- Journal of RNA and Genomics (2006) Research Article
Delivery of RNAi reagents in murine models of obesity and diabetes
| Denise M Wilcox1*, Ruojing Yang1, Sherry J Morgan1, Phong T Nguyen1, Martin J Voorbach1, Paul M Jung1, Deanna L Haasch1, Emily Lin1,2, Eugene N Bush1, Terry J Opgenorth1, Peer B Jacobson1, Christine A 1Metabolic Disease Research, in vivo Chemical Genomics, Department R4CY, 100 Abbott Park Road, Abbott Laboratories, Abbott Park, IL 60064, USA; 2UIC College of Medicine, Chicago, IL 60612-7302, USA; 3Present address: Hoffmann-La Roche Inc., Nutley, NJ 07110, USA; 4Present address: Eli Lilly, Inc., Indianapolis, IN 46285, USA. |
| *Correspondence to: Denise Wilcox, Email: denise.m.wilcox@abbott.com, Tel: +847 937 5790, Fax: +847 938 1656 |
| Journal of RNAi and Gene Silencing (2007), 3(1), 225-236 |
| © Copyright The Authors |
| (Received 25 September 2006; Revised 13 November 2006; Accepted 15 November 2006; Published online 29 November 2006) |
Abstract
RNA interference (RNAi) is an exciting new tool to effect acute in vivo knockdown of genes for pharmacological target validation. Testing the application of this technology to metabolic disease targets, three RNAi delivery methods were compared in two frequently utilized preclinical models of obesity and diabetes, the diet-induced obese (DIO) and B6.V-Lep
Keywords |
| siRNA, shRNA, obesity, adenovirus, murine, steatosis, liver toxicity |
Introduction |
| The advent of RNA interference (RNAi) technology has created new opportunities for preclinical target validation in murine models of disease. To maximize the potential utility of RNAi, safe and effective in vivo delivery methods are of critical importance. A potential complication is the possible impact of the disease state itself on the tolerability and efficacy of a particular RNAi delivery method. |
| RNAi in vivo studies published to date have predominantly focused on exogenously applied short-interfering (si) or endogenous short-hairpin (sh)RNA delivery to normal, wild-type animals or to specific regions or organs (Hannon and Rossi, 2004; Dykxhoorn and Lieberman, 2005; Shankar et al, 2005). For liver gene knockdown, examples include the delivery of siRNAs or shRNA expression constructs to wild-type mice with the high-pressure “hydrodynamic” intravenous (i.v.) tail injection technique (Lewis and Wolff, 2005) resulting in protection from hepatitis (Song et al, 2003), viral infection (Giladi et al, 2003; Tompkins et al, 2004) and tumor progression (Lakka et al, 2004; Duxbury et al, 2005; Sumimoto et al, 2005). Successful in vivo knockdown has also been reported with a more benign intraperitoneal (i.p.) injection of siRNAs, although results have varied widely (Sorensen et al, 2003). Alternatively, viral vectors administered through low pressure, small volume i.v. tail vein injection have been reported to successfully deliver shRNA expression constructs, resulting in sustained gene knockdown of liver-expressed genes (Xia et al, 2002; Huang et al, 2004; Lin et al, 2005). |
| While these various methods of RNAi delivery have been reported as safe and effective in animal models with normal liver function, these methods may lose their utility in animal models with compromised livers, such as the leptin-deficient, severely steatotic ob/ob mouse and the moderately steatotic high fat diet-induced obese (DIO) mouse. Both of these models are used extensively in metabolic disease research for assessment of potential drug targets, with many published examples of genetic knockouts backcrossed to the ob/ob background and knockout resistance to high fat diet-induced weight gain (Clee et al, 2005 ; Moraes et al, 2003; Haluzik et al, 2004). Acute gene knockdown through RNAi in DIO and ob/ob models would represent a powerful way to quickly establish relevance for potential drug discovery targets in obesity, diabetes, or non-alcoholic fatty liver disease, if a method for well-tolerated and effective delivery could be established. |
| In the current study, i.p. injection of siRNA, hydrodynamic i.v. injection of siRNA, and (standard) i.v. injection of adenovirus expressing shRNA were evaluated in DIO, ob/ob, and normal (lean) control mice. Observations included assessment of tolerability, degree of knockdown, and treatment-related physiological changes. Since these three RNAi delivery methods have been reported to target the liver (Dykxhoorn and Lieberman, 2005; Lewis and Wolff, 2005), particular attention was paid to iatrogenic hepatic effects, which could seriously compromise analysis and conclusions drawn for any liverexpressed gene. With recent attention focused on cJun Nterminal kinase 1 (JNK1) activity in the liver as a possible small molecule target for diabetics, we selected this gene for our pilot si/shRNA in vivo knockdown studies. Previously published systemic knockout (Hirosumi et al, 2002), inhibitory peptide (Kaneto et al, 2004; Nakatani et al, 2004), and dominant negative studies (Kaneto et al, 2004; Nakatani et al, 2004) were considered helpful context for interpretation of any physiological changes observed. |
Materials and Methods |
| Generation of synthetic siRNAs, vectors expressing shRNAs and recombinant adenoviruses |
| Two siRNAs for mouse JNK1 (Genbank accession: NM_016700; ORF 192-1346) used in this study were designed according to previously described selection criteria (Boese et al, 2005) and synthesized by Dharmacon, Inc (Boulder, CO). The siRNAs, designated siJNK1-2 and siJNK1-5, each consist of a 21 nucleotide (nt) long-siRNA duplex with a 3’-dTdT overhang. Their sequences are as follows: |
| siJNK1-2 (sense/antisense): |
| 5’-GCAGAAGCAAACGUGACAACA-3’/ |
| 5’-UGUUGUCACGUUUGCUUCUGC-3’ |
| siJNK1-5 (sense/antisense): |
| 5’-GCACACAAUAGAGGAGUGGAA-3’/ |
| 5’-UUCCACUCCUCUAUUGUGUGC-3’ |
| The 21 nt non-targeting “Universal Control” siRNA used here is commercially available from Dharmacon (Catalog #: D-001220-01) and was designed to minimize targeting of any known human, mouse, or rat genes. Its sequence is as follows: |
| Control siRNA (sense/antisense): |
| 5’-GGGCGUCGAUCCUAACCGG-3’/ |
| 5’-CCGGUUAGGAUCGACGCCC-3’ |
| Identity of all siRNAs provided by Dharmacon is confirmed by MALDI-TOF mass spectrometry (individual strand composition) and non-denaturing gel electrophoresis (duplex formation). |
| To create JNK1-2 shRNA, sense and antisense sequences defined by siJNK1-2 were separated by an eight-base linker (TCAAGACT) to give the following sequence: |
| 5’-GCAGAAGCAAACGTGACAACATCAAGACTTGTT GTCACGTTTGCTTCTGC-3’ |
| This was cloned into a pENTRTM vector driven by a U6 promoter (Invitrogen, Carlsbad CA). The resulting shRNAexpressing plasmid was tested for its ability to knock down JNK1 protein in HEK293 cells co-transfected with a vector-overexpressing mouse JNK1 cDNA (pAd/CMV/V5- DEST; Invitrogen, Carlsbad, CA) (Yang R and Wilcox DM, submitted). Knockdown of JNK1 protein was evaluated by western blot analysis as described below. A second shRNA, JNK1-5 shRNA, was prepared in parallel based on sequences defined in siJNK1-5 but did not knock down JNK1 as effectively as JNK1-2 shRNA in vitro (Yang R and Wilcox DM, submitted). With knockdown efficacy established for the JNK1-2-based insert, the targeting construct was transferred to an adenoviral pAd/BLOCK-ITTM-DEST vector by Gateway LR recombination (Invitrogen, Carlsbad CA). Recombinant adenovirus expressing JNK 1-2 shRNA was then generated by transfection of adenoviral-based construct containing JNK1-2 shRNA into HEK293 cells as described in the manufacturer’s instructions for the pAd/BLOCK-ITTM system (Invitrogen, Carlsbad CA). Virus destined for in vivo studies was then amplified in HEK 293 cells and then purified using cesium chloride gradient centrifugation followed by gel filtration desalting, following published procedures (Becker et al, 1994). The negative control adenovirus utilized was designed to express shRNA specific for green fluorescent protein (GFP) and does not target any known eukaryotic gene. Construct shGFP (Huang et al, 2004) consisted of the following sequence: |
| 5’-GAAGCAGCACGACTTCTTCTTCAAGAGAGAAGA AGTCGTGCTGCTTC-3’ |
| Animal protocols |
| The Abbott Institutional Animal Care and Use Committee approved all protocols involving rodents in advance and all studies were conducted in an Association for Assessment and Accreditation of Laboratory Animal Careapproved barrier facility. All solutions to be injected were determined to be negative for endotoxin by a Limulus assay system (Cambrex, Walkersville MD). |
| Intraperitoneal delivery study |
| Male C57Bl/6J (Jackson Laboratories, Bar Harbor ME) mice were made obese by spontaneous overfeeding a high fat diet (D12492; 60% kcal from fat) for 20 weeks (Bush et al, 2006). Normal (lean) control mice were fed a corresponding low fat diet (D12450Bi; 10% kcal from fat). All animals were individually housed in micro-isolator cages for one week prior to the study for acclimation. The diet-induced obese (DIO) mice were assigned to treatment groups such that mean body weights were similar among groups. Mice were given daily i.p. injections with saline, control siRNA, or siJNK1-5 for four days (25 mg/kg, total volume approximately 0.45 ml/injection). Animals were humanely euthanized by CO2 inhalation and plasma and liver samples collected on day five. |
| Hydrodynamic delivery study |
| Male B6.V-Lep<ob>/J (ob/ob) mice (5-6 weeks of age) purchased from Jackson Laboratories (Bar Harbor, ME) were allowed food (Purina 5015 Diet) and water ad libitum. DIO mice were as described above under i.p. delivery methods. For each study, mice were assigned to treatment groups such that mean body weights (and blood glucose for ob/ob) were similar among groups. Mice were restrained for a single, pressurized i.v. injection of 2 ml saline, control siRNA, siJNK1-5 or siJNK1-2 into the lateral tail vein within 10 seconds (Lewis and Wolff, 2005). Animals were monitored daily for up to 8 days following treatment for adverse effects. Animals were humanely euthanized by CO2 inhalation for end of study plasma and liver sample collection. |
| Adenovirus-mediated delivery study |
| Male DIO mice were assigned to treatment groups as above such that mean body weights were similar among groups. Mice were restrained for a single 100 Ql lateral tail vein injection of either saline, 2 x 1011 viral particles of adenovirus expressing nontargeting control shGFP or 2 x 1011 viral particles expressing shRNA targeting JNK1 (shJNK1-2). Five days post-injection, animals were humanely euthanized by CO2 inhalation and plasma and liver samples were collected for analysis. |
| Body weight, food intake, blood chemistries and histological preparation |
| Body weight and food intake were monitored in each study. Postprandial blood glucose was assessed in all cases by tail snip (Precision PCx, Abbott Laboratories, Abbott Park, IL). Plasma analyzed for insulin (ALPCO Diagnostics, Windham NH) and alanine aminotransferase/aspartate aminotransferase (ALT/AST) (Sigma Diagnostics, St Louis MO) levels were measured according to manufacturer’s instructions from postprandial study samples. Liver samples were flash frozen in liquid nitrogen for western blot analysis of JNK1 protein levels. Samples for histology were immersion fixed in 10% (v/v) formalin for at least 24 hr. After wet cutting, sections were processed and embedded in paraffin and 5-6 μm sections were cut and stained with hematoxylin and eosin. A single board certified pathologist conducted histological evaluation of histopathology on all studies. |
| Western blot analysis |
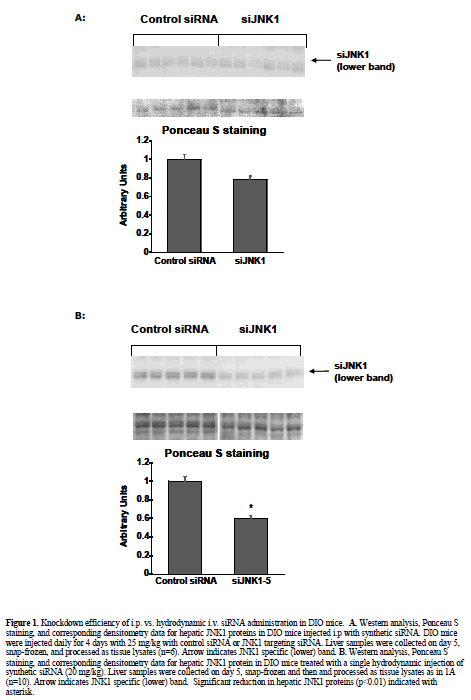
| Knockdown potency of JNK1 siRNA and shRNA constructs was initially confirmed prior to use in vivo by western blot analysis of cell lysates derived from either in vitro transfected or viral-transduced primary murine hepatocytes. Liver and fat tissues obtained from in vivo siRNA and shRNA treatments were pulverized and homogenized in lyses buffer as previously described (Yang and Newgard, 2003). Protein content of lysates was determined using a BCA assay kit (cat. #23227, Pierce Biologicals, Rockford IL). For western blot analysis, equal amounts of protein were loaded onto SDS gels, electrophoresed and subsequently transfered to nitrocellulose membranes. The membranes were stained with Ponceau S for 15 minutes to confirm equal loading and transfer of samples. The JNK antibody utilized for all westerns, sc571, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Protein-antibody complexes were visualized using ECL plus (PerkinElmer Life Science, Wellesley MA) following the manufacturer’s instructions, and quantitated by densitometry (GE Healthcare, Uppsala, Sweden). A typical western blotting pattern with sc571 includes an upper band of nonspecific/unknown origin, and a JNK1- specific lower band. |
| Real-time Quantitative PCR |
| Viral DNA was detected in collected tissues by duplex real-time quantitative PCR and normalized to content of murine DNA. The oligonucleotide primers and probes for the murine and viral genes were designed using the Primer Express software program (Applied Biosystems, Framingham MA). The viral TaqMan probe was 5S labeled with the reporter 6-carboxyfluorescein and 3S labeled with the quencher tetramethylrhodamine. The TaqMan probe for the murine reference gene carried a 5’- hexachlorofluorescein reporter and a 3’- tetramethylrhodamine quencher. |
| Specific primers and probes for the viral E2A DBP gene (AP_000213): |
| 5S-CCAACTGCGACTTCAAGATATCG-3S (forward) |
| 5S- CACAGGCTGCGCACCAT-3S (reverse) |
| 5S-CGCCCGACCTGCTAAACGCG-3S (probe) |
| Specific primers and probes for the murine Birc5 gene (NM_009689): |
| 5S-GCTGAGATAACTTGGACCTGAGTGA-3S (forward), 5S-GAGGCCCTGGCTGGAAAA-3S (reverse) 5S-TGCCACATCTAAGCCACGCATCCC-3S (probe) |
| The viral primers and probes were purchased from Operon Biotechnologies, Inc (Huntsville, AL). The murine primers and probes were purchased from Integrated DNA Technologies, Inc (Coralville, IA). |
| The PCR assay was carried out at standard temperatures and times using the ABI AmpliTaq Gold DNA Polymerase Kit with Buffer II, 1.25 U polymerase and 5.5 mM MgCl2 in 25 Ql reactions. The viral primers were used at 400 nM each and probe at 100 nM. The concentrations of the murine reference gene primers and probe were 100 nM and 200 nM, respectively. The fluorescence intensity of the reporter labels were normalized to Texas Red, a passive reference label added to the buffer at 25 uM. PCR reactions were performed in an ABI-PRISM 7900HT Sequence Detection System (Applied Biosystems, Framingham MA). Viral DNA levels were calculated by the relative standard curve method and were normalized to content of the murine gene Birc5. |
| Statistical Analysis |
| Data are expressed as the mean ± S.E.M. Statistical significance was determined by a Dunnett one-way ANOVA using the statistics module of GraphPad Instat (San Diego CA) with significance assumed at p < 0.05. |
Results |
| Comparison of siRNA delivery methods in steatotic murine models of diabetes and obesity |
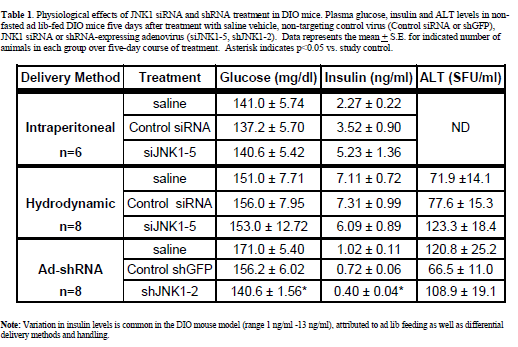
| Intraperitoneal injection of diet-induced obese (DIO) mice Intraperitoneal injection (i.p.) of naked siRNAs is one of the most benign methods reported for RNAi delivery (Sorensen et al, 2003). To assess the efficacy of this method in a murine metabolic disease model, DIO mice were injected i.p. with 10 ml/kg (0.45 ml/mouse) daily for 4 days with saline, control non-targeting siRNA, and siRNA targeting murine JNK1 (siJNK1-5). Previous studies in primary hepatocytes (Yang et al, submitted) using siJNK1-5 had demonstrated potent knockdown of JNK1 protein expression when transfected in vitro. The i.p. injection procedure was well tolerated by the mice, with no significant changes observed in food intake, body weight or plasma glucose. Increases in insulin levels observed in all groups reflected values typically observed for daily i.p. injected animals (1.2-6.8 ng/ml,) and were not significantly different from vehicle vs. siRNA treated groups (Table 1). Liver histology appeared unchanged by this regimen. Despite its good potency as an in vitro knockdown agent, western blot analysis of liver extracts revealed only minimal knockdown of hepatic JNK1 by the JNK1-5 siRNA construct (Figure 1A). |
| Hydrodynamic injections in diet-induced obese (DIO) mice |
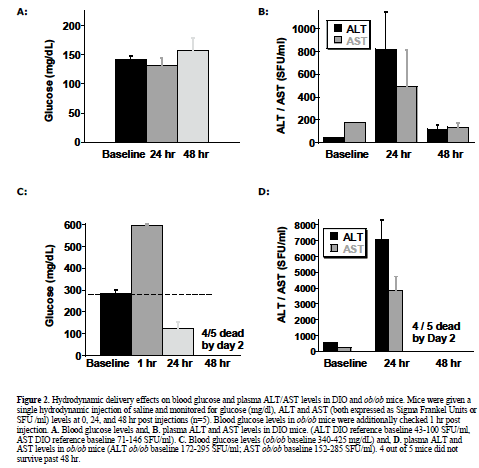
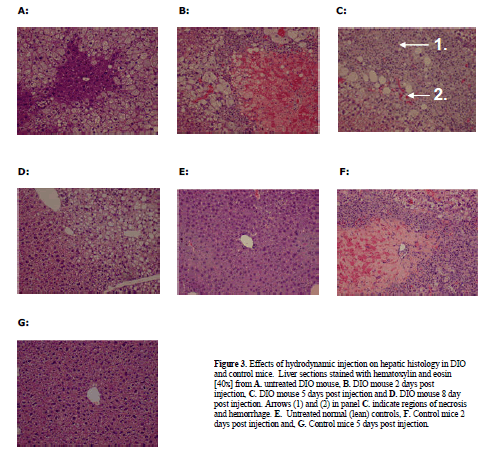
| To observe injection method-related effects, DIO and normal (lean) control mice were given a single, 2 ml pressurized tail vein injection of saline vehicle as described and reviewed (McCaffrey et al, 2002; Song et al, 2003; Zhang et al, 2004; Dykxhoorn and Lieberman, 2005; Lewis and Wolff, 2005). In addition, DIO mice were injected with control non-targeting siRNA, or two different JNK1 siRNAs (siJNK1-2 or siJNK1-5). Animals were observed both acutely and 5-8 days post injection. Both liver (targeted organ) and adipose tissue (control) were assayed by western blot analysis using JNK antibody for evidence of knockdown. As expected (Becker et al, 1994; Song et al, 2003), JNK1 levels were not diminished in the collected adipose samples nor in any samples from animals treated with the non-targeting siRNA. The siJNK1-5 mildly but significantly knocked down hepatic JNK1 by 40% compared to control siRNA treated mice (Figure1B), while the siJNK1-2 knocked down hepatic JNK1 by only 25% (data not shown). While there were no acute effects on plasma glucose in these mice (Figure 2A), there were other significant delivery method-associated tolerance issues observed. Saline-treated DIO animals had a 20-fold increase in plasma alanine aminotransferase(ALT) levels 24 hr after injection, subsiding to 3-fold above baseline by 48 hr (Figure 2B). A similar pattern of response was observed with aspartate aminotransferase (AST) (Figure 2B). Examination of liver histology from both treated DIO and control mice revealed significant hydrodynamic injection-related hepatic injury (Figure 3). On day 2-post injection, areas of hemorrhage and peripheral inflammation were evident in livers of both DIO and control mice (Figure 3B and 3F). By day 5, extensive regions of focal inflammation were still apparent in the DIO mice (Figure 3C), while the non-steatotic livers of the normal (lean) control mice appeared to have largely recovered (Figure 3G). By day 8, the livers from the treated DIO animals had regained their expected pretreatment histology (Figure 3D). Measurements of insulin, glucose, and ALT taken on day 5 are summarized in Table 1. Insulin levels were very high (6.09-7.31 ng/ml) across all groups 5 days post injection, with no significant difference observed for JNK1-2 siRNA vs. vehicle treatment. Taken together, these results indicate that although DIO mice can recover from hydrodynamic injection, there are significant effects on liver function and histology up to 8 days post injection. The non-steatotic normal control mice appear to recover days earlier than steatotic animals, implying that the procedure induces greater toxicity in animals with fatty livers (Figure 3E, 3F, and 3G). |
 |
 |
 |
| Hydrodynamic injections in ob/ob mice |
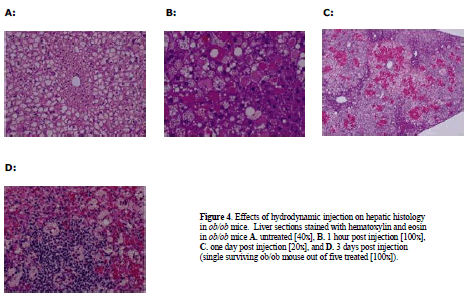
| Given the results above with the moderately steatotic DIO mouse, it was important to test the effects of hydrodynamic injection in the severely steatotic ob/ob animal. These mice proved to be even more sensitive to hydrodynamic injection than the DIO mice. Administration of saline vehicle only, utilizing the same injection volume and pressure as in the previous DIO experiment, resulted in dramatic fluctuations in glucose and subsequent death for 4/5 treated ob/ob animals (Figure 2C). Plasma glucose increased from 282 mg/dl to 596 mg /dl one hour post injection (Figure 2C), subsequently dropping to 124 mg/dl (less than half of baseline ob/ob levels) one day post-injection. Most mice did not survive past day 2. Both plasma ALT and AST levels were markedly affected, rising one day after injection to 7000U/ml and 3800U/ml, respectively (Figure 2D). Histological examination revealed extensive liver injury (Figure 4). One hour after injection, cellular swelling and necrosis were already apparent (Figure 4B). By 24 hr, livers of treated animals exhibited regions of extensive hemorrhage and inflammatory cell infiltration (Figure 4C). Only one ob/ob mouse out of the five animals that received hydrodynamic injection survived to 3 days post treatment (Figure 4D). |
 |
| Histological examination of the single surviving ob/ob mouse revealed significant hepatic inflammation. The severe response of ob/ob mice suggests that the degree of steatosis is correlated with the degree of liver damage incurred by hydrodynamic tail vein injection. |
| Adenovirus mediated delivery in ob/ob and diet-induced obese (DIO) mice |
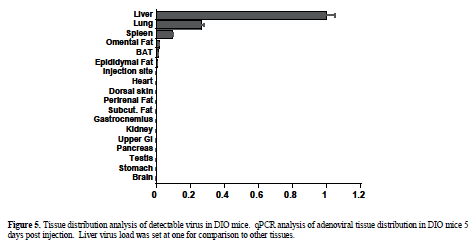
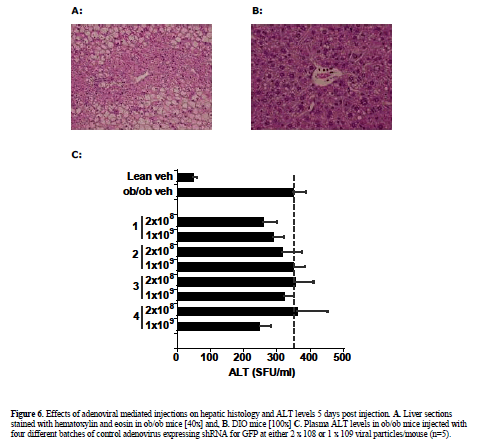
| Viral expression vectors have been utilized by several investigators to deliver shRNA expression constructs to wild type mice without reports of significant injury (Xia et al, 2002; Huang et al, 2004). To verify relative tissue selectivity of adenoviral-mediated shRNA delivery in DIO and ob/ob mice, the tissue distribution of injected adenovirus was determined by qPCR 5 days post injection. Tissue distribution analysis revealed detectable virus in liver, lung (27% of liver levels), and spleen (9% of liver levels) with minimal distribution of virus found in the other organs analyzed (<1% of liver, limit of detection) (Figure 5). Liver histology from shGFP-adenoviral-treated mice (1 x108 or 2 x108) appeared normal when analyzed by H&E staining (Figure 6A) and unlike the hydrodynamically-injected animals, no increases in plasma ALT enzyme levels were observed in ob/ob mice in response to treatment (Figure 6B). |
 |
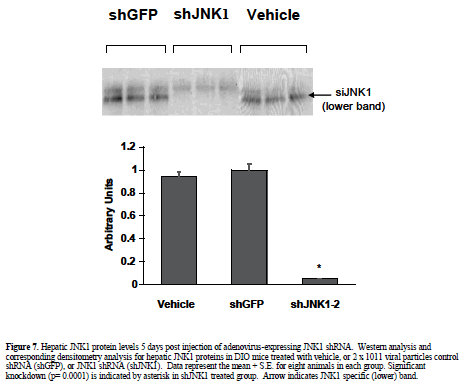
| Having established the adenovirus-mediated delivery of shRNA via normal tail-vein delivery is well-tolerated in ob/ob and DIO mice, we pursued further studies with an shRNA construct designed to target JNK1 (shJNK1-2) as described in material and methods. DIO mice were given a single i.v. injection of saline or 2 x 1011 viral particles of adenovirus containing shGFP or adenovirus containing shJNK1-2, and assessed 5 days later for molecular and metabolic changes. Hepatic knockdown was confirmed by Western blot analysis, and showed almost complete silencing (94%) of the JNK1 mRNA in the shJNK1-2 treated mice, with no knockdown observed in the shGFP - treated mice (Figure 7). There was no evidence of liver damage in any of the adenovirus-shRNA treated animals, with no changes observed in either liver histology (data not shown) or liver enzyme levels (Table 1). Insulin levels for these mice (post single tail vein injection) were much lower (0.4-1.02 ng/ml) than that observed in the previous 5 day i.p. injected and hydrodynamic-treated animals. Lack of overt toxicity and potent knockdown of hepatic JNK1 enabled preliminary targeting construct-specific effects to be observed: A 10% reduction in blood glucose levels, and a 45% decrease in plasma insulin levels (Table 1). |
Discussion |
| The application of RNAi to new therapeutic target discovery and validation is nothing short of revolutionary. While the use of RNAi for screens and in vitro target validation has been very successful and is in widespread use, application of RNAi to in vivo target validation has been a more difficult challenge. In order to utilize this new technology to characterize and validate potential obesity and diabetes drug targets, it is essential to establish methodology that would be both efficacious for gene knockdown and safe in the murine disease models used for metabolic disease studies, including the ob/ob and DIO mouse. |
| Repeated i.p. injections of anti-sense oligonucleotides have been used successfully in various animal models for gene knockdown studies (Zinker et al, 2002; Yu et al, 2005), although phenotypic changes have typically been reported after multiple (3 to 6 weeks) weeks of treatment. This injection method appears to be well tolerated by steatotic animal models like DIO and ob/ob mice. Following a 5-day regimen reported with anti-sense oligonucleotide (ASO) treatment in which protein levels of PTP1B were significantly reduced (Zinker et al, 2002), we chose a comparable short course of four consecutive days of i.p. dosing for naked siRNA administration. No hepatic JNK1 knockdown was observed. Therefore, although well-tolerated and safe, a short i.p. injection course of naked siRNA was ineffective for siRNA delivery in our hands. Recent successes have been reported using direct administration of cholesterol-coupled siRNAs (Soutschek et al, 2004). We have not investigated these carriers in detail, however, as they are not without toxicities and were considered potentially problematic with our steatotic animal models (Sorensen et al, 2003). |
 |
 |
 |
| The hydrodynamic or high pressure tail vein injection technique (Liu et al, 1999; Lewis and Wolff, 2005) has been the most commonly used technique for siRNA delivery. Injected volumes of fluid corresponding to up to 10% of total body weight are injected over 5-10 seconds, disrupting inter-hepatocyte tight junctions and facilitating siRNA uptake (Zhang et al, 2004). High incidence of cardiac events reported in hydrodynamic-injected mice (Zhang et al, 2004; Zhang et al, 2004) may be due to the addition of a 2 ml fluid volume into an approximately 2.5 ml circulating blood volume over a short period of time. Hydrodynamic tail vein injection has been apparently used successfully in mouse models with normal liver function, however, consistent with our findings, these reports also indicate transient increases in ALT/AST levels and evidence of liver damage 2 days post injection (Liu et al, 1999; Zhang, 1999; Lewis and Wolff, 2005). Here we have shown that two of the steatotic mouse models used most widely in diabetes and obesity research, the DIO and ob/ob, are very sensitive to this technique. DIO mice receiving hydrodynamic treatment suffered extensive, differentially reversible liver damage accompanied by ALT/AST spikes 24 hr post treatment. Histological examination of livers at varying times post injection revealed extensive hemorrhage and necrosis after two days that appeared to be resolving by day 5 and virtually normalized by day 8. In contrast, lean control animals with normal livers showed initial hepatic injury on day 2 but this appeared to be fully resolved by day 5. In the more extensively steatotic ob/ob mouse, hydrodynamic injections resulted in lethal complications. Blood glucose levels increased one hour after the injection and then fell well below baseline. Many of treated ob/ob died immediately following injection, and most did not survive 3 days post-injection. Liver damage in the ob/ob animals was more extensive than that observed in the DIO mice, possibly indicating delayed or reduced hepatic repair capabilities. The degree of injury and differential recovery rate between normal controls, moderately steatotic DIO and severely steatotic ob/ob mice, may suggest that the successful hydrodynamic injection reported in the literature (Giladi et al, 2003; Song et al, 2003; Tompkins et al, 2004) are due to the ability of normal animals, with healthy livers, to recover from the hepatic insult that occurs post-injection. |
| In the DIO mice studies reported here, hydrodynamic injection-derived liver damage prevented any conclusions regarding JNK1 knockdown-related effects. Such deliveryrelated side effects would be particularly problematic with novel, less well-characterized targets. Therefore, hydrodynamic injection of siRNA should be considered unsuitable for in vivo target validation using the DIO and ob/ob mouse models. Given these findings, it may be important to consider background liver damage/repair even in the interpretation of hydrodynamic injection-based studies in normal animals. Depending on experimental time-line or the target being manipulated, contribution to overall phenotype could be substantial. |
| For target validation studies, a viable alternative to i.p. and hydrodynamic siRNA delivery methods is adenovirus-mediated shRNA delivery. Adenoviral shRNA expression constructs continuously express shRNA, allowing for sustained knockdown for up to two weeks (Xu et al, 2005; Yamazaki et al, 2005), advantageous for eliciting physiological change and modeling small molecule activity. Adenovirus is particularly attractive for in vivo studies due to its episomal, non-integrating mode of expression and typically high titer. Immune response to adenovirus challenge is well-documented and cannot be entirely avoided, but properly controlled studies limited to the 5 to 14 day response time-frame can allow dissection of viral-mediated vs. gene knockdown-mediated changes. Tissue distribution analysis by qPCR carried out 5 days post tail vein injection indicated that the liver was the major site of viral deposition, with some virus detected in the lung and spleen (27% and 9% of liver levels, respectively). Given these findings, shRNA delivery via adenovirus seemed particularly appropriate for shortterm validation studies of liver-expressed targets. |
| In contrast to the hepatotoxic responses seen with hydrodynamic injection, no evidence of histological liver damage or overt stress was observed in either adenovirusshRNA treated DIO or ob/ob animals. Animals appeared healthy when monitored for up to 14 days post-injection. Knockdown efficacy was also achieved, with JNK1 protein levels reduced in the DIO mice by 94% when assayed five days post-injection. Recently, other studies of ours utilizing adenovirus-mediated delivery of shRNA have shown equivalent knockdown efficacy achieved in ob/ob mice for other liver-expressed targets (Xu et al, 2005; Xu et al, 2006). |
| In the absence of delivery method-associated effects, physiological changes observed could be preliminarily attributed to the JNK1-targeting construct. In DIO mice, plasma insulin was reduced 45% and non-fasting glucose was reduced by 10% in shJNK1-2 treated animals compared to the non-targeting shRNA-treated controls. Confirmation of the effects of JNK1 knockdown via shRNA will await follow-up studies with additional knockdown positive constructs/viruses. |
| Identification of safe and efficacious delivery methods that will allow siRNA or shRNA to be applied either systemically or directly to target organs remains a significant hurdle for both preclinical and clinical applications of RNAi. In order to apply this approach to in vivo target validation, disease model-specific responses need to be assessed and accommodated. In this study, the steatotic livers of ob/ob and DIO mice were particularly sensitive to the otherwise widely reported hydrodynamic tail vein injection technique. Hepatic damage resulting from injection eliminated this method as a viable approach for validating liver expressed diabetes and obesity targets. In contrast, adenoviraldelivered shRNA allowed effective knockdown in the absence of hepatic damage in these mouse models, enabling the observation and measurement of targetspecific findings. While direct treatment of siRNA would obviate potential complications with viral infection and provide an opportunity to utilize chemically modified controls, a suitable delivery method has yet to become widely available. Of the currently available alternatives, viral delivery of shRNA provides optimal gene knockdown with minimal side effects, permitting conclusive target validation studies to be conducted with novel targets for diabetes and obesity. |
Conclusions |
| • Delivery of naked siRNA by i.p. injection was welltolerated, but resulted in insufficient target gene knockdown when tested in DIO mice. |
| • Although modestly effective at target gene knockdown, hydrodynamic delivery of siRNA resulted in severe liver damage compromising any interpretation of study results in DIO and ob/ob mice. Degree of damage was correlated with the degree of steatosis in the two models. |
| • Adenoviral vector delivery of JNK1 shRNA via tail vein injection resulted in effective target gene knockdown and no overt toxicities in DIO mice. This RNAi delivery method also proved to be well-tolerated in control experiments with ob/ob mice, making this mode of RNAi delivery the method of choice for target validation studies in these two models of diabetes and obesity. |
Acknowledgements |
| We are grateful to Steven Postl for expert technical assistance in histology analysis, and Dr Regina Reilly, for critical reading of the manuscript and expert advice. Research work reported in the article was funded by Abbott Laboratories. |
Statement Of Compteting Interests |
| Denise Wilcox, Ruojing Yang, Sherry Morgan, Phong Nguyen, Martin Voorbach, Paul Jung, Deanna Haasch, Emily Lin, Eugene Bush, Terry Opgenorth, Peer Jacobson, Christine Collins, Cristina Rondinone, Terry Surowy and Katherine Landschulz, are either employees or potentially own stock and/or hold stock options in Abbott Laboratories. |
List Of Abbreviations |
| DIO; diet induced obese |
| i.p.; intraperitoneal |
| i.v.; intravenous |
| ALT; alanine aminotransferase |
| si; short-interfering |
| sh; short-hairpin |
| JNK1; cJun N-termal kinase |
| Ad; adenovirus |
| GFP; green fluorescence protein |
| AST; aspartate aminotransferase |
| qPCR; quantitative poymerase chain reaction |
References
|