Research Article - Biomedical Research (2017) Volume 28, Issue 6
Dexmedetomidine attenuates lung injury induced by liver ischemiareperfusion injury in rats
Taylan Şahin1*, Zekine Begeç2, Hülya Elbe3, Nigar Vardı4, Mahmut Durmuş2 and M. Ozcan Ersoy21Department of Anaesthesiology and Reanimation, Faculty of Medicine, Yeniyuzyıl University, Istanbul, Turkey
2Department of Anaesthesiology and Reanimation, Faculty of Medicine, Inonu University, Malatya, Turkey
3Department of Histology and Embryology, Faculty of Medicine, University of Mugla Sitki Kocman, Muğla, Turkey
4Department of Histology and Embryology, Faculty of Medicine, Inonu University, Malatya, Turkey
- *Corresponding Author:
- Taylan Şahin
Department of Anesthesiology and Reanimation
Faculty of Medicine, Yeniyuzyıl University, Turkey
Accepted date: October 28, 2016
Abstract
Objectives: It was aimed to evaluate histological effects of different doses of dexmedetomidine on lung injury induced by liver ischemia-reperfusion in rats.
Materials and Methods: Forty rats were included into the study in Inonu University Animal laboratory at 2013, In Group 1, the liver was manipulated and no occlusion of the vessels of the liver was performed. In IR Group 2, 60 min of ischemia and 60 min of reperfusion were applied. In Group 3, 10 μg/kg of dexmedetomidine was injected into the peritoneal cavity 30 min before ischemia. In Group 4, 100 μg/kg of dexmedetomidine was administered via intraperitoneal route 30 min before ischemia. Further procedures in groups 3 and 4 were the same as those of group 2. After the experiment was completed, the rats were killed and then histologic assessments were performed to the lung tissues.
Results: Histopathological damage score in group 2 was higher than in group 1. Although lung damage was recognized as alleviated in group 3, the lesions did not completely improve. However, treatment with 100 μg/kg of dexmedetomidine was more effective than 10 μg/kg of dexmedetomidine injection in respect to protection of alveolar structures. The difference was found to be statistically significant between group 3 and group 4 in terms of histopathological damage score.
Conclusions: The present study suggests that dexmedetomidine administration may be beneficial for preventing lung injury induced by hepatic IR.
Keywords
Liver ischemia-reperfusion injury, Dexmedetomidine, Lung injury.
Introduction
The liver is highly sensitive to ischemia/reperfusion injury (IR), which occurs clinically during hemorrhagic shock, disseminated intravascular coagulation, liver transplantation and surgery involving this organ [1]. Liver IR injury causes a systemic inflammatory response [2]. The intensity of this inflammatory reaction in postischaemic tissue can be so great that the injury response to reperfusion is also manifested in remote organs. These remote effects of IR are most frequently observed in the lung and can result in the development of acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) both of which are important causes of mortality in critically ill patients for which the mortality rate is 40-50% [3].
Dexmedetomidine is a potent and selective α2-adrenergic receptor agonist with sedative, analgesic and hemodynamic properties. It has been used as an adjunct to anesthesia, analgesia and intensive care unit sedation. Previous studies showed that, in addition to it’s anesthetic property, dexmedetomidine also has anti-inflammatory and antiapoptotic effects [4,5]. Despite it’s increasing clinical use, the effects of dexmedetomidine on lung injury induced by liver ischemia-reperfusion have yet to be adequately investigated [6]. The purpose of this experimental study was to evaluate histological effects of different doses of dexmedetomidine on lung injury induced by liver ischemia-reperfusion in rats.
Methods
In this forty Spraguee Dawley male rats with mean weight of 250-350 g were used as prospective at Inonu University, Faculty of Medicine, Multidisciplinary Laboratory of Experimental Animals in 2013, with permission no. 2013/A-80 from Inonu University, Medical School, Ethical Committee of Laboratory Animal Research. The rats were housed in transparent polycarbonate cages in a gloomy room with air conditioning and temperature ranging 22°C ± 2°C, and a rotation of light illumination for 12 h and dark illumination for the other 12 h. All rats were fed with standard pelleted feeds, and provided with fresh tap water. Rats were anesthetized as with urethane (1.2 g/kg) route intraperitoneally (i.p.). The rats were allocated randomly to one of four groups.
Sham control Group (Group 1, n=10): The liver was manipulated following laparotomy and no occlusion of the vessels of the liver was performed. IR Group (Group 2, n=10): The segmental (70%) hepatic warm ischemia model was used after the laparotomy [7]. Following an ischemia period of 60 min, the atraumatic microvascular clamp was removed. Reperfusion period was maintained for 60 min. Drugs were administered as double blind, IR+10 μg/kg of dexmedetomidine Group (Group 3, n=10): 10 μg/kg of dexmedetomidine was injected into the peritoneal cavity 30 min before ischemia. Further procedures were the same as those of group 2. IR+100 μg/kg of dexmedetomidine Group (Group 4, n=10): 100 μg/kg of dexmedetomidine was administered via intraperitoneal route 30 min before ischemia. Further procedures were the same as those of Group 2. The removed lung tissues were stored in a deep freezer at -80°C after the reperfusion period until histological assessments were performed. At the end of the procedures, the animals were sacrificed via cardiac puncture.
Histopatological evaluations
The lung tissues were fixed in 10% formalin for 24 h and were embedded in paraffin. Paraffin blocks were cut at 5 μm, mounted on slides and stained with hematoxylin-eosin (H-E). Sections were examined under X20 magnification for severity of lung injury such as inflammatory cell infiltration, hemorrhage, thickened of alveolar wall and congestion. Lung damage was semi-quantitatively graded for this analysis as absent (0), mild (1), moderate (2), and severe (3), for each criterion. The maximum score was 12. All sections were examined by a histologist blinded to the grouping of the animals using a Leica DFC280 light microscope and a Leica Q Win and Image Analysis system (Leica Micros Imaging Solutions Ltd., Cambridge, UK).
Statistical evaluation
Statistical analysis was carried out using the SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL., USA) statistical program. All data are expressed as median ± (minimum-maximum). Normality values for continued variables in groups were determined by the Shapiro Wilk test. The variables did not show normal distribution (P<0.05). Kruskal-Wallis and Mann-Whitney U tests were used for comparison of variables among the studied groups. P <0.05 was regarded as significant.
Results
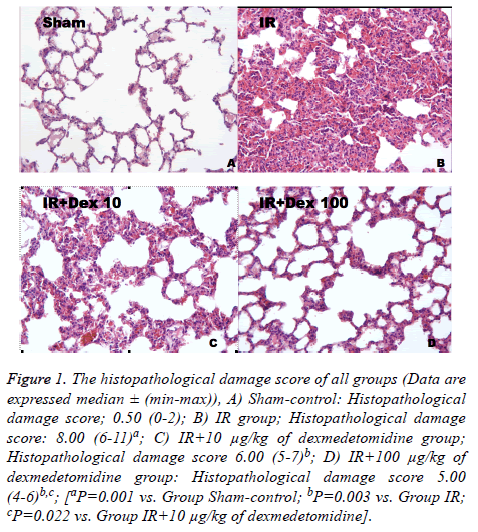
The Sham-control Group (Group 1) showed a normal pulmonary structure (Figure 1A). However, in the IR group (Group 2), histopathological changes were observed such as hemorrhage, interstitial congestion and accumulation of inflammatory cell. Moreover the alveolar septa were markedly thickened (Figure 1B). A statistically significant increase in histopathological damage score was found in the IR Group (Group 2) in comparison with the Sham-control Group (P=0.001).
Figure 1. The histopathological damage score of all groups (Data are expressed median ± (min-max)), A) Sham-control: Histopathological damage score; 0.50 (0-2); B) IR group; Histopathological damage score: 8.00 (6-11)a; C) IR+10 μg/kg of dexmedetomidine group; Histopathological damage score 6.00 (5-7)b; D) IR+100 μg/kg of dexmedetomidine group: Histopathological damage score 5.00 (4-6)b,c; [aP=0.001 vs. Group Sham-control; bP=0.003 vs. Group IR; cP=0.022 vs. Group IR+10 μg/kg of dexmedetomidine].
On the other hand, although lung damage was recognized as alleviated in IR+10 μg/kg of dexmedetomidine Group (Group 3), the lesions did not completely improve. Degenerative alterations like interstitial congestion, inflammatory cell infiltration and thickening of the inter-alveolar septain some areas were still present in this Group (Figure 1C). However, reduction in thickening of interalveolar septa is seen in treatment with 100 μg/kg of dexmedetomidine. Therefore, treatment with 100 μg/kg of dexmedetomidine was more effective than treatment with 10 μg/kg of dexmedetomidine injection in respect to protection of alveolar structures (Figures 1C and 1D). The difference was found to be statistically significant between group 3 and group 4 in terms of histopathological damage score (P=0.022). The histopathological damage score was demonstrated in Figure 1.
Discussion
It has been demonstrated in this study that hepatic IR caused structural changes in the lungs whereas dexmedetomidine significantly decreased these changes. However, 100 μg/kg dose of dexmedetomidine was more effective than 10 μg/kg dose of dexmedetomidine. Liver transplantation and surgery involving this organ, hemorrhagic shock, disseminated intravascular coagulation may lead to hepatic IR injury [1]. Lung injury is one of the most severe complications after hepatic IR and a major concern in the ICU [8-10]. Lung injury pathogenesis following hepatic IR is observed to be multifactorial. Inflammatory leukocytes and reactive oxygen species (ROS) are IR-induced remote organ injury mediators. Remote organ injury is caused by ROS either directly via oxidative stress or indirectly through the release of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 from the reperfused liver [11,12]. Inflammatory mediators are generated via postischaemic tissues which can activate the circulating neutrophils. The activated neutrophils are accepted as mediators of IR induced remote organ injury [12]. We showed a significant increase inflammatory cell in the lungs after liver IR.
It is not clear how dexmedetomidine provides a protective effect against lung injury induced by liver ischemiareperfusion. We are aware of the various physiological changes induced by dexmedetomidine suggesting a powerful antiinflammatory effect. As an example, the administration of dexmedetomidine decrease cytokine production in experimental models such as tumor necrosis factor-α and interleukin-6 [13,14]. The aforementioned data are in accordance with those of various clinical studies carried out on critically ill septic or postoperative major surgery patients [15,16]. Therefore, the anti-inflammatory effects of dexmedetomidine might be responsible for the prevention of liver IR induced lung injury in rats. In our study we investigated whether or not dexmedetomidine prevents lung injury induced by liver IR. We found that dexmedetomidine decreases hemorrhage, interstitial congestion and accumulation of inflammatory cells. It is suggested as a result of these data that modulation of cytokine production might be one of the mechanisms of anti-inflammatory effects of dexmedetomidine.
Tüfek et al. [6] have not determined any statistically significant difference hystopathologically between IR and IR +dexmedetomidine Groups in their study in which they examined the effects of dexmedetomidine on liver and remote organs against hepatic IR. The reason for these results is thought to be due to variations in the experimental design, included differences in; the duration of ischemia; and the ketamine (ischemia and reperfusion durations are 30 minutes each. Whereas the durations in our study were 60 minutes each. In addition, they have used ketamine which has antiinflammatory capacity in all groups as anesthetic). The optimal dose of dexmedetomidine for a specific therapeutic effect without adverse reactions is unknown. For the antiinflammatory actions of dexmedetomidine, Nishina et al. [17] found that clinically relevant doses of dexmedetomidine did not affect chemotaxis or phagocytic action but that high doses induced neutrophil apoptosis. The dose of dexmedetomidine was chosen based on our previous published study protocol.
This study has certain limitations. The first limitation is that there was no negative control since it is not possible to perform this type of surgical intervention in rats without anesthesia. Second, we chose to use i.p. injections of dexmedetomidine rather than the systemic administration of this drug with intravenous infusion. However, the systemic effects with i.p. injections of dexmedetomidine have been shown to be effective [18,19]. In conclusion, the present study suggests that dexmedetomidine administration may be beneficial for preventing lung injury induced by hepatic IR. Measurement of biochemical markers of liver functions and cytokines are needed to better clarify the role of dexmedetomidine on lung injury induced by liver ischemia-reperfusion.
References
- Abu-Amara M, Yan Sy, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: process in inflammatory networks-a review. Liver Transpl 2010; 16: 1016-1032.
- Wanner Ga, Ertel W, Müller P, Höfer Y, Leiderer R, Menger Md, Messmer K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock 1996; 5: 34-40.
- Nakamura K, Yazawa T, Kawaguchi Y, Baba Y, Kitaoka R, Morimura N, Goto T, Yamada Y, Kurahashi K. High tidal volume ventilation induces lung injury after hepatic ischemia-reperfusion. Am J Physiol Lung Cell Mol Physiol 2007; 292: 625-631.
- Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci 2014; 10: 19-24.
- Cai Y, Xu H, Yan J, Zhang L, Lu Y. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury. Mol Med Rep 2014; 9: 1542-1550.
- Tüfek A, Tokgöz O, Aliosmanoglu I, Alabalık U, Evliyaoglu O, Ciftci T, Güzel A, Yıldırım ZB. The protective effects of dexmedetomidine on the liver and remote organs against hepatic ischemia reperfusion injury in rats. Int J Surg 2013; 11: 96-100.
- Karaman A, Fadillioglu E, Turkmen E, Tas E, Yılmaz Z. Protective effects of leflunomide against ischemia-reperfusion injury of the rat liver. Pediatr Surg Int 2006; 22: 428-434.
- Shen H, Jin L, Zhuang X, Zhou Y. A single small dose of ketamine prevents lung injury following hepatic ischemia-reperfusion in rabbits. J Chin Med Assoc 2011; 74: 350-356.
- Hong Sk, Hwang S, Lee Sg, Lee Ls, Ahn Cs, Kim Kh, Moon Db, Ha Ty. Pulmonary complications following adult liver transplantation. Transplant Proc 2006; 38: 2979-2981.
- Bozbas SS, Eyuboglu FO, Ozturk Ergur F, Gullu Arslan N, Sevmis S, Karakayali H, Haberal M. Pulmonary complications and mortality after liver transplant. Exp Clin Transplant 2008; 6: 264-270.
- Uchiyama M, Tojo K, Yazawa T, Ota S, Goto T, Kurahashi K. Edaravone prevents lung injury induced by hepatic ischemia-reperfusion. J Surg Res 2015; 194: 551-557.
- Carden D, Ranger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000; 190: 255-266.
- Can M, Gul S, Bektas S, Hanci V, Acikgoz S . Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol Scand 2009; 53: 1068-1072.
- Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth 2008; 22: 221-228.
- Memiş D, Hekimoğlu S, Vatan I, Yandim T, Yüksel M, Süt N. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth 2007; 98: 550-552.
- Venn Rm, Bryant A, Hall Gm, Grounds Rm. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth 2001; 86: 650-656.
- Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, Niwa Y. The effects of clonidine and dexmedetomidine on human neutrophil functions. Anesth Analg 1999; 88: 452-458.
- Engelhard K, Werner C, Kaspar S, Möllenberg O, Blobner M, Bachl M, Kochs E. Effect of the alpha2-agonist dexmedetomidine on cerebral neurotransmitter concentrations during cerebral ischemia in rats. Anesthesiology 2002; 96: 450-457.
- Sahin T, Begeç Z, Toprak Hi, Polat A, Vardi N, Yücel A, Durmuş M, Ersoy Mö. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J Surg Res 2013; 183: 385-390.