- Biomedical Research (2007) Volume 18, Issue 2
Decrease in the activities of lysosomal enzymes may contribute to the urotoxicity of cyclophosphamide in the rat
Premila Abraham1,*, Indirani Kanakasabapathy2 and Emila Sugumar11Departments of Biochemistry, Christian Medical College, Bagayam, Vellore – 632002, Tamil Nadu, India.
2Departments of Anatomy, Christian Medical College, Bagayam, Vellore – 632002, Tamil Nadu, India.
- *Corresponding Author:
- Premila Abraham
Departments of Biochemistry
Christian Medical College Bagayam
Vellore 632002, Tamil Nadu, India
Tel: 0091-416-2284267, 0416-2284458
Fax: 0416-2262788
E-mail: premilaabraham@yahoo.com; premilaabraham@cmcvellore.ac.in
Accepted date: April 17 2007
Abstract
One of the most common adverse side effects of cyclophosphamide, a potent anticancer drug which limits its clinical utility is its urotoxicity. The mechanism of cyclophosphamide induced urotoxicity (cystitis) is poorly understood. In the present study the possible role of lysosomal enzymes in cyclophosphamide induced hemorrhagic cystitis was investigated using rat model. Adult female Wistar rats weighing 200-250 gm were used for the study. The rats were administered single intraperitoneal injection of cyclophosphamide at the dose of 150 mg/ kg body wt and sacrificed at various time intervals 6 hr, 16 hr or 24 hr after the dose of cyclophosphamide. The control rats were administered saline alone. The urinary bladder was removed, weighed and used for histological examination and assay of lysosomal enzymes namely acid phosphatase, β glucuronidase and N acetyl glucosaminidase. The weight of the urinary bladder (g/ 100 g body wt) of the drug treated rats was significantly higher compared to that of the untreated rat (0.12± 0.01 Vs 0.064 ± 0.005, P≤ 0.004). Histologically, all the characteristic features of hemorrhagic cystitis and hematuria were observed in the drug treated rats. The extent of damage to the bladder increased with time after treatment. A highly significant increase in protein content (mg/ g wt) was observed in the bladders of the cyclophosphamide treated rats as compared with the control (16.4± 3.0 Vs 6.5 ± 1.7, P ≤0.009). A significant drastic decrease in the activities of the entire lysosomal enzyme studied was observed in the bladders of drug treated rats at 16 hrs and 24 hrs. Acid phosphatase activity was decreased by 437% (P≤0.004); N acetyl glucosaminidase activity was decreased by 403% (P≤ 0.05) and glucuronidase activity by 392% (P≤ 0.009) as compared with the control.Decrease in the activities of lysosomal enzymes in the urinary bladder of cyclophosphamide treated rat may contribute to the urotoxicity of cyclophosphamide.
Keywords
Cyclophosphamide; urotoxicity; lysosomal enzymes; protein content; rat
Introduction
Cyclophosphamide (CYP) is a drug with a wide spectrum of clinical uses, and it has been proved to be effective in the treatment of cancer and nonmalignant disease states. However, this drug may induce acute inflammation of the urinary bladder (cystitis) and therefore the therapeutic use of CYP is limited [1,2]. Cystitis is manifested by marked congestion, edema, extravasation of the urinary bladder, as well as marked desquamative damage to the urothium, severe inflammation in the lamina propria and focal erosions.
Although there are some preliminary clinical studies and animal studies [3-5], there does not appear to be an extensive examination of mechanism of cystitis induced by cyclophosphamide. It is important to find out the mechanism by which CYP produces cystitis in order to prevent/ treat the patients who develop cystitis as a result of CYP therapy.
Cyclophosphamide is cytotoxic to the transitional epithelium lining the urinary bladder, and also causes hemorrhage from the sub epithelial blood capillaries. Under normal conditions, the main function of the transitional epithelium is to act as protective lining to the bladder. The normal epithelium contains variable number of lysosomes, which are most numerous in the superficial cells. By electron microscopy, vesicular or heterogeneously dense lysosomes have been demonstrated in rat and human epithelial cells; it has also been suggested that epithelial cells actively form autophagolysosomes [6]. Electron microscopic investigations of the cyclophosphamide-induced lesions of the urinary bladder of the rat suggest that lysososomes may be involved in its urotoxic effect [7]. Acid phosphatase activity [8,9] and glucuronidase activity [10] have been demonstrated in these lysosomes and their normal function in the superficial cells is to breakdown surplus or damaged plasma membrane [9].
Lysosomes contain hydrolases that breakdown unwanted macromolecules including proteins. Therefore decrease in the activities of lysosomal enzymes can result in the accumulation of abnormal amounts of proteins and other macromlecules within the bladder causing damage.
Lysosomes are reported also to play a role in cell death and tissue damage due to drugs e.g. paracetamol or gentamicin [11-14] and toxins [15,16]. We hypothesised that cyclophosphamide induced urotoxicity may be due to alteration in function of the lysosomal enzymes. Thus far there are no studies reported on the role of lysosomal enzymes in cyclophosphamide induced urinary bladder damage to the best of our knowledge.
The rat is a suitable model for the study of urinary bladder toxicity of the chemotherapeutic agent because after the administration of CYP, the rats present with histological evidence of urinary bladder injury similar to that seen in humans [17,18].Therefore, in the present study we investigated whether there is any alteration in the activities of the lysosomal enzymes in the urinary bladder of rats after treatment with cyclophosphamide.
Materials and Methods
Cyclophosphamide (Endoxan) was purchased from Baxter oncology GmbH, Germany. All the other chemicals were obtained from Sigma Chemical Company U, S, A.
Animals: Adult Female Wistar rats (180-220g) were used for the experiments. The study was approved by animal ethics Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA), Government of India. The guidelines were followed.
Animal treatment
Dosage and route of administration of cyclophosphamide were determined from those described in literature as causing consistent cystitis in normal rats [17]. The rats were divided into two groups and treated as follows.
Group I: The rats in this group (n = 21) received single intraperitoneal injection of cyclophosphamide in saline at the dose of 150 mg/ kg body weight.
Group II: The rats in this group (n=6) received saline alone. Rats were killed 6 hours, 16 hours or 24 hours after the dose of CYP or vehicle.
Tissue procurement
Rats were killed by exsanguination. The urinary bladder was removed and blotted dry before weighing. A part of the bladder was used for biochemical assays and another part for histological assessment.
Histology
The dissected bladder were fixed overnight in buffered neutral formalin, processed to paraffin wax, sectioned at 3 to 4 μm, and stained with haematoxylin and eosin for examination by light microscopy.
Biochemical assays
Urinary bladder homogenates was prepared as described by Kyaw et al. [19]. Portions of urinary bladder were homogenized in 10 ml of ice cold 0.25 M sucrose-1mM Na2EDTA in a Potter-Elvehjem homogeniser. The homogenate was centrifuged at 4 °C at 11,000 x g for 30 minutes to remove unlysed particles. The supernatant was used for the assay of glucuronidase [20], N acetyl glucosaminidase [20], acid phosphatase [21] and protein content [22].Enzyme activities are expressed as milliunit per gm weight of the bladder.
Statistical Analysis
The results are expressed as mean ± S.D. Comparison between groups was done using Mann-Whitney U test. A P value of ≤ 0.05 was considered as statistically significant.
Results
Histology:
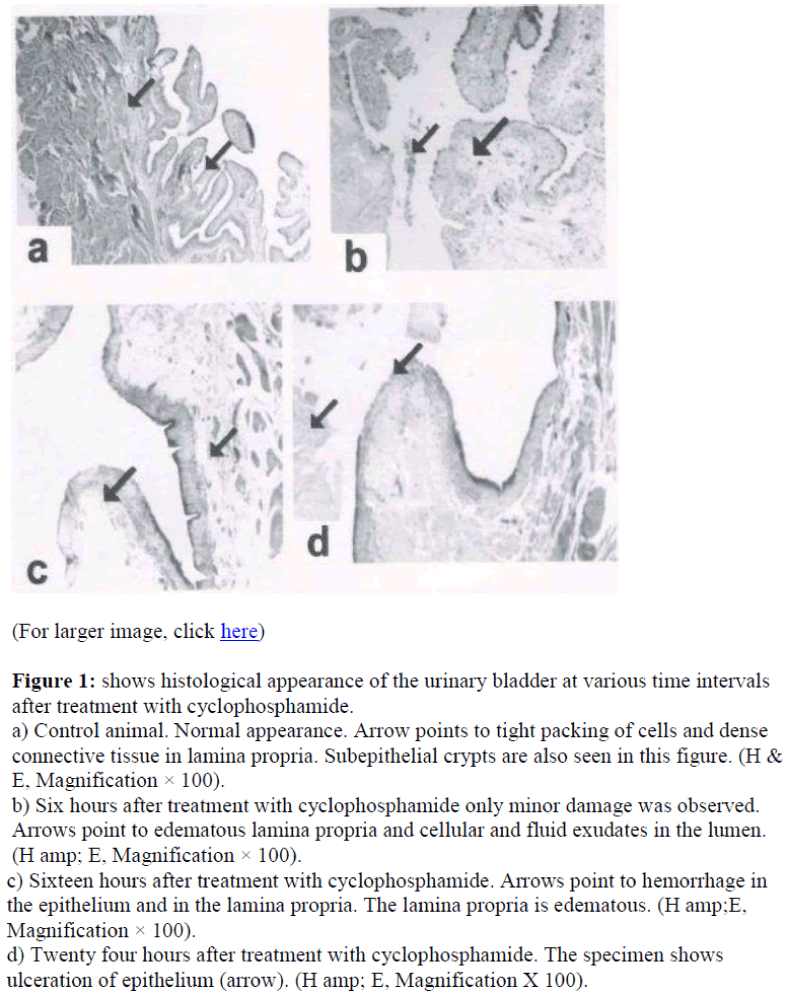
Figure 1 shows the histology of the urinary bladder of control rats and cyclophosphamide treated rat. In control animal, the urinary bladder had normal morphology (Figure 1a).
Figure 1: shows histological appearance of the urinary bladder at various time intervals after treatment with cyclophosphamide.
a) Control animal. Normal appearance. Arrow points to tight packing of cells and dense connective tissue in lamina propria. Subepithelial crypts are also seen in this figure. (H & E, Magnification × 100).
b) Six hours after treatment with cyclophosphamide only minor damage was observed. Arrows point to edematous lamina propria and cellular and fluid exudates in the lumen. (H amp; E, Magnification × 100).
c) Sixteen hours after treatment with cyclophosphamide. Arrows point to hemorrhage in the epithelium and in the lamina propria. The lamina propria is edematous. (H amp;E, Magnification × 100).
d) Twenty four hours after treatment with cyclophosphamide. The specimen shows ulceration of epithelium (arrow). (H amp; E, Magnification X 100).
Contrary to the normal picture presented above, the bladder wall in treated animals showed damages, which became severe with increased time after treatment with the drug (Fig 1). In six-hour case the mucosa become edematous and the cells of the urothelium were not compact. There seemed to be cellular exudates in the lumen. Mucosal content formed follicular cystitis .However hemorrhage was not seen in this group (figure 1b). The condition became worse in sixteen hours, where edema of lamina propria with epithelial and subepithelial hemorrhage was seen (arrows in figure 1c). In animals twenty four hours after treatment with the drug, epithelium showed patches of ulceration (arrow in figure 1d). The lamina propria was edematous and there were exudates in the lumen (arrow in figure 1d).
Biochemical Parameters
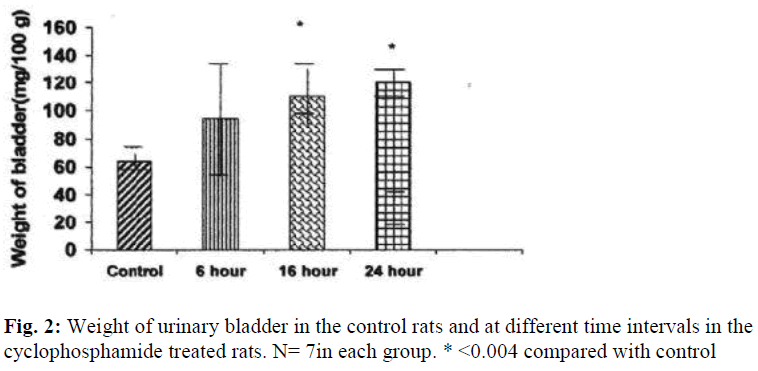
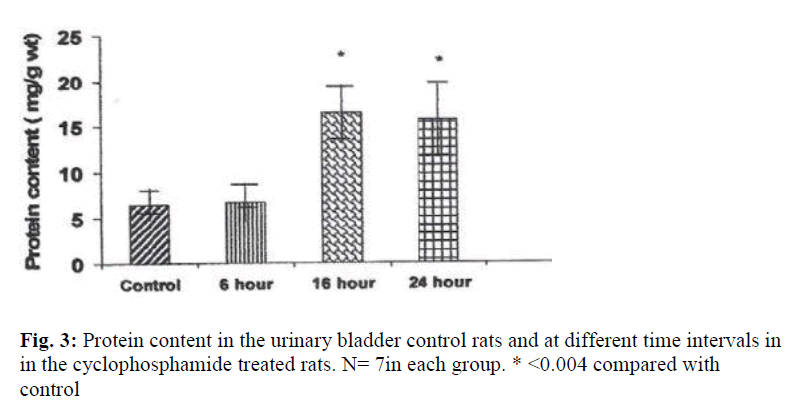
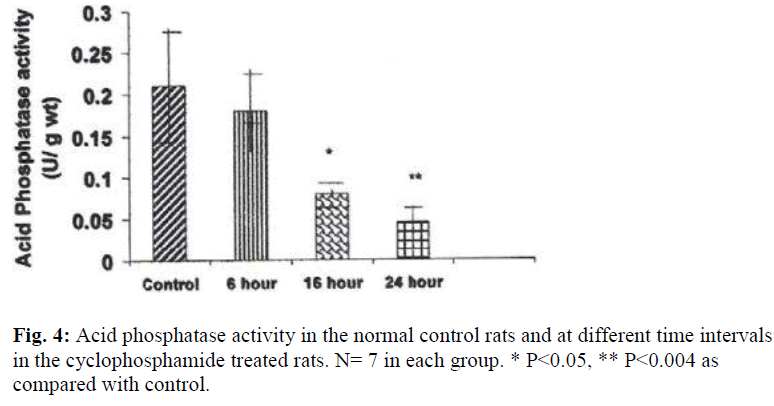
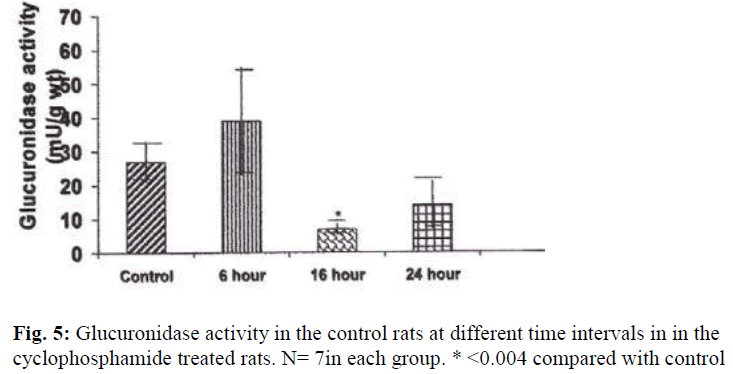
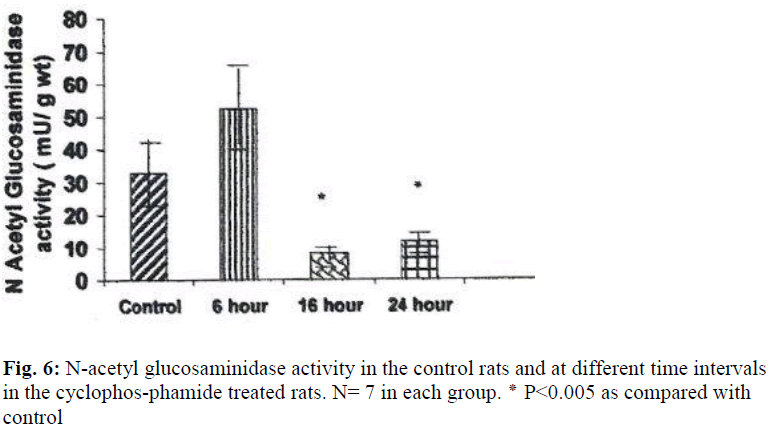
The weights of the urinary bladder, protein content, and activities of the lysosomal enzymes in the CYP treated rat and control rat is shown in the figures 2-6. Since the protein content in the bladders of CYP treated rats were much higher than that of control rats, the activities of the enzymes are expressed here as mU or U per gm weight of the urinary bladder.
The weight of the urinary bladder of the drug treated rats was increased by 172% (P≤ 0.004) at 16 hours and by 185% (P≤ 0.004) at 24 hours as compared to that of the control rat (Fig.2).
A highly significant increase in protein content was observed in the bladders of CYP treated rats at 16 hours and 24 hours after treatment. Protein content was increased by 252% at 16 hour and by 240% at 24 hour (Fig.3).
A significant decrease in the activities of lysosomal enzymes was observed in the bladders of drug treated rats at 16 hrs and 24 hrs. Acid phosphatase activity was decreased by 263% at 16hr (P≤ 0.05) and by 437% (P≤ 0.004) at 24 hr after treatment with CYP (Fig. 4). With regard to glucuronidase activity, the activity was decreased by 253% (P≤ 0.009) at 16 hours and by 51% at 24 hours by as compared with the control (Fig.5).
N acetyl glucosaminidase activity was decreased by 403% (P ≤ 0.05) at 16 hours and by 275%(P ≤ 0.05) at 24 hours (Fig. 6). An increase in the activities of N acetyl glucosaminidase and glucuronidase was observed in the urinary bladders of cyclophosphamide treated rats 6 hour after treatment, but the increase was not statistically significant.
Discussion
Significant increase in weight of the urinary bladder and protein content was observed in the cyclophosphamide treated rats. In general, the levels of proteins within the cells are determined not only by rates of synthesis, but also by rates of degradation. Therefore, increased protein content in the urinary bladder may be due to a decrease in cell protein degradation or an increase in protein synthesis. Normally proteins are degraded by two major path ways1. Lysosomal proteolysis and 2. ubiquitin-proteosome pathway. Lysosomes which are major machinery for recycling intra and extracellular material have long been regarded as “suicide bags” [23]. In the present study, protein accumulation in the urinary bladder is associated with significant decrease in the activities of all the lysosomal enzymes that were studied at 16 hours and 24 hours after treatment with cyclophosphamide, suggesting that lysosomal dysfunction may contribute to accumulation abnormal amounts of proteins and hence cyclophos-phamide induced damage to the urinary bladder.
Cyclophosphamide treatment results in hemorrhage from sub epithelial capillaries, and many erythrocytes which pass into the epithelium are phagocytosed by the epithelium [24]. In tissues where erythrophagocytois normally takes place, e.g. the spleen, liver , bone marrow and lymph nodes [25], the red blood cells are engulfed by the phagocytic reticuloendothelial cells and subsequently digested by them [26,27]. Two methods of digestion have been described in these tissues: the first one is by fusion of lysosomes with the phagocytic vacuole with the subsequent lysis of the erythrocytes [25] and the second one by fragmention of the ingested red blood cells followed by the subsequent breakdown of the fragments [26]. The lysosomal pathway of digestion of red blood cells plays a main role in the clearance of engulfed red blood cells. Therefore, the increased red blood cells observed in the urinary bladder of cyclophosphamide treated rats of the present study may be due to lack of the degrading enzymes of lysosomal origin.
The mechanism of cyclophosphamide induced urotoxicity appears to be different from that of cyclophosphamide induced hepatotoxicity. Cyclophosphamide induces progressive damage to hepatocyte membranes by the destabilization of lysosomal membrane and activation of lysosomal enzymes [28] while in the urinary bladder it appears to cause decrease in the activities of lysosomal enzymes, as has been revealed in the present study.
We suggest that the urotoxicity of cyclophosphamide may be attributed at least in part to the decrease in the activities of lysosomal enzymes thereby (1). Decreasing the degradation of proteins leading to accumulation of proteins in abnormal amounts (2). Decreasing digestion of the red blood cells in the urinary bladder transitional epithelium.
It is concluded that cyclophophamide induced damage to the urinary bladder may be due at least in part to the decreased activities of the lysosomal enzymes. In future we intend to study the mechanism by which cyclophosphamide causes decrease in the activities of lysosomal enzymes in the urinary bladder and, once the mechanism is elucidated it will pave way for the prevention of the damage. The results of the present study may be useful for researchers who are interested in preventing cystitis a common side effect of cyclophosphamide therapy in patients on cyclophosphamide therapy.
Acknowledgements
The funding by CMC fluid research committee is acknowledged.
References
- Frasier LU, Sarathchandra K, Kehrer JP. Cyclophosphamide toxicity: Characterising and avoiding the problem. Drugs 1991; 42: 781-795.
- Grinberg-Funes D, Sheldon C, Weiss?. The use of prostaglandin F2 for the prophylaxis of cyclophosphamide-induced cystitis in the rat. J Urol 1990; 44: 1500-1504.
- Menetrey D, Bon K, Michiels JF, Lanteri-Minet M. The uroprotection of mesna on cyclophosphamide cystitis in rats. Its consequences on behaviour and brain activities. CR Acad Sci III 1999; 322:505-515.
- Alferi AB and Cubeddu LX. La liberacion de oxido nitrico inducida por ciclofosfamida en la rata puede ser prevenida con un antagonista de los receptors NK1. Proceedings of the XV Congreso Latinoamericano de Farmacologia, VI Congreso Interamericando de Farmacologia Clinica y Terapeutica 1997: Cartagena de Indias, Colombia. Abraham/ Kanakasabapathy/ Sugumar
- Alferi AB, Gardner CJ. The NKI antagonist, GR203-040, inhibits cyclophosphamide-induced damage in the rat and ferret bladder. Gen Pharmacol 1997; 29: 245-250.
- Tokunaga H, Waguri S, Sato N, Ohsawa Y, Banya Y, Kominami E, Uchiyama Y. Lysosomal cysteine and aspartic proteinases and ubiquitin in rat and human urinary bladder epithelium. Arch Histol Cytol. 1996; 59: 249-260.
- Themann H, Oberdorf E, Brock N, Pohl J. Electron microscopic investigations of the cyclophosphamide-induced lesions of the urinary bladder of the rat and their prevention by mesna. Urol Int. 1987;42: 37-43
- Novikoff AB. Lysosomes and related particles. In the cell, vol 2 (ed.J Brachet & AE Mirsky), New York and London; Academic Press, 1961, pp 423-488.
- Hicks RM. The function of the Golgi complex in transitional epithelium. Synthesis of the thick cell membrane. J Cell Biol 1966; 30: 623-643.
- Kanzack NM, Krall JI, Hayes ER l. Lysosomal fractions from transitional epithelium. J Cell Biol 1965; 24: 259-266.
- Khandkar MA, Parmar DV. Is activation of lysosomal enzymes responsible for paracetamol induced hepato-toxicity and nephrotoxicity? J Pharm Pharmacol 1996; 48: 437-440.
- Vengerovaski AI, Saratikov AS. The effect of hepatotoxins on the activity of organelle- specific enzymes and metabolism of liver lipids. Vopr Med Khim 1989; 35: 87-91.
- Whiting PH, Brown PA. The relationship between enzymuria and kidney enzyme activities in experimental gentamicin toxicity. Ren Fail 1996; 18: 899-909.
- Olbricht CJ, Fink M. Alterations in lysosomal enzymes of the proximal tubule in gentamicin neprotoxicity. Kidney Int 1991; 39: 639-646.
- Dianzani MU. Lysosome changes in liver injury. In: Ciba Foundation Symposium on Lysosomes. Eds. A.V. S. deReuck and MP Cameron, Little, Brown and Co., Boston. 1963; 335-351
- Younes M, Albrecht M, Siegers CP. Interrelation between lipid peroxidation and lysosomal enzyme release in the presence of carbon tetrachloride, cumene hydroperoxide or thioacetamide. J Appl Pharmacol 1983; 40: 121-132.
- Ahluwalia A, Maggi CA, Santiccioli P, Lecci A, Giuliani S. Characterisation of the Capsaicin-sensitive component of cyclophosphamide-induced inflammation in the rat urinary Bladder. Br J Pharmacol 1994; 111: 1017-1022.
- Abd-Allah AR, Gado IS, Al-Majed AAA, Al-Yahya AA, Al-Shabanah OA. Protective effect of taurine against cyclophosphamide-induced urinary bladder toxicity in rats. Clin Exp Pharmacol Physiol 2005; 32: 167-72.
- Kyaw AA, Aung T, Htut T. Lysosomal enzymes activities in normals and in patients with chronic liver disease. Clin Chim Acta 1983; 131: 317-323.
- Kawai Y, Anno K. Mucopolysaccharide-degrading enzymes from the liver of the squid, Ommastrephes sloami pacificus I. Hyaluronidase. Biochim Biophys Acta 1971; 242: 428-436.
- Rosenblit PD, Metzger RP, Wick AN. Effect of strepto-zotocin diabetes on acid phosphatase and selected glycosidase activities of serum and various rat organs. Proc Soc Exp Biol Med 1974; 145: 244-248.
- Lowry OH, Rosebrough MJ, Farr AL. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- DeDuveC, WattiauxR. Functions of lysosomes. Annu Rev Physiol. 1966;28: 435-439
- Wakefield JJ, Hicks RM. Erythrophagocytosis by the epithelial cells of the bladder. J Cell Sci 1974; 15: 555-573.
- 25. Simon GT, Burke JS. Electron microscopy of the spleen. III. Erythro-leucophagocytosis. Am J Path 1970; 58: 451-470.
- Marton PF. Erythrophagocytosis in the human bone marrow. Scand J Haemat 1970; 7: 177.
- Edwards VD, Simon GT. Ultrastructural aspects of red cell destruction in the normal rat spleen. J Ultrastruct Res 1970; 33:187-201.
- Grek OR, Mishenina SV, Pupyshev AB. Protective effect of enterosgel on rat liver lysosomes during cytostatic treatment. Bull Exp Biol Med. 2002; 134: 355-358.