% only (Table 4).
Inflammatory cytokine assay
IL-6 secretion significantly correlates with Ulcerative Colitis patients' disease activity [21]. IL-6 also synergistically promotes secretion of pro-inflammatory cytokine IL-17, which leads to the production of inflammatory cytokines and tissue inflammation [21]. In addition, IL-1β is also responsible for inflammation in IBD patients [22, 23]. Consumption of ginsenosides can promote secretion of anti-inflammatory cytokine IL-10 in the intestinal tract of rats [16]. After three months of supplementation with APS, pro-inflammatory cytokines IL-6, IL-17, and IL-1β decreased by 70.1%, 66.9%, and 44.6%, respectively, and anti-inflammatory cytokine IL-10 increased by 17.9% in the APS group. On the other hand, pro-inflammatory factors IL-6, IL-17, and IL-1β decreased by 19.6%, 11.9%, and 22.2%, respectively, and anti-inflammatory factor IL-10 decreased by 19.2% in the placebo group (Table 5). We speculate APS supplementation has an anti-inflammatory effect in IBD patients based on the noticeable difference in inflammatory response between the APS and placebo groups.
| APS (N=5) | P-value | Placebo (N=3) | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| pg/ml | 0M | 1.5M | 3M | 1.5M/ 3M | 0M | 1.5M | 3M | 1.5M/ 3M |
| IL-6 | 1.44±3.59 | 0.69±1.54 | 0.43±0.64 | 0.374/ 0.511 | 8.10±14.04 | 5.27±8.56 | 6.51±11.27 | 0.466/ 0.423 |
| IL-17A | 1.72±3.25 | 0.58±0.70 | 0.57±0.83 | 0.493/ 0.618 | 8.51±14.75 | 6.03±9.61 | 7.50±12.99 | 0.493/ 0.423 |
| IL-1β | 0.92 ±0.59 | 0.78±0.52 | 0.51±0.47 | 0.125/ 0.063 | 0.36±0.62 | 0.55±0.95 | 0.28±0.49 | 0.423/ 0.423 |
| IL-10 | 0.28± 0.21 | 0.29± 0.21 | 0.33± 0.22 | 0.675/ 0.078 | 0.26±0.23 | 0.29±0.27 | 0.21±0.19 | 0.423/ 0.423 |
Values were expressed as Mean ±S.D. A p-value less than 0.05 were considered statistically significant.
Table 5. The percentage change of various inflammatory cytokines in the peripheral blood of UC patients.
Gut microflora study
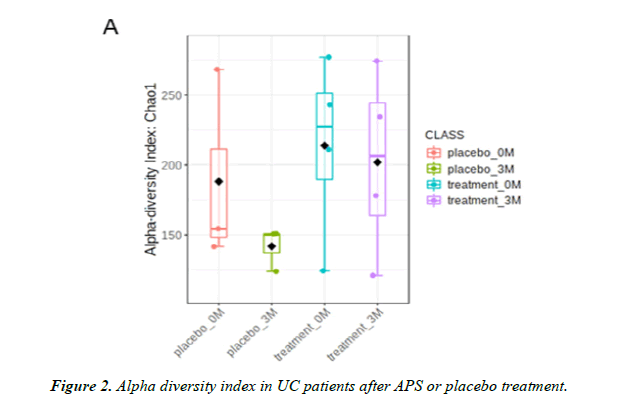
Lozupone, Stombaugh, Gordon, Jansson and Knight [25] Point out that the more microbiota species, the higher the diversity and the healthier the gut environment. In the alpha diversity analysis of the intestinal biodiversity the diversity index of the placebo group showed a decreasing trend while the diversity index in the APS group attenuated this decreasing trend (up 60%) (Figure 2). Even though the comparison between the two groups does not reach a statistical difference due to the small sample size, we speculate that there will be significant difference if the sample size is increased (Figure 2).
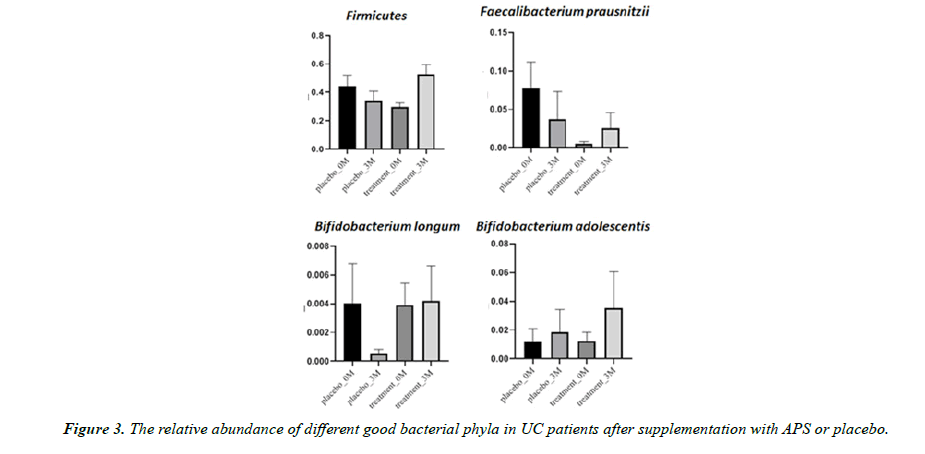
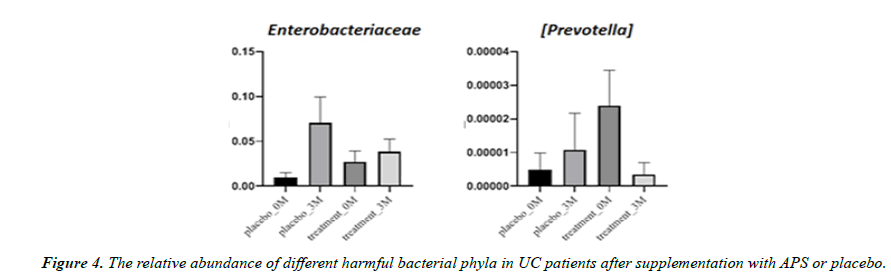
Aside from microbiota diversity, it is equally if not more important to investigate whether APS supplementation can alter the population of certain specific microbiota species that are critical to human health, such as firmicutes, etc. After three month of trial, the good bacteria Firmicutes decreased by 23% in the placebo group but increased by 77% in the APS group. Faecalibacterium prausnitzii decreased by 52% in the placebo group but increased by 420% in the APS group. The good bacteria Bifidobacterium longum decreased by 87% in the placebo group but remained unchanged in the ASP group. On the other hand, Bifidobacterium adolescentis increased by 57% in the placebo group, but increased by 181% in the APS group (Figure 3). In addition, harmful bacteria Enterobacteriaceae increased by 614% in the placebo group but increased only slightly by 14% in the APS group. Harmful bacteria Prevotella increased by 27% in the placebo group, but significantly decreased by 98% in the APS group (Figure 4).
Immune activation
Although the amount of calprotectin in feces provides a noninvasive quantitative measure of neutrophil flux to the intestine, the immune cells of peripheral blood also serve as important indicators of immune function. After three months of APS supplementation, neutrophil and lymphocyte counts increased by 11.7% and 20.5%, respectively, in the APS group while neutrophil count decreased by 9.3% and lymphocyte count only increased by 7.0% in the placebo group. We speculated that APS supplementation may have beneficial effects on immune functions in UC patients (Table 6).
| APS (N=5) Placebo (N=3) | ||||||||
|---|---|---|---|---|---|---|---|---|
| (%) | 0M | 1.5M | 3M | 1.5M/ 3M | 0M | 1.5M | 3M | 1.5M/ 3M |
| Neutrophils | 59.39±12.87 | 64.16±9.09 | 66.32±7.82 | 0.414/ 0.225 | 62.97±12.87 | 62.63±11.82 | 57.13±14.82 | 0.972/ 0.224 |
| Lymphocytes | 25.02±7.43 | 27.52±8.35 | 30.16±11.33 | 0.139/ 0.279 | 28.90±12.67 | 27.8±9.06 | 30.93±10.72 | 0.886/ 0.28 |
Values were expressed as Mean ±S.D. A p-value less than 0.05 were considered statistically significant.
Table 6. The percentage change of immune cells in the peripheral blood of UC patients.
Appetite study
Loss of appetite is a common symptom of Ulcerative Colitis, and individuals with Ulcerative Colitis frequently experience eating difficulties. Eating is often associated with symptoms such as nausea, pain, bloating, and diarrhea. Complications of Ulcerative Colitis, such as mouth sores, can also prevent people with Ulcerative Colitis from eating certain types of foods.
Ghrelin, an appetite-increasing hormone, acts in the brain to regulate food intake, body weight, adiposity, and glucose metabolism [28]. Subsequently, Ghrelin’s central and peripheral actions include stimulation of gut motility and gastric acid secretion [29].
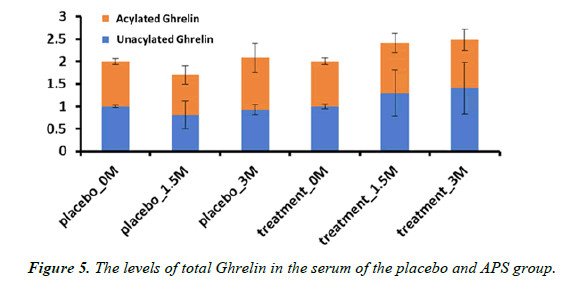
The total amount of acylated Ghrelin (A-Ghr) and nonacylated Ghrelin (NA-Ghr) in the participants supplemented with APS had an increasing tendency. In contrast, there was no change in the placebo group (Figure 5). Notably, acylated ghrelin (A-Ghr) and nonacylated ghrelin (NA-Ghr) are the ghrelin gene's main products. The total amount of Ghrelin (A-Ghr + NA-Ghr) is regarded as an appetite-increasing hormone. The current study indicated that APS supplementation increases appetite.
Discussion
To the best of our knowledge, this is the first human trial that examined the effect of the APS supplementation on Ulcerative Colitis patients. The current study showed, after three month trial, participants who were supplemented with APS had improved arginine absorption, intestinal permeability, microbiota population, immune cell count, and appetite.
The colon tissue biopsy Activity score was considerably decreased with APS supplementation, which denoted the intestinal repairing ability of APS in UC patients. MPO, a biomarker of UC [14], was significantly reduced (58%) in the APS group, demonstrating the anti-oxidative and gastroprotective properties of APS. Wu [30] found that astragalin could reduce the inflammatory markers (TNF-α, IL-1β, IL-6 and MPO) in LPS-induced colonocytes and DSS-induced UC mice. Evidence showed fecal calprotectin concentrations demonstrate a good correlation with intestinal inflammation. The current study indicated fecal calprotectin concentration significantly decreased (60.9%) in the APS group compared to the placebo group (10.9%). Evidence showed amount of calprotectin in feces provides a noninvasive quantitative measure of neutrophil flux to the intestine [1]. The current study also showed immune cell neutrophil and lymphocyte counts increased by 11.7% and 20.5%, respectively, in the APS group; while the neutrophil count decreased by 9.3% and lymphocyte count only increased by 7.0% in the placebo group. Furthermore, we found that APS supplementation decreases the pro-inflammatory cytokines IL-6, IL-17, and IL-1β, while increases the anti-inflammatory cytokine IL-10 in the APS group. In addition, the leaky gut test indicated the ratio of lactulose/mannitol was significantly reduced in the APS group. We speculate APS supplementation improved the intestinal mucosal integrity.
Li, Dong, Wang, Xu, Yang, Tang, Qiao and Cong [31] Found that administration of Astragalus (1g/kg/day) for 15 days can increase the diversity of intestinal microbiota in type 2 diabetic mice. In the current study, the microbiota gut diversity has a downward trend in the placebo group while APS supplementation attenuated this trend, which suggests that APS supplementation improved gut dysbiosis. Furthermore, Firmicutes, Faecalibacterium prausnitzii, Bifidobacterium longum, and Bifidobacterium adolescentis counts in the placebo group were decreased while Enterobacteriaceae and Prevotella counts were increased. Notably, after APS supplementation, Firmicutes, Faecalibacterium prausnitzii, Bifidobacterium longum, and Bifidobacterium adolescentis counts were increased, and Enterobacteriaceae and Prevotella counts were decreased in the APS group. All of these suggest APS supplementation has beneficial effects in Firmicutes [31] and probiotics Lactobacillus and Bifidobacterium [16, 31] in UC patients.
Previous studies indicate more than half of UC patients have malabsorption. In the current study, the absorption of L-arginine assay indicated the Cmax concentration and the AUC of L-arginine in the APS group was significantly higher than the placebo group, the Tmax time was earlier than that the placebo group. Zhu, Wang, Li, Zhu, Hu and Chen [17] Used indomethacin (6mg/kg, i.h.) to induce intestinal mucosal injury in rats and administered rat’s ginseng extract for seven days (5g/kg or 15 g/kg per day). Their findings showed significant improvement in mucosal tissue after ginseng extract treatment. In addition, Lee, Tsai, Lin, Ahmetaj-Shala, Huang, Chang and Chang [7] found that Astragalin in TNBSinduced UC mice can promote intestinal epithelial repair by promoting intestinal arginine absorption. We speculate that supplementation of APS can help repair intestinal mucosa in UC patients and increase the absorption of L-arginine.
Loss of appetite is a common symptom of Ulcerative Colitis, and individuals with Ulcerative Colitis frequently experience eating difficulties. The Ghrelin content assay, an appetiteincreasing hormone, indicated APS supplementation increased the total amount of Ghrelin.
The limitation of this study was the small sample size. Therefore, the size of this trial should be increased in future studies. This would better elucidate the effects of the APS. More in-depth studies are needed to verify our findings.
Conclusion
After a 3-month trial supplementing APS, the fecal calprotectin decreased significantly by 60.9% (P=0.015) suggesting decreased intestinal inflammation. In the leaky gut test, the APS group's ratio of lactulose/mannitol significantly reduced by 40% (P=0.02), suggesting improved intestinal mucosal integrity. Furthermore, there was a significant 58% decrease in MPO (P=0.046), a decreasing trend in intestinal tissue inflammation (activity score downward by 66.7%), and a significant increase in L-arginine absorption by 49.7% compared to the placebo group (P=0.008). After a 3-month trial, the alpha diversity index of the placebo group had a downward trend; in contrast, the APS group had an upward trend (up 60%). The results of the study showed that intestinal beneficial bacteria Firmicutes, Faecalibacterium prausnitzii, Bifidobacterium longum, and Bifidobacterium adolescentis counts were increased and the harmful bacteria Enterobacteriaceae and Prevotella counts (reduced/ decreased) in the APS group. Furthermore, the total amount of appetite-increasing hormones Ghrelin increased by 48% in the APS group. In conclusion, APS supplementation can improve arginine absorption, intestinal permeability, microbiota population, immune cell count, and appetite in patients with Ulcerative Colitis
Conflicts of Interest
All authors declare that there is no conflict of interest with respect to the publication of this research article.
Acknowledgments
The authors would like to thank NuLiv Science USA Inc (Brea, CA, USA) for providing the study with ASP (AstraGin®) and placebo capsules.
Funding statement
This trial was funded by Chung Shan Medical University.
References
- Bjarnason I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol. 2017;13(1):53.
- Xie T, Zhao C, Ding C, et al. Fecal calprotectin as an alternative to ulcerative colitis endoscopic index of severity to predict the response to corticosteroids of acute severe ulcerative colitis: A prospective observational study. Dig Liver Dis. 2017;49(9):984-90.
- Bello C, Roseth A, Guardiola J, et al. Usability of a home-based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig Liver Dis. 2017;49(9):991-6.
- Puolanne AM, Kolho KL, Alfthan H, Ristimäki A, Mustonen H, Färkkilä M. Rapid fecal calprotectin test and symptom index in monitoring the disease activity in colonic inflammatory bowel disease. Digestive diseases and sciences. 2017 Nov;62:3123-30.
- Morris Jr SM. Arginine metabolism revisited. J Nutr. 2016;146(12):2579S-86S.
- Agarwal U, Didelija IC, Yuan Y, et al. Supplemental citrulline is more efficient than arginine in increasing systemic arginine availability in mice. J Nutr. 2017;147(4):596-602.
- Lee SY, Tsai WC, Lin JC, et al. Astragaloside II promotes intestinal epithelial repair by enhancing L-arginine uptake and activating the mTOR pathway. Sci Rep. 2017;7(1):12302.
- Chami B, Martin NJ, Dennis JM, et al. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch Biochem Biophys. 2018;645:61-71.
- Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715.
- Norris V, Molina F, Gewirtz AT. Hypothesis: bacteria control host appetites. J Bacteriol. 2013;195(3):411-6.
- Van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-gut-brain axis: modulator of host metabolism and appetite. J Nutr. 2017;147(5):727-45.
- Abdalla MM.Ghrelin–physiological functions and regulation. Eur Endocrinol. 2015;11(2):90.
- Liu X, Guo Y, Li Z, et al. The role of acylated ghrelin and unacylated ghrelin in the blood and hypothalamus and their interaction with nonalcoholic fatty liver disease. Iranian Journal of Basic Medical Sciences. 2020;23(9):1191.
- Guo Z, Lou Y, Kong M, et al. A systematic review of phytochemistry, pharmacology and pharmacokinetics on astragali radix: Implications for astragali radix as a personalized medicine. International journal of molecular sciences. 2019;20(6):1463.
- Fu J, Wang Z, Huang L, Zheng S, Wang D, Chen S, Zhang H, Yang S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother Res. 2014;28(9):1275-83.
- Sun Y, Chen S, Wei R, et al. Metabolome and gut microbiota variation with long-term intake of Panax ginseng extracts on rats. Food Funct. 2018;9(6):3547-56.
- Zhu Y, Wang A, Li R, et al. Total ginsenosides promote the IEC-6 cell proliferation via affecting the regulatory mechanism mediated by polyamines. Saudi Pharm J. 2021;29(10):1223-32.
- Ahn S, Simu SY, Yang DC, et al. Effects of Ginsenoside Rf on dextran sodium sulfate-induced colitis in mice. Food and Agricultural Immunology. 2021;32(1):360-72.
- Hansberry DR, Shah K, Agarwal P, et al. Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus. 2017;9(1).
- mith LA, Gaya DR. Utility of faecal calprotectin analysis in adult inflammatory bowel disease. World J Gastroenterol. 2012 Dec 12;18(46):6782.
- Mudter J, Neurath MF.Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13(8):1016-23.
- Mao L, Kitani A, Strober W, et al. The role of NLRP3 and IL-1β in the pathogenesis of inflammatory bowel disease. Front Immunol. 2018;9:2566.
- Vounotrypidis P, Kouklakis G, Anagnostopoulos K, et al. Interleukin-1 associations in inflammatory bowel disease and the enteropathic seronegative spondylarthritis. Auto Immun Highlights. 2013;4(3):87-94.
- Sequeira IR, Lentle RG, Kruger MC, et al. Standardising the lactulose mannitol test of gut permeability to minimise error and promote comparability. PloS one. 2014 Jun 5;9(6):e99256.
- Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220-30.
- Tamanai-Shacoori Z, Smida I, Bousarghin L, et al. Roseburia spp.: a marker of health?. Future Microbiol. 2017;12(2):157-70.
- Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease . Front Microbiol. 2018;9:2247.
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908-13.
- Masuda Y, Tanaka T, Inomata N, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276(3):905-8.
- Wu S, Chen Z.Astragaloside IV alleviates the symptoms of experimental ulcerative colitis in vitro and in vivo. Exp Ther Med. 2019;18(4):2877-84.
- Li C, Dong Y, Wang L, et al. Ginsenoside metabolite compound K induces apoptosis and autophagy in non-small cell lung cancer cells via AMPK–mTOR and JNK pathways. Biochem Cell Biol. 2019;97(4):406-14.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref