Research Article - Biomedical Research (2022) Volume 33, Issue 5
Early reperfusion with hemoglobin vesicles into tracheal subepithelial capillaries in a mouse tracheal transplant model.
Hiroto Onozawa1, Mitsutomo Kohno2*, Kana Oiwa1, Ryo Hashimoto1, Masatoshi Yamaguchi2, Tai Hato2, Masazumi Watanabe3, Hirohisa Horinouchi4, Hiromi Sakai5, Koichi Kobayashi3, Masayuki Iwazaki1
1Department of Surgery, Tokai University School of Medicine, Isehara, Japan
2Department of General Thoracic Surgery, Saitama Medical Center, Saitama, Japan
3Department of General Thoracic Surgery, Keio University School of Medicine, Tokyo, Japan
4Department of General Thoracic Surgery, Saitama City Hospital, Saitama, Japan
5Department of Chemistry, Nara Medical University, Kashihara, Nara, Japan
- Corresponding Author:
- Mitsutomo Kohno
Department of General Thoracic Surgery
Saitama Medical University
Kawagoe, Saitama
Japan
Accepted date: June 6, 2022
Abstract
Introduction: Hemoglobin Vesicles (HbVs) have been developed as artificial oxygen carriers in the form of liposomes containing concentrated hemoglobin extracted from outdated human Red Blood Cells (RBC). Because HbVs have a small particle size of 250 nm in diamete, rthey can efficiently perfuse capillaries.
Objectives: To evaluate the reperfusion of capillaries with HbVs in a tracheal transplant model and compare it with that of RBC.
Methods: Isogenic mice were used as both donors and recipients in a parallel trachea transplant model, which realizes simultaneous histological observation of the recipient and the grafted tracheas. Both ends of the donor trachea were anastomosed end-laterally to the recipient trachea to form in parallel. After transplantation, 0.3 ml of HbV solution (Hb concentration, 10 g/dl) was administered via the tail vein. The recipients were sacrificed 1, 4, 6, and 8 h after surgery. The tracheas were harvested, and the reperfusion of tracheal Subepithelial Capillaries (SEC) was histologically evaluated.
Results: A significant number of particles defined as HbV by electron microscopy were observed in the SEC of the grafted tracheas 4 h after the transplant surgery and HbV administration when no RBC were found in the SECs. The number increased 6 h later, and HbVs were observed in the SEC 8 h later.
Discussion: Our experiment with a tracheal transplantation model suggests that HbVs, which are smaller than RBC, can reperfuse the capillaries of grafts earlier than RBC after transplantation and contribute to the oxygenation of transplanted tissues.
Keywords
Hemoglobin vesicle, Organ transplantation, Trachea, Ischemia, Reperfusion.
Introduction
Suture failure of anastomosis and dysfunction of grafted organs are major complications in transplantation. Blood flow disorders are more likely to occur, and ischemia at the anastomotic site can cause dehiscence and other serious problems. Especially in lung transplantation, because bronchial arteries are not anastomosed, the blood flow of capillaries in the bronchi is supplied from the collateral circulation from the pulmonary arteries and veins [1,2]. Attention should always be paid to the blood flow and oxygen supply at the anastomotic site of the bronchi.
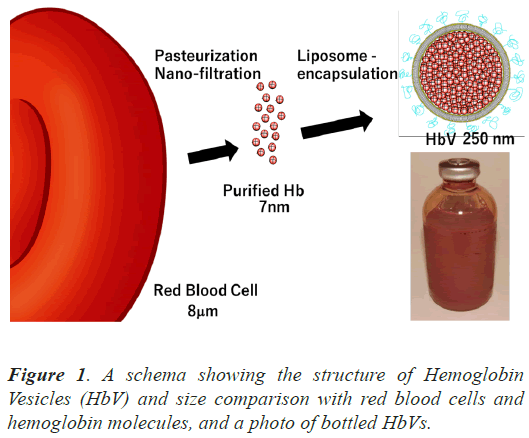
Hemoglobin Vesicles (HbVs) have been developed as blood transfusion substitutes, which are liposomes containing human hemoglobin extracted from expired Red Blood Cells (RBC) [3]. The 250 nm particles have a lipid bilayer membrane with a surface modified with polyethylene glycol to prolong the blood retention time (Figure 1). Various animal studies have demonstrated the efficacy and safety of HbVs in resuscitation for hemorrhagic shock and their ability to deliver oxygen [4-6]. They are expected to be used as alternatives to RBC transfusion in emergency medicine, large-scale disasters, and remote areas [7,8]. Clinical trials were initiated with the support of the Japan Agency of Medical Research and Development model.
(AMED) in 2021. Because HbVs are non-infectious, do not require cross-matching, and can be stored at room temperature for more than 2 years, they are ideal for quick use in the operating room [9]. They can be administered without delay for abrupt massive intraoperative bleeding. In the acute and long-term phases of an animal model of lung resection, circulation was stabilized with HbV administration, and there were no significant adverse longterm effects on postoperative recovery in our previous investigation [10,11].
HbVs have various clinically useful features, including their small particle size of 250 nm, much smaller than RBC (Figure 1) [3]. We hypothesized that HbVs would reperfuse capillaries in a grafted trachea earlier than RBC could in a mouse tracheal transplant model in which blood vessels nourishing the trachea are not anastomosed. We aimed to evaluate the reperfusion of capillaries with HbVs in a tracheal transplant model and compare it with that of RBCs. In this model, the capillaries of the transplanted trachea were elongated at the anastomotic site, forming an anastomosis with the recipient’s capillaries and resuming blood flow.
Materials and Methods
All experimental protocols were reviewed by the Committee on the Ethics of Animal Experiments at the School of Medicine, Tokai University and were conducted in accordance with the Guidelines for the Care and Use of Animals for Scientific Purposes at Tokai University Experimental Animal Center.
Mice
Specific pathogen-free, inbred, 6-week-old male C57BL/6 mice weighing approximately 20 g were purchased from CLEA Japan (Tokyo, Japan). They were kept on a 12 h light/dark cycle with free access to food and water. They were brought to the hospital a week before surgery, and isogeneic tracheal transplants were performed using C57BL/6J mice as recipients and donors.
Tracheal transplant model
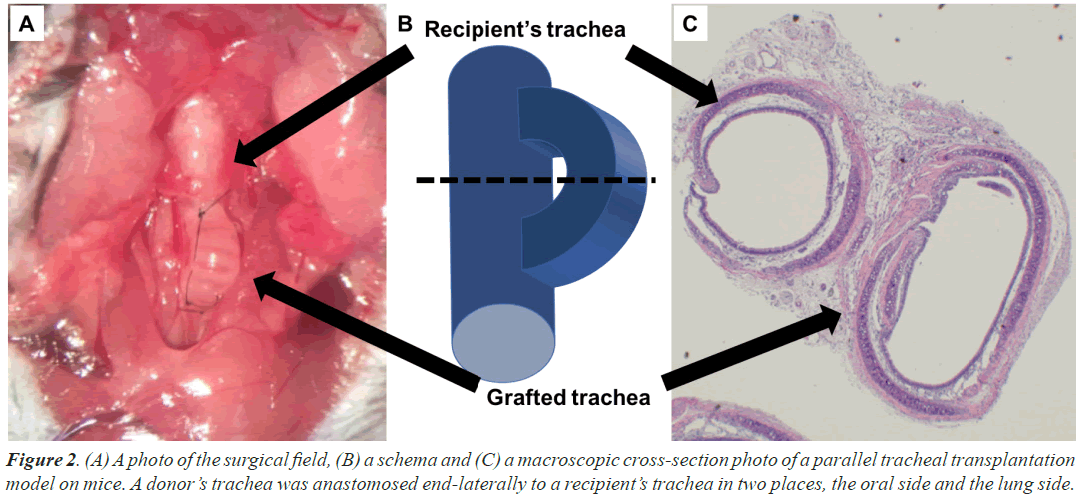
The double-lumen orthotopic tracheal transplant mouse model was established according to the procedures previously reported by Ohtsuka et al. [12]. A donor mouse was deeply anesthetized by intraperitoneal injection of ketamine and xylazine, followed by inhalation of sevoflurane in the supine position, thoracotomized, and harvested from the cricoid cartilage to the bifurcation of the carina. After flushing the RBCs, the trachea was wrapped in gauze that was moistened with normal saline and placed at a cold temperature (4°C). Subsequently, the recipient mice were anesthetized in the same manner. The neck was opened, and two orifices were provided for anastomosis on the recipient trachea at 8 mm intervals to prepare anastomosis. The trachea of the donor and recipient were anastomosed by the method of the end-to-side anastomosis with approximately 3-4 interrupted sutures using 10-0 nylon to ensure airway continuity (Figures 2A and 2B). All surgeries were performed aseptically using a surgical microscope at 5-20 × magnification (surgical scope M680; Leica Microsystems Inc., Bannockburn, IL, USA). The cold and warm ischemic times were approximately 10 min and 15 min, respectively.
Preparation and systemic administration of HbVs
HbVs have been developed as artificial oxygen carriers in the form of liposomes containing concentrated hemoglobin, extracted from outdated human RBC prepared for transfusion, purified, virus-inactivated, and encapsulated in liposomes (Figure 1) [3]. They were prepared under sterile conditions at Nara Medical University, as described previously, and suspended in saline at a hemoglobin concentration of 10 g/dL. The physicochemical parameters of HbVs were as follows: particle diameter, 251 ± 80 nm; methemoglobin concentration, <2%; and oxygen affinity (P50), 28 Torr. They can be preserved for years, used regardless of blood type, and do not incur a risk of infection.
After closing the cervical wound and completing the transplant surgery, 0.3 ml of HbVs (30 mg Hb/body) were intravenously administered from the tail vein with a 29-gauge needle at 0.1 ml/min. The mice were awakened from anesthesia.
Histological evaluation of reperfusion of HbVs in tracheal Subepithelial Capillaries (SECs)
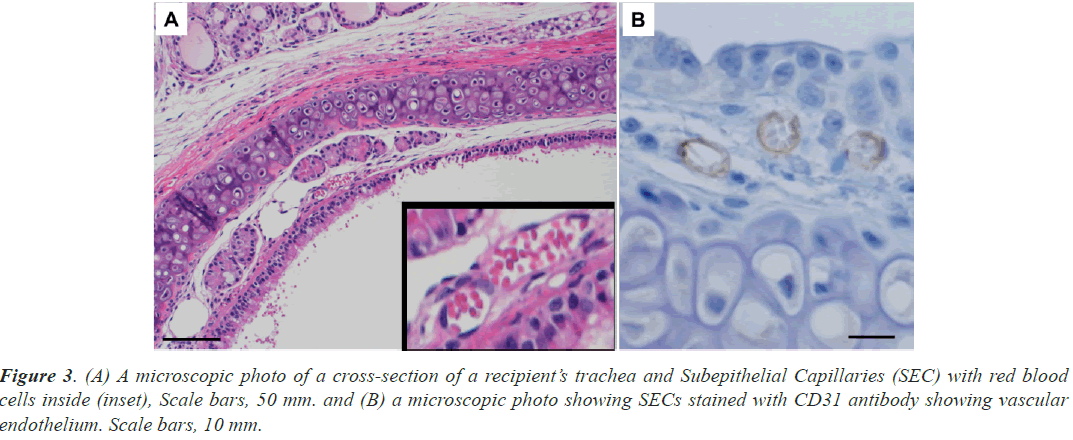
Recipient mice were euthanized under deep anesthesia at 1, 4, 6, and 8 h after transplantation. The graft and recipient tracheas were harvested in a lump, and the tissue was fixed with a 5% formalin solution. Paraffin-embedded tissues were sectioned at 5 mm thickness in a plane perpendicular to the long axis of the trachea. Sections taken from the middle of the tracheal graft and adjacent recipient’s tracheas with preserved luminal structures were stained with Hematoxylin and Eosin (HE) and CD31 for vascular endothelium to identify tracheal SECs (Figure 2C).
Histological evaluation with transmission electron microscope
Tracheal grafts and recipient tracheas were immediately fixed in 2.5% glutaraldehyde in phosphate buffer (0.05 mol/L, pH 7.4) for 2 h and post-fixed in 1% osmium tetroxide in the same buffer for 1 h at 4°C. The specimens were dehydrated in a graded series of ethanol, dehydrated in acetone, and embedded in a Quetol 812 epoxy resin mixture (Nisshin EM, Tokyo, Japan). Ultrathin sections were prepared and stained with uranyl acetate and lead citrate. Sections were examined using a JEM 1400 electron microscope (JEOL, Tokyo, Japan) operating at 80 kV.
Quantitative analysis of HbVs perfusing SECs
The time course of the number of HbVs per unit crosssection of SEC was compared between the recipient’s tracheas and the grafted tracheas using electron microscope images. Vessels with preserved luminal structures were randomly selected for measurements. The subepithelial region was tracked using computer-assisted image analysis system image j, and the vessel area and the number of HbV particles were measured. The areas of RBC and white blood cells were subtracted from the vessel area.
Statistical analysis
The results are presented as mean ± standard deviation. A comparison of values between the groups was performed using unpaired t-tests. All data were analyzed using EZR, version 1.54 software (Saitama Medical Center, Jichi Medical University, Saitama, Japan). Differences were considered statistically significant at P<0.05.
Results
Identification of tracheal SEC and their perfusion with RBC
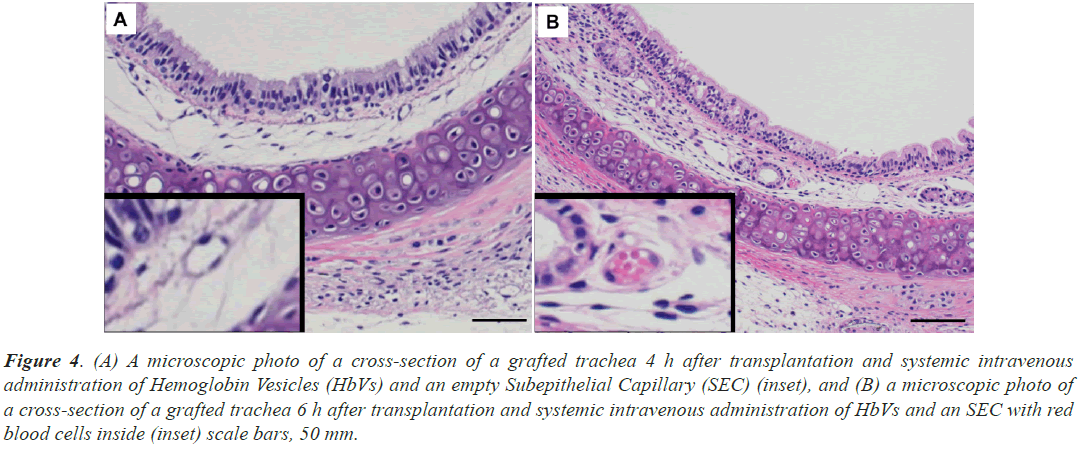
The graft trachea and the recipient’s trachea were excised next to each other (Figure 2C). In the trachea of the recipient, HE staining revealed ring-shaped structures with a diameter of approximately 10 μm under the ciliated epithelium of the recipient’s tracheas and RBC (Figure 3A). Because the cells forming these structures were positive for CD31 and were identified as vascular endothelium (Figure 3B), the structures were identified as blood vessels. RBCs were continuously observed in the SECs of the recipient’s tracheas from 1 to 8 h after surgery and HbV administration. Similar ring-shaped structures were also observed in the grafted tracheas (Figures 4A and 4B), and they could be easily recognized as subepithelial blood vessels from their morphology at each time point from 1 h to 8 h after the surgery and HbV administration. RBCs were not observed at 1 h and 4 h after surgery and HbV administration in the SECs of the grafts (Figure 4A) and then appeared 6 h and 8 h after surgery and HbV administration (Figure 4B).
Figure 4: (A) A microscopic photo of a cross-section of a grafted trachea 4 h after transplantation and systemic intravenous administration of Hemoglobin Vesicles (HbVs) and an empty Subepithelial Capillary (SEC) (inset), and (B) a microscopic photo of a cross-section of a grafted trachea 6 h after transplantation and systemic intravenous administration of HbVs and an SEC with red blood cells inside (inset) scale bars, 50 mm.
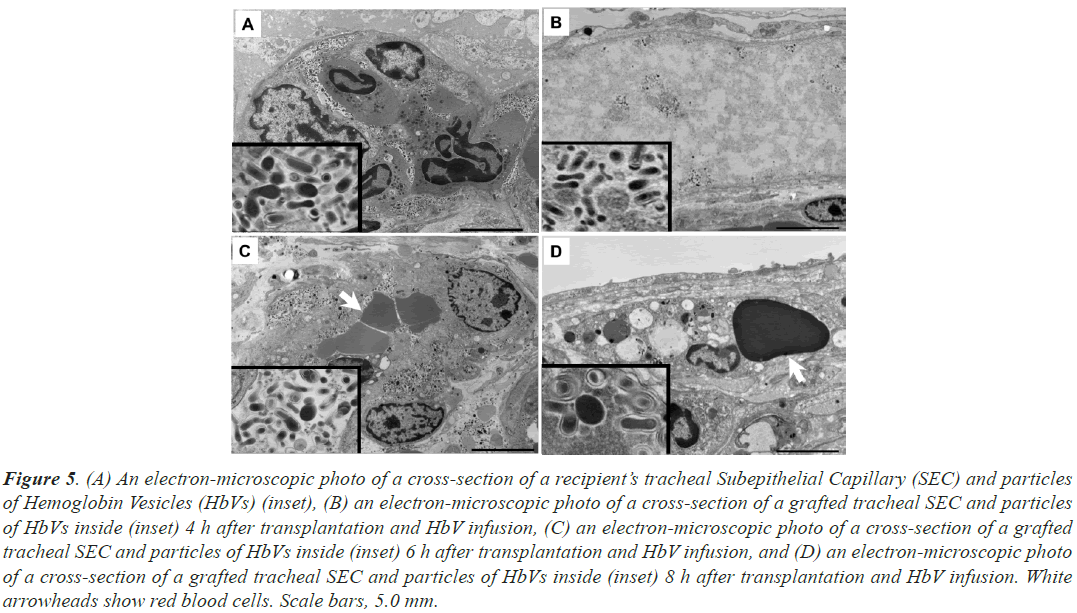
Cognition of hemoglobin vesicle morphology by transmission electron microscope
A large number of fine particles with a diameter of approximately 250 μm were observed in the SECs of the recipient trachea (Figure 5A). Since the particles were spherical or disc-shaped with a smooth surface and had the same morphology as HbVs observed under electron microscopy in our previous studies [13], it was confirmed that HbVs perfused the SECs of the recipient’s trachea at every time point, 1 to 8 h after the surgery and HbV administration.
Figure 5: (A) An electron-microscopic photo of a cross-section of a recipient’s tracheal Subepithelial Capillary (SEC) and particles of Hemoglobin Vesicles (HbVs) (inset), (B) an electron-microscopic photo of a cross-section of a grafted tracheal SEC and particles of HbVs inside (inset) 4 h after transplantation and HbV infusion, (C) an electron-microscopic photo of a cross-section of a grafted tracheal SEC and particles of HbVs inside (inset) 6 h after transplantation and HbV infusion, and (D) an electron-microscopic photo of a cross-section of a grafted tracheal SEC and particles of HbVs inside (inset) 8 h after transplantation and HbV infusion. White arrowheads show red blood cells. Scale bars, 5.0 mm.
In the SECs of the grafted tracheas, at 1 h after implantation, no particles were found, but particles were identified at 4, 6, and 8 h after surgery and HbV administration (Figures 5B-5D). The dispersion of HbV was diluted in capillaries without RBCs 4 h after surgery and HbV administration (Figure 5B) but became dense 6 h after surgery and was mixed with RBCs (Figure 5C).
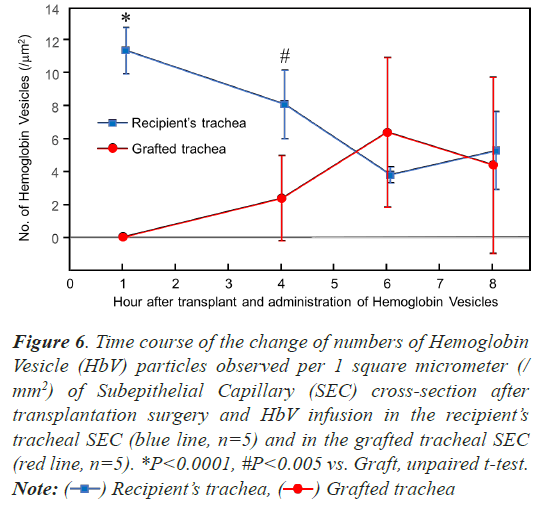
Quantitative analysis of HbVs perfusing tracheal SEC
To investigate whether HbV was reperfused over time, a quantitative evaluation was performed using electron microscopy. The time course of the number of HbVs per unit cross-section of SECs was compared between the recipient and the grafted tracheas (n=5) (Figure 6). On the recipients’ tracheas, 11.4 ± 1.4 HbV particles were observed per 1 square micrometer (/mm2) of SEC crosssection at 1 h after surgery and HbV infusion, and then the number of the particles decreased over time, 8.1 ± 2.1 (/ mm2) after 3 h, 3.8 ± 0.5 (/mm2) after 6 h, and 5.3 ± 2.4 (/mm2) after 8 h. On the graft tracheas, no particle was found on the SEC cross-section at 1 h after surgery and HbV infusion, and then 2.4 ± 2.6 (/mm2) of HbV particles were observed 4 h after, 6.4 ± 4.6 (/mm2) after 6 h, and 4.4 ± 5.4 (/mm2) after 8 h. There was a significant difference between the recipient’s and graft trachea at 1 h and 4 h after surgery (P<0.0001 and P<0.005, respectively, unpaired t-test), although no significant difference was detected 6 and 8 h after surgery and HbV administration.
Figure 6: Time course of the change of numbers of Hemoglobin
Vesicle (HbV) particles observed per 1 square micrometer (/
mm2) of Subepithelial Capillary (SEC) cross-section after
transplantation surgery and HbV infusion in the recipient’s
tracheal SEC (blue line, n=5) and in the grafted tracheal SEC
(red line, n=5). *P<0.0001, #P<0.005 vs. Graft, unpaired t-test.
Note:  Grafted trachea
Grafted trachea
Discussion
This is the first study to observe capillaries in transplanted tissue reperfused with HbVs (an artificial oxygen carrier) using an electron microscope. It was a surprising result that a considerable number of 250 nm HbV particles could be found in the SECs of the grafted trachea as early as 4 h after transplantation, at a time when RBC reperfusion had not started.
It is important to avoid anastomotic insufficiency or suture failure during surgery, including organ transplantation. Because most suture failures are caused by poor blood circulation and ischemia between tissues, surgeons should always pay attention to blood flow and oxygen supply at the anastomotic site. It is thought that blood vessels must be connected and share blood flow between tissues as soon as possible. In this experiment of a tracheal transplant model without vascular anastomosis, it was revealed that the anastomosis of capillaries through which HbV particles with a diameter of 250 μm could pass started within 4 h after transplantation, while the passage of RBC was confirmed after 6 h. Various animal models have confirmed that HbV has oxygen-carrying ability in the living body; therefore, if HbV supplies oxygen to ischemic tissue faster than RBCs, it may help prevent suture failure and dysfunction of transplanted organs.
The number of HbVs in SECs changed over time, and different trends were observed between the recipient and transplanted tracheas. The maximum number of HbVs was first observed and then decreased in the recipient trachea. This is because the HbV retention time in blood was designed to be shorter than that of RBC [13]. They are trapped in the liver and spleen over time, decompose, and metabolize as well as RBC. Meanwhile, in the graft trachea, the number of HbVs was small after 4 h, but it increased as the reanastomosis of the capillaries probably progressed after 6 h and subsequently decreased by being trapped in the liver and spleen.
It has been reported that the ischemic state of the tissue triggers the induction of angiogenesis and the establishment of a new vascular network. Sprouting, stretching, and branching from injured vessels promote vascular anastomosis and lead to reperfusion. It is also known that vascular endothelial growth factor is a potent factor that promotes endothelial cell proliferation and migration and is likely to be associated [14]. Plock et al. previously reported that HbV administration improved tissue survival and wound healing in critically ischemic skin flaps [15,16]. They suggested that hemodilution with HbV stimulates neovascularization through endothelial Nitric Oxide Synthase (eNOS). These effects of HbV on wound healing may be expected in organs other than the skin. In our tracheal transplant model, capillary elongation and reanastomosis probably occurred within 4 h by a similar mechanism. The location of the reanastomosis of capillaries is considered to be the end-to-side anastomosis between the recipients’ and the grafted trachea, but it is also possible that the reanastomosis occurred between the graft trachea and surrounding tissues, such as fat tissue.
A limitation of this study is that it did not evaluate whether oxygen delivery by HbVs contributes to transplanted tracheal engraftment and anastomotic wound healing. The amount of HbVs required to contribute to wound healing or engraftment of transplanted organs has not yet been investigated. In future studies, we will consider experiments to examine the functional effectiveness of early perfusion with HbVs for organ transplantation. Additionally, the effects of HbVs on additional functions should be verified. High oxygen affinity HbV is achieved by changing the allosteric effectors to reduce P50 to 9 Torr, which can carry more oxygen and contribute to tissue oxygenation [11]. Carbon monoxide-bound HbVs (CO-HbV) have antioxidant and anti-inflammatory model.
functions [17]. They may also be effective in oxygenating transplanted tissues in microcirculatory failure and may provide additional benefits.
Conclusion
In conclusion, it was revealed that reperfusion in the capillaries of the transplanted tissue started as soon as 4 h after transplantation and that HbVs passed through the reanastomosis site before RBC. As HbV has a similar oxygen-carrying capacity to RBC, it may reduce ischemic damage and contribute to the healing of transplanted tissues.
Acknowledgment
The authors thank the Support Center for Medical Research and Education, Tokai University School of Medicine, for their assistance with animal experiments.
References
- Date H, Trulock EP, Arcidi JM, Sundaresan S, Cooper JD, Patterson GA. Improved airway healing after lung transplantation. An analysis of 348 bronchial anastomoses. J Thorac Cardiovas Surg 1995; 110: 1424-1433.
[Crossref] [Google Scholar] [PubMed]
- Ishihara T, Nemoto E, Kikuchi K, Kato R, Kobayashi K. Does pleural bronchial wrapping improve wound healing in right sleeve lobectomy? J Thorac Cardiovasc Surg 1985; 89: 665-672.
[Google Scholar] [PubMed]
- Sakai H, Hamada K, Takeoka S, Nishide H, Tsuchida E. Physical properties of hemoglobin vesicles as red cell substitutes. Biotechnol Prog 1996; 12: 119-125.
[Crossref] [Google Scholar] [PubMed]
- Izumi Y, Sakai H, Kose T, Hamada K, Takeoka S, Yoshizu A, Horinouchi H, Kato R, Nishide H, Tsuchida E, Kobayashi K. Evaluation of the capabilities of a hemoglobin vesicle as an artificial oxygen carrier in a rat exchange transfusion model. ASAIO J 1997; 43: 289-297.
[Google Scholar] [PubMed]
- Sakai H, Masada Y, Horinouchi H, Yamamoto M, Ikeda E, Takeoka S, Kobayashi K, Tsuchida E. Hemoglobin-vesicles suspended in recombinant human serum albumin for resuscitation from hemorrhagic shock in anesthetized rats. Crit Care Med 2004; 32: 539-545.
[Crossref] [Google Scholar] [PubMed]
- Seishi Y, Horinouchi H, Sakai H, Kobayashi K. Effect of the cellular-type artificial oxygen carrier hemoglobin vesicle as a resuscitative fluid for prehospital treatment: experiments in a rat uncontrolled hemorrhagic shock model. Shock 2012; 38: 153-158.
[Crossref] [Google Scholar] [PubMed]
- Tsuchida E. Introduction, overview and perspective: In artificial red cells. 1995; 1-20.
- Sakai H. Overview of potential clinical applications of hemoglobin vesicles (HbV) as artificial red cells, evidenced by preclinical studies of the academic research consortium. J Funct Biomater 2017; 8: 10.
[Crossref] [Google Scholar] [PubMed]
- Kohno M, Hashimoto R, Onozawa H, Oiwa K, Horinouchi H, Sakai H, Kobayashi K, Iwazaki M. Hemoglobin vesicles as a transfusion alternative for perioperative hemorrhage in thoracic surgery. World Scientific Publishing 2022; 823-835.
- Kohno M, Ikeda T, Hashimoto R, Izumi Y, Watanabe M, Horinouchi H, Sakai H, Kobayashi K, Iwazaki M. Acute 40% exchange-transfusion with hemoglobin-vesicles in a mouse pneumonectomy model. PloS one 2017; 12: e0178724.
[Crossref] [Google Scholar] [PubMed]
- Hashimoto R, Kohno M, Oiwa K, Onozawa H, Watanabe M, Horinouchi H, Sakai H, Kobayashi K, Iwazaki M. Immediate effects of systemic administration of normal and high O2-affinity haemoglobin vesicles as a transfusion alternative in a rat pneumonectomy model. BMJ Open Respir Res 2020; 7.
- Ohtsuka T, Changelian PS, Bouis D, Noon K, Harada H, Lama VN, Pinsky DJ. Ecto-5'-nucleotidase (CD73) attenuates allograft airway rejection through adenosine 2A receptor stimulation. J Immunol 2010; 185: 1321-1329.
[Crossref] [Google Scholar] [PubMed]
- Sakai H, Horinouchi H, Tomiyama K, Ikeda E, Takeoka S, Kobayashi K, Tsuchida E. Hemoglobin-vesicles as oxygen carriers: influence on phagocytic activity and histopathological changes in reticuloendothelial system. Am J Pathol 2001; 159: 1079-1088.
[Crossref] [Google Scholar] [PubMed]
- Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009; 153: 347-358.
[Crossref] [Google Scholar] [PubMed]
- Plock JA, Tromp AE, Contaldo C, Spanholtz T, Sinovcic D, Sakai H, Tsuchida E, Leunig M, Banic A, Erni D. Hemoglobin vesicles reduce hypoxia-related inflammation in critically ischemic hamster flap tissue. Crit Care Med 2007; 35: 899-905.
[Crossref] [Google Scholar] [PubMed]
- Plock JA, Rafatmehr N, Sinovcic D, Schnider J, Sakai H, Tsuchida E, Banic A, Erni D. Hemoglobin vesicles improve wound healing and tissue survival in critically ischemic skin in mice. Am J Physiol Heart Circ Physiol 2009; 297: H905-H910.
[Crossref] [Google Scholar] [PubMed]
- Watabe Y, Taguchi K, Sakai H, Enoki Y, Maruyama T, Otagiri M, Kohno M, Matsumoto K. Bioinspired carbon monoxide delivery using artificial blood attenuates the progression of obliterative bronchiolitis via suppression of macrophage activation by IL-17A. Eur J Pharm Biopharm 2022; 170: 43-51.
[Crossref] [Google Scholar] [PubMed]