Research Article - Journal of Orthopedic Surgery and Rehabilitation (2017) Volume 1, Issue 1
Development of Zebrafish (Danio rerio) as a natural model system for studying scoliosis
Accepted date: February 03, 2017
Citation: Tomasiewicz HG, Johnson BA, Liu XC, et al.. Development of Zebrafish (Danio rerio) as a natural model system for studying 10 scoliosis . J Orthop Surg Rehabil 2017;1(1):9-14.
Abstract
Background: Idiopathic scoliosis in humans is an abnormal 3-dimensional curvature of the spine with unknown cause. The lack of a model system with naturally occurring spinal curvatures has hindered research on the etiology of idiopathic scoliosis. Recently, it has become clear that zebrafish and other fish species exhibit spinal curvatures similar to those found in humans, making them a good animal model system for studying idiopathic scoliosis. Methods: Founder fish with spinal curvatures were outcrossed with a wild type zebrafish line (AB) and the resulting siblings (F1 generation) crossed and their offspring (F2 generation) examined for signs of spinal curvature beginning at 14 days post fertilization (dpf). Spinal curvatures of the affected fish were visualized using either a Faxitron or by Alizarian red staining of the skeletons and the curvature measured from the resulting images in the thoracic, thoracolumbar, or lumbar regions. Affected and normal zebrafish were sectioned and stained with hemotoxylin and eosin. Findings: The degrees of curvatures in affected zebrafish ranged from 18° to 40°. Histological data demonstrated structural changes as compared to normal fish spine. Out of 212 individuals in the F2 generation 28, or 13.2%, were observed to have spinal deformities at 21 dpf. Importantly, we did not observe spinal deformities in the F1 generation fish and similar age wild type fish, indicating the observed spinal deformities were due to a recessive mutation(s). Conclusion: We have noticed several fish in our zebrafish colony with spinal curvatures reminiscent of human idiopathic scoliosis. We suspect that “scoliosis” in these zebrafish results from mutation(s) in the zebrafish genome. And these fish can be the source of a zebrafish line in which the offspring exhibit a predictable frequency of scoliosis which can be used to study the etiology and progression of scoliosis.
Keywords
Idiopathic scoliosis model, Zebrafish, Mutants, Histo-pathology, Faxitron.
Abbreviations
AB: A Wild Type Zebrafish Line; F1 generation: First Filial Generation, obtained by crossing two parental lines; F2 generation: Second Filial Generation, obtained by intercrossing or self-crossing the F1; dpf: Days Post Fertilization; IS: Idiopathic Scoliosis; SD: Spinal Deformed; PCR: Polymerase Chain Reaction; EF1a: Elongation Factor 1 Alpha Gene
Introduction
Idiopathic scoliosis (IS) is a three-dimensional deformity of the spine with unknown etiology. The spinal deformities include a lateral translation in the coronal plane, a flat back with hypokyphosis in the sagittal plane, vertebral rotation, and wedging of the vertebrae [1-3]. Curve progression was found to be related to the curve pattern, curve magnitude, age, the Risser sign, and menarchal status [1]. In school screening studies when only curves of more than 10 degrees are considered, the prevalence of the IS were approximately 1.5% to 3% of pediatric population. The prevalence rate varies with the curve magnitude, being 2% to 3% for curves over 10 degrees, 0.3% to 0.5% for curves over 20 degrees, and 0.2% to 0.3% for curves over 30 degrees [2,4]. Clinical case studies reported a family of IS and suggests the genetic components linked with chromosomes 6, 9, 16 and 17, respectively [5,6]. The pathogenesis and etiology of IS were still not understood.
The lack of a good vertebrate animal model for studying the progression and etiology of scoliosis has hindered progress in understanding the underlying cause of the spinal curvatures observed in scoliosis patients. Most of the animal models used to date involve experimental scoliosis, where the spinal curvature is artificially induced [7]. Some examples of experimentally induced scoliosis include spinal offset tethering in pigs [8], pinealectomized chicken, rat, goat and mouse.
A variety of fish models have been utilized as a resource for the study of scoliosis [9]. 82% of pinealectomized Atlantic salmon develop lateral and dorso-ventral spinal curvatures [10].
Selenium as a teratogenic environmental contaminant also had an impact on the Sacramento splittail, causing pericardial edema, loss of tail, scoliosis, and kyphosis [11]. The best genetic animal model, to date, for IS was the curveback guppy [12]. The curveback guppy exhibited a polygenic mode of inheritance with more females than males exhibiting the phenotype. The polygenic nature of the disorder is reflected in the highly variable phenotype observed in affected fish, which mimics the wide variation of observed spinal curvatures found in human IS patients. The genetic locus, as determined in a qualitative study, contains over 100 genes [13].
Interestingly, one mutant zebrafish line has been identified to date that exhibits a spontaneous occurrence of an abnormal spinal curve without anthropomorphic assistance in impairing normal anatomical structure or inducing the deformity. The humpback mutant line of zebrafish exhibits an abnormal spinal curvature in the proximal part of the spinal cord located over the pectoral fins [14].
Having a model system naturally exhibiting the defects observed in scoliosis in humans would be instrumental in determining the factors causing scoliosis, whether it is genetic, environmental or a gene-environment interaction. We have found nine zebrafish in our colony with curved spines characteristic of scoliosis observed in humans. We have used these as prospective founder fish for lines of zebrafish with an increased risk for developing spinal deformities. Presently, we have focused on one of these lines or strains. Towards the goal of developing these fish as a model system for studying the etiology of scoliosis with the explicit objective of identifying factors causing the development of scoliosis in humans, we have been characterizing this strain of zebrafish with an eye towards using it to develop therapies for treating scoliosis in humans.
Methods
Design of the study
A perspective study was designed for Zebrafish in our colony where the zebrafish were screened for the presence of spinal deformities. Fish with spinal deformities were used as the basis for a genetic screen for mutations involved in spinal deformities.
Animal husbandry
Zebrafish (Danio rerio) were raised under standard laboratory conditions [15]. Fish were maintained at 28?C on a 14 hour light, 10 hour dark cycle. The adult fish were fed freshly prepared live artemia nauplii twice daily and flake food. Larval zebrafish were fed freshly prepared live artemia nauplii twice daily. Adult zebrafish were euthanized using an overdose of MS-222 (Tricaine) per established procedures described in The Zebrafish Book [15].
Microsporidia
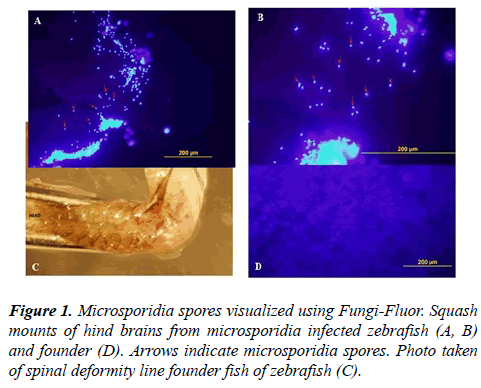
The spinal cord and hind brain was removed from euthanized zebrafish. Squash mounts were prepared from the hind brains,which were stained for microsporidia using the Fungi-Fluor kit as described by the manufacturer. Microsporidia were visualized by epifluorescence illumination using an Olympus BX60 microscope equipped with a DAPI filter. Images were taken using an Olympus DP70 color camera. For microsporidium, polymerase chain reaction (PCR) was performed using universal panmicrosporidium specific rRNA primers [16] under conditions described in Mathews et al. [17].
Faxitron
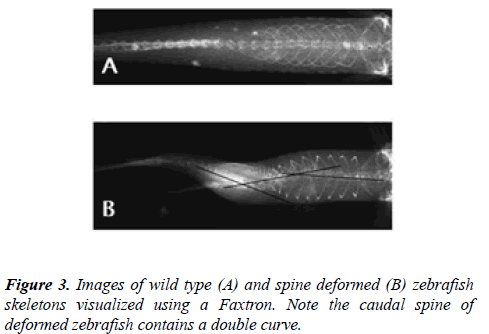
To further characterize the nature of the spinal curvatures in the fish and as a means of comparing them with those observed in humans, radiographies of normal and fish with curved spines were taken using a Faxitron Image System (Faxitron X-ray LLC, Lincolnshire, IL). Radiographies of fish were taken in the anterior-posterior view. The spinal curvatures of the affected fish were measured based upon the Ferguson’s method. Pictures of the resulting radiographs were taken using a UVP Biochemi gel documentation system equipped with a high resolution 12 bit CCD Camera.
Bone staining
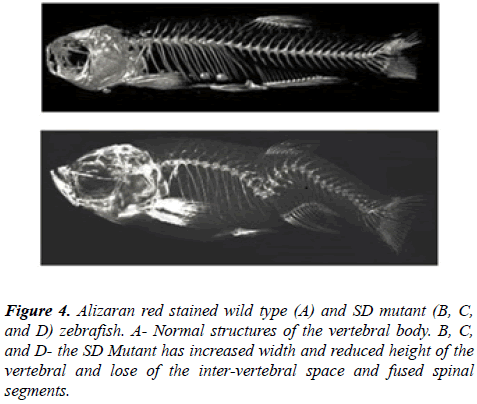
The bones of euthanized adult zebrafish were stained using Aliziran red (Sigma) and the cartilage with alcian blue (Sigma) [18].
Histology and cytology
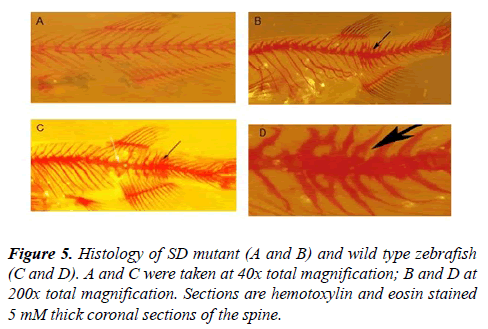
Both wild-type and spinal deformed (SD) zebrafish, were euthanized using a lethal dose of MS-222 (Tricaine), fixed in 4% paraformaldehyde in 100 mM phosphate buffer (pH 7.3) at 4?C for 48 hrs, decalcified, embedded in parafilm, sectioned at 4 μm thickness and stained with hemotoxylin and eosin [17,19]. Images of the stained sections were captured on an Olympus BX60 microscope equipped with an Olympus DP70 camera. The tissues were examined for histopathology characteristic of either scoliotic tissue in humans or parasitic infections (e.g. inclusion bodies, parasites or cysts). Since mycobacteria infection in zebrafish results in the formation of granuloma, the sections will also be examined for the presence of these structures, as well as inflammation [20]. As a negative control, the same regions of the spinal cord from health fish were treated similarly.
Squash mounts of affected regions of spinal cords were examined as wet mounts for microsporidia spores. Some wet mounted tissues was stained for microsporidia spores using a fluorescent stain, Fungi-Fluor (Polysciences, Inc., Warrington, PA) as described in Mathews et al. [17].
Morphology of the vertebrae was investigated via microscopic examination, including the cortical and cancellous bone, disc, and endochondral growth plate. Any fusion, bony or cartilage fracture, orientation of the annular fibers, zones of chondrocytes, and density were examined.
Micro CT scans
Both wild type and spinal deformed zebrafish were scanned nondestructively with a Zeiss Xradia Versa 410 3D X-ray microscope (Micro CT). The resulting images were used to create a 3D image of the fish.
Results
To date, eleven zebrafish with abnormal spinal curvatures were observed in our zebrafish colony. To determine if the observed spinal curvatures had a genetic cause, these fish with spinal curvatures were setup to spawn with their wild type counterparts. One of the spawning resulted in the production of a viable F1 generation of fish that were phenotypically normal, despite the founding male fish had a severe spinal curvature.
While the F1 generation was growing and maturing they were observed for signs of spinal deformities. No spinal deformities were observed in the F1 generation. During this time, the founder fish, and other fish with spinal curvatures were examined for infection by both microbacteria and Pseudolomas neurophilia, a microsporidia, pathogens that are known to cause abnormal spinal curvatures in zebrafish. Examination of stained sections of the individual zebrafish spinal cords failed to reveal any microbacteria (HGT, Data not shown), indicating microbacteria were not the cause of the spinal curvatures.
Since microsporidia infections are known to cause spinal deformities in zebrafish, spinal cord and hind brain tissue from the founder male zebrafish was tested for the presence of microsporidia by two independent methods. The first method involved examining squash mounts of the hindbrain stained with a fluorescent dye, Fungi-Fluor, which binds specifically to the spores of microsporidia found in infected tissue. As a positive control we used the hindbrain from an emaciated zebrafish with an obvious microsporidia infection. As a negative control we used several normal looking wild type fish from our colony. As shown in Figures 1A and 1B, the spores could be clearly seen in the microsporidia infected fish, but not in the founder fish (Figures 1C and D).
The second method for detecting microsporidia in spinal cord tissue involved using the polymerase chain reaction to detect microsporidia specific DNA in the spinal cord tissue of zebrafish [17]. DNA containing lysates were prepared from the spinal cord of several zebrafish including the spinal deformity founder zebrafish, the microsporidia infected positive control and uninfected wild type fish. The lysates were used in PCR reactions containing primers specific for microsporidia ribosomal RNA genes. Only the DNA from the microsporidia infected fish (Lane Sk) generated PCR products that were visible on agarose gels stained with ethidium bromide (Figure 2).
Figure 2: Results of PCR analysis of DNA isolated from the spinal cord of zebrafish with deformed spines (S), “skinny” zebrafish (Sk) or wild type zebrafish (W). Image of ethidium bromide stained agarose gel containing PCR products. M- lane loaded with 100 bp ladder. Arrowhead points to PCR product from microsporidia infected “skinny” zebrafish.
Importantly, each DNA sample when used as templates in PCR reactions containing primers to the zebrafish Elongation Factor 1alpha (EF1a) gene, an appropriate size PCR product was generated, indicating the DNA was capable of supporting the PCR (HGT, data not shown).
Spinal curvatures of the affected individual fish were visualized using either a Faxitron (Figure 3), micro CT (Figure 4) or by Alizarian red staining (Figure 5) of the skeletons and the curvature measured from the resulting images in the thoracic, thoracolumbar, or lumbar regions. The degrees of curvatures ranged from 18° to 40° in the thoracolumbar and lumbar region (Figure 3).
Spinal transverse is asymmetrical, as one side is compressed and other sides are more open with the fins also being are asymmetrical and rotated, coupled with axial rotation of the vertebral body (personal communication, BAJ).
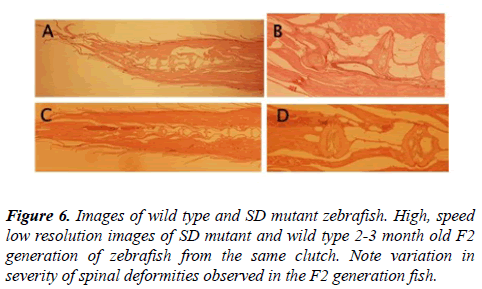
Histological examination of the affected spine of the zebrafish with spinal deformities revealed structural changes in the vertebral bodies that were consistent with the changes observed in both the Aliziran red stained and faxitron and micro CT images of the zebrafish with spinal curvatures (Figure 6).
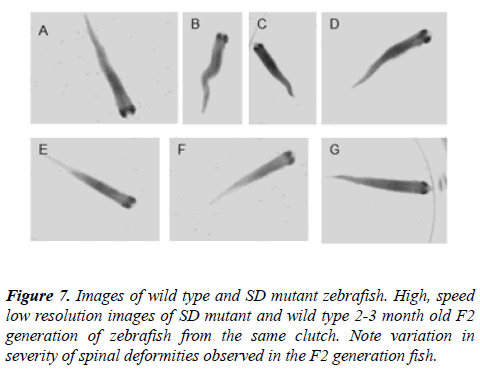
While the F1 generation of the spinal deformity mutant line remained free of spinal deformities, some members of the F2 generation begin to show small spinal deformities or curvatures beginning at about 21 days post fertilization. Of the 212 individuals examined 28, or about 13%, exhibited spinal curvatures. The spinal curvatures in some of these individuals got progressively worse but did not reach the severity observed in the line’s founding father (Figure 7). All of the deformities occurred in the caudal part of the spine, distal to the dorsal fin.
Discussion
The lack of a good animal model system has hindered studying the etiology of idiopathic scoliosis. Recently, it has become clear that some individual fish of several fish species appear to exhibit spinal curvatures. To determine if the affected tissue in zebrafish with spinal deformities resembles the pathology observed in pediatric populations with scoliosis we have developed a line of zebrafish with an increased occurrence of spinal deformities.
As some spinal curvatures observed in zebrafish are due to infection by the microsporidia, Pseudolomas neurophilia, tissue from both normal and zebrafish with deformed spines were examined for the presence of this pathogen. Both PCR analysis and histological examinations were negative for microsporidia, confirming that these fish with spinal curvature were not infected by microsporidium and myxosporan parasites. Because fish infected with these pathogens exhibit overt symptoms, such as emaciation [17], not observed in the fish we have, we fully expect most of the fish to be free of these pathogens and to provide a stock for future studies and the beginning of a strain of fish with scoliosis.
Spinal curvatures of the affected fish were visualized using either a Faxitron, Micro CT or by Alizarian red staining of the skeletons and the curvature measured from the resulting images in the thoracic, thoracolumbar, or lumbar regions. The degrees of the curvatures ranged from 18° to 40° Histological data demonstrated structural changes as compared to normal fish spine.
Potential founder fish with spinal curvatures were outcrossed with a wild type zebrafish line (AB) and the resulting siblings (F1 generation) crossed and the offspring (F2 generation) examined for signs of spinal curvature beginning at 14 days post fertilization. Out of 212 individuals in the F2 generation 28, or 13.2%, were observed to have spinal deformities at 21 days post fertilization. Importantly, we did not observe spinal deformities in the F1generation fish and similar age wild type fish, indicating the observed spinal deformities were due to a recessive mutation(s). The spinal deformities occurred in the same distal region of the spine in all fish examined, supporting the notion of a genetic defect causing the spinal deformity. Interestingly, Konig et al. [14] reported the occurrence of a mutant line of zebrafish, humpback, which exhibited a spinal curvature in the proximal part of the spine. The humpback mutant mapped to the guanylate kinase gene, DLG3 [14]. Yet the observed defects are less severe than those observed in the founder fish at a similar age, suggesting that an unknown environmental agent is exacerbating the spinal deformity observed in the founder fish. In an attempt at identifying candidate agents, we have begun assaying the water and food for potential contaminants that may act to exacerbate the spinal curves caused by this mutation. We have crossed the founder fish with WIK and TU strains of zebrafish to generate the lines of zebrafish we plan to use in mapping the mutation observed in the affected fish relative to unaffected fish from the same clutch.
Recently, investigators have identified mutations in several genes in zebrafish encoding proteins that are involved either in cilia formation or motility [21-23]. Defective cilia result in a decrease in cerebral spinal fluid flow that causes spinal deformities. Interestingly, all of these cilia mutations are either embryonic lethal or temperature-sensitive, which requires compensatory injection or expression of the normal gene product or raising the embryos at the permissive temperature then shifting the embryos to the non-permissive temperature to reveal the scoliosis phenotype. The embryonic lethality of these mutations provides both an obvious explanation for the rarity for the adult zebrafish with spinal deformities, and a test for identifying heterozygous carriers of the mutant scoliosis genes, sco/+. If the sco mutation is an embryonic lethal then crosses of male and female sco/+ mutant fish should result in 25% of the resulting offspring dying as embryos. This is more complicated if the sco mutant protein is temperature sensitive, as the mutant phenotype would only reveal itself when the embryos are raised at the non-permissive temperate of 30?C. These properties may explain why it has been so difficult to identify and raise sco mutants.
However, once a sco mutant family has been identified, the real power of using zebrafish as a model system becomes apparent. Some of the advantages the zebrafish had over the guppy as a model system was the ability to use the availability of a well annotated genome with a full suite of tools for doing genome manipulations, including the ability to create transgenic fish to express proteins of interest in specific cell populations and at define developmental periods and creation targeted knockouts of genes of interest (e.g. using the CRISPRCAS- 9 system [24]). Once the gene(s) implicated as playing a role in the spinal deformities is identified a CRSPR can be designed to knockout the candidate gene and the effect on spinal column development and stability can be assessed. This technology is not available for use in live bearing fish, e.g. guppies.
We have isolated strains of zebrafish that have an increased susceptibility to spinal deformities that is exacerbated by an unknown environmental agent. These fish will serve as a natural model system for studying gene-environment interactions in spinal deformities, and for testing environmental factors that may play a causative role in human juvenile idiopathic scoliosis.
Acknowledgement
The authors wish to express their gratitude to Dr. Toth for the use of the Faxtron used in generating x-ray images of the zebrafish used in this study and the staff of the Aquatic Animal Facility at the UWM NIEHS CEHSC center for excellent fish care. Thanks to Sam Fellow and Emily Gilles for excellent technical assistance. We are grateful for pilot grants from the UWM NIEHS CEHSC Center (P30 ES004184) and the Department of Orthopaedic Surgery, Medical College of Wisconsin.
Funding
The study was funded by pilot project grants from the Dept. of Orthopaedic Surgery, Medical College of Wisconsin and the University of Wisconsin-Milwaukee NIEHS Children’s Environmental Health Sciences Core Center (ES004184-24).
References
- Lonstein J, Carlson M. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am 1984; 66: 1061-71.
- Lonstein J. Scoliosis. Lovell and winter’s pediatric orthopaedics. Editors: R.Morrissy and S. Weinstein. IV. Edition. New York: Raven Press; 1996; 625-737.
- Harrington P. The etiology of idiopathic scoliosis. Clin Ortho Rel Res 1977; 126:17-25.
- Weinstein SL, Ponseti IV. Curve progression in idiopathic scoliosis. J Bone Joint Surg Am 1983; 65: 447-55.
- Miller N, Justice C, Marosy B, et al. Identification of candidate regions for familial idiopathic scoliosis. Spine 2005; 30:1181-1187.
- Carr AJ. Adolescent idiopathic scoliosis in identical twins. J Bone Joint Surg Br 1990, 72:1077.
- Ouellet J, Odent T. Animal models for scoliosis research: State of the art concepts and future perspective applications. Eur Spine J 2013;2:S81-95.
- Odent T, Cachon T, Peultier B, et al. Porcine model of early onset scoliosis based on animal growth created with posterior mini-invasive spinal offset tethering: A preliminary report. Eur spine J 2011; 20: 1869-76.
- Gorman KF, Breden F.Teleosts as models for human vertebral stability and deformity. Comp Biochem. Physiol. C toxicolo. Pharmacol 2007; 145:28-38.
- Grotmol PG, Kryvi S, Gjerdet H, et al.Pinealectomy induces malformation of the spine and reduces the mechanical strength of the vertebrae in Atlantic salmon, Salmosalar. Journal of Pineal Research 2004;36:132-39.
- Teh SJ, Deng X, Teh FC, et al. Selenium-induced teratogenicity in Sacramento splittail (Pogonichthysmacrolepidotus. Marine Environmental Research 2002; 54:605-08.
- Gorman KF, Breden F. Disproportionate body lengths correlate with idiopathic-type curvature in the curveback guppy. Spine 2010; 35: 511-16.
- Gorman KF, Christains JK, Parent J, et al. A major QTL controls susceptibility to spinal curvature in the curveback guppy. BMC Genet 2011; 12:16.
- Konig C, Yan YL, Postlethwait J, et al. A recessive mutation leading to vertebral ankylosis in zebrafish is associated with amino acid alterations in the homologue of the human membrane-associated guanylate kinase DLG3. Mech. Dev 1999; 86:17-28.
- The Zebrafish Book. A guide to the laboratory use of zebrafish (Daniorerio). Ed. Monte Westerfield: University of Oregon Press, 2000.
- Vossbrinck CR, Baker MD, Didier ES, et al. Ribosomal DNA sequences of Encephalitozoonhellum and Encephalitozooncuniculi: species identification and phylogenetic construction. J. Eukaryot. Microbiol 1993; 40: 354-62.
- Matthews JL, Brown AM, Larison K, et al.Pseudolomaneurophilia n. g., n. sp., a new microsporidium from the central nervous system of the zebrafish (Daniorerio). J EukaryotMicrobiol 2001; 48:227-33.
- Gavaia PJ, Sarasquete C, Cancela ML. Detection of mineralized structures in early stages of development of marine teleoststei using a modified Alcian blue-Alziran red double staining technique for bone and cartilage. Biotechnic&Histochemistry 1999; 75:79-84.
- Moore JL, Aros M, Steudel KG, et al. Fixation and decalcification of adult zebrafish for histological, immunocytochemical, and genotypic analysis. Biotechniques 2002; 32:296-98.
- Swaim LE, Connolly LE, Volkman HE, et al. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infection and Immunity 2006; 74:6108-117.
- Hayes M, Gao X, Yu LX, et al. ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicates dysregulatedWnt signaling in disease. Nat. Comm. 2014, 5:4777-787.
- Buchan JG, Gray RS, Gansner JN, et al.Kinesin family member 6 (lif6) is necessary for spine development in zebrafish. Dev. Dyn. 2014; 243: 1646-657.
- Grimes DT, Boswell CW, Morante NFC, et al.Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spinal curvature. Science 2016; 352: 1341-344.
- Li M, Zhao L, Page-McCaw PS, et al. Zebrafish Genome Engineering Using the CRISPR-Cas9 System. Trends in Genet. 2016; 32: 815-27.