Research Article - Biomedical Research (2017) Volume 28, Issue 11
Correlation between cerebral damage due to hypoglycemia in new-born and spasms in infancy
Tianming Jia*, Ling Gan, Xiaoli Zhang, Kaixian Du, Yan Dong, Shuai Liu, Li Shen and Miaomiao Liu
Department of Pediatric Neurology, the Third Affiliated Hospital of Zhengzhou University, Zhengzhou, PR China
- *Corresponding Author:
- Tianming Jia
Department of Pediatric Neurology
Third Affiliated Hospital of Zhengzhou University, PR China
Accepted date: March 25, 2017
Abstract
This study aimed to investigate the clinical features, imaging findings, electrocardiogram characteristics, treatment and prognosis of infantile spasms children with hypoglycemic cerebral damage in neonatal period, and the correlation between hypoglycemic cerebral damage and infantile spasms. One hundred and six children with infantile spasms including 21 cases with history of hypoglycemic cerebral damage in neonatal period were enrolled in this study. One hundred and six children patients without infantile spasms were selected as control. The general information and electrocardiogram and imaging data of 21 cases with history of hypoglycemic cerebral damage were collected. The single variable logistic regression analysis was performed to investigate the risk factors of infantile spasms in 212 children. In 21 infantile spasms children with history of hypoglycemic cerebral damage, the age of hypoglycemia onset was 1-8 days (3.26 ± 1.73 days). The blood glucose level was 0.2-2.1 mM/L (1.20 ± 0.54 mM/L). The duration of hypoglycemia was 2 h-5 d. The age of infantile spasms onset was 0.5-30 months (7.21 ± 6.68 months). Sixteen children (76.19%) had obvious imaging performance of occipital softening or glial scar. The control rate was 4.76% after 6 months of treatment using steroid hormone combined with antiepileptic drugs. The single variable logistic regression analysis showed that the neonatal hypoglycemia was a risk factor of infantile spasms in 212 children. The imaging of hypoglycemic cerebral damage is mainly manifested with occipital softening or glial scar. The occipital lobe injury induced by neonatal hypoglycemia can easily cause the infantile spasms. The neonatal hypoglycemia is a risk factor of infantile spasms.
Keywords
Spasms, Infancy, Cerebral damage, Hypoglycemia, Imaging
Introduction
Neonatal hypoglycemia is a common disease. The occurrence rate of hypoglycemia in healthy full-term child is 1%-5%, and that in small gestational-age and preterm children is 15%-25% [1]. Severe hypoglycemia can cause brain cell death, leading to irreversible brain dysfunction [2]. Harris et al. [3] find that, the neurologic dysfunction caused by hypoglycemia mainly includes the attack of difficulty in motion development, vision, learning and behavior and long-term epilepsy. Infantile spasms are the catastrophic epilepsy syndrome, and the typically characterized seizures include nodding- or huddle-like spasms, Electrocardiogram (ECG) hypsarrhythmia and psychomotor development delay. The prognosis of this disease is poor, which can lead to severe neurocognitive dysfunction [4]. The cause of infantile spasms is not very clear. The patients with infantile spasms are divided into symptomatic cases and cryptogenic cases, and the symptomatic cases account for 60%-85% [5]. Currently, there are more than 200 known etiological factors for infantile spasms, including perinatal cerebral damage, congenital brain abnormality, brain trauma, intracranial hemorrhage, and genetic metabolic diseases (including tuberous sclerosis), chromosome abnormalities (including Down syndrome) and infections [6]. In this study, we analysed the clinical features, imaging findings, EEG characteristics, treatment and prognosis of infantile spasms children with history of hypoglycemic cerebral damage in neonatal period. The objective was to provide a basis for prevention and treatment of this disease.
Subjects and Methods
Subjects
One hundred and six children diagnosed with infantile spasms from July 2013 to April 2015 in the Third Affiliated Hospital of Zhengzhou University were selected. The data of the perinatal period and growth and development were collected. The corresponding physical examination and laboratory tests, including imaging (brain MRI or CT), EEG, blood biochemistry and metabolic screening were performed. Finally 21 children with early hypoglycemia brain injury were screened. In these 21 patients, there were 13 males and 8 females. The birth weight was 2.200-4.950 kg, with average 3.106 kg. The gestational age was 37-41 weeks (including 1 case of twins). All 21 patients had no sepsis, intracranial infection, electrolyte imbalance or genetic metabolic disease. There were 3 cases of hypoglycemia complicated with asphyxia. One hundred and six children patients without infantile spasms in our hospital were selected as control for investigating the hypoglycemia history. This study was approved by the ethics committee of The Third Affiliated Hospital of Zhengzhou University. Written informed consent was obtained from the family of all patients.
Inclusion criteria of hypoglycemia
The inclusion criteria of hypoglycemia were as follows: i) The blood glucose level within 24 h after birth was less than 2.2 mM/L, and that after 24 h was less than 2.2-2.8 mM/L [4]; ii) The patients had one or more neonatal hypoglycemia manifestations including low reaction weakness, paroxysmal cyanosis, convulsions, irritability, apnea, lethargy, refusing milk, and so on.
Diagnostic criteria of hypoglycemic cerebral damage
The diagnostic criteria of hypoglycemic cerebral damage were as follows: (i) The patients had clinical non-specific symptoms of hypoglycemia, or had severe hypoglycemia (0-1.7 mM/L) history; (ii) The blood glucose level was less than 2.0 mM/L; (iii) The neurological dysfunction (coma, convulsions, etc.) appeared during the period of hypoglycemia or after a period from hypoglycemia correction; (iv) The skull imaging examination or brain function examination showed changes in brain structure or function; (v) The brain injury caused by hypoxic-ischemic cerebropathia, severe infection or abnormal brain development was excluded.
Statistical analysis
All statistical analysis was carried out using SPSS17.0 software (SPSS Inc., Chicago, IL, USA). Data were presented with descriptive statistics including mean, median, and standard deviation. The single variable logistic regression analysis was performed to investigate the risk factors of infantile spasms in children patients.
Results
General information of 21 infantile spasms children with history of hypoglycemic cerebral damage
In 21 infantile spasms children with history of hypoglycemic cerebral damage, the age of hypoglycemia onset was 1-8 days (3.26 ± 1.73 days). The blood glucose level was 0.2-2.1 mM/L (1.20 ± 0.54 mM/L). The duration of hypoglycemia was 2 h-5 d. The age of infantile spasms onset was 0.5-30 months (7.21 ± 6.68 months). The development situation included poor motion coordination, language lag, motion development lag, dull eyes, no language, cortical blindness, etc. (Table 1 and Figures 1 and 2).
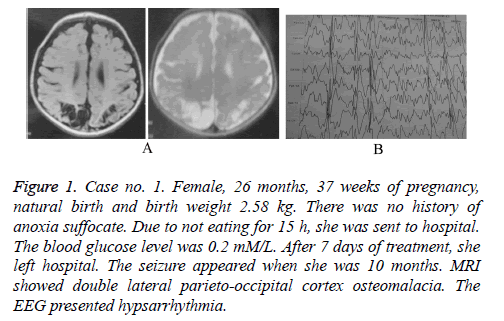
Figure 1: Case no. 1. Female, 26 months, 37 weeks of pregnancy, natural birth and birth weight 2.58 kg. There was no history of anoxia suffocate. Due to not eating for 15 h, she was sent to hospital. The blood glucose level was 0.2 mM/L. After 7 days of treatment, she left hospital. The seizure appeared when she was 10 months. MRI showed double lateral parieto-occipital cortex osteomalacia. The EEG presented hypsarrhythmia.
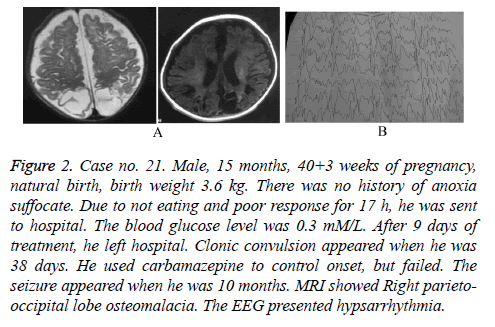
Figure 2: Case no. 21. Male, 15 months, 40+3 weeks of pregnancy, natural birth, birth weight 3.6 kg. There was no history of anoxia suffocate. Due to not eating and poor response for 17 h, he was sent to hospital. The blood glucose level was 0.3 mM/L. After 9 days of treatment, he left hospital. Clonic convulsion appeared when he was 38 days. He used carbamazepine to control onset, but failed. The seizure appeared when he was 10 months. MRI showed Right parietooccipital lobe osteomalacia. The EEG presented hypsarrhythmia.
| No. | Gender | Asphyxia at birth | Age of hypoglycemia onset (days) | Blood glucose (mM/L) | Duration of hypoglycemia | Age of infantile spasms onset (months) |
|---|---|---|---|---|---|---|
| 1 | Female | No | 6 | 0.2 | 7 h | 10 |
| 2 | Male | No | 2 | 1 | 2 h | 2 |
| 3 | Female | No | 8 | 0.6 | 20 h | 10 |
| 4 | Male | Yes | 3 | 1.7 | 7 h | 6 |
| 5 | Male | No | 2 | 2.1 | Unknown | 3 |
| 6 | Male | No | 1 | 1.1 | 16 h | 9 |
| 7 | Female | No | 3 | 1.8 | 3 h | 30 |
| 8 | Male | No | 3 | 1 | 2 h | 4 |
| 9 | Male | No | 4 | 1.2 | 5 d | 1 |
| 10 | Female | No | 4 | 0.6 | 3 d | 13 |
| 11 | Female | Yes | 2 | 0.8 | 14 h | 5 |
| 12 | Male | No | 5 | 1.1 | 10 h | 5 |
| 13 | Male | Yes | 1 | 1.3 | 15 h | 3 |
| 14 | Female | No | 3 | 1.3 | Unknown | 2 |
| 15 | Male | No | 2 | 1 | 20 h | 4 |
| 16 | Female | No | 1 | 1.8 | Unknown | 12 |
| 17 | Male | No | 3 | 2 | Unknown | 7 |
| 18 | Female | No | 4 | 1.8 | Unknown | 14 |
| 19 | Male | No | 2 | 1.5 | Unknown | 0.5 |
| 20 | Male | No | 2 | 1.1 | Unknown | 1 |
| 21 | Male | No | 2 | 0.3 | 17 h | 10 |
Table 1. General information of patients.
Imaging and EEG results of 21 infantile spasms children with history of hypoglycemic cerebral damage
In 21 infantile spasms children with history of hypoglycemic cerebral damage, 20 cases were performed with routine cranial MRI examination and 1 case had routine head CT examination. The age of examination was 4-60 months (median 22 months). Sixteen cases (76.19%) exhibited occipital cortex and cortical softening or glial scar; two cases exhibited the abnormal signals in frontal and parietal lobe; one case showed mild cerebral atrophy; one case showed less white matter than normal; one case showed abnormal signal adjacent lateral ventricle before and after angle. The EEG for twenty-one children with infantile spasms occurrence showed high hypsarrhythmia. After treatment, nine cases showed high hypsarrhythmia; seven cases exhibited epilepsy wave distribution in the occipital lobe; four cases showed full breadth of high-amplitude slow wave distribution; one case showed normal EEG (Table 2).
| No. | Brain MRI or CT | EEG |
|---|---|---|
| 1 | Double lateral parieto-occipital cortex osteomalacia | Hypsarrhythmia |
| 2 | Mild cerebral atrophy | Hypsarrhythmia |
| 3 | Parieto-occipital cortex atrophy | Occipital sharp and spine wave |
| 4 | Bilateral parieto-occipital atrophy associated with osteomalacia coordination difficulty | Hypsarrhythmia |
| 5 | Bilateral parieto-occipital flake osteomalacia | Hypsarrhythmia |
| 6 | Mild hydrocephalu; metabolites of frontal lobe and occipital lobe on the left above the right | Hypsarrhythmia |
| 7 | Bilateral parieto-occipital atrophy; rear corpus callosum atrophy | Generalized slow wave |
| 8 | Right occipital lobe osteomalacia | Paroxysmal slow and sharp wave |
| 9 | Less white matter | Occipital spine slow wave |
| 10 | Bilateral parieto-occipital atrophy | Occipital sharp wave |
| 11 | Occipital lobe softening | Occipital sharp and slow wave |
| 12 | Bilateral occipital lobe osteomalacia | Occipital sharp and slow wave |
| 13 | Bilateral parieto-occipital atrophy | Paroxysmal slow and sharp wave |
| 14 | Bilateral temporo-parietal cortex osteomalacia | Hypsarrhythmia |
| 15 | Bilateral parieto-occipital atrophy | Sharp and spine wave |
| 16 | Abnormal white matter signal of next to the anterior and posterior horns of the lateral ventricle | Hypsarrhythmia |
| 17 | Bilateral parieto-occipital atrophy | Paroxysmal occipital slow and sharp wave |
| 18 | Bilateral parieto-occipital anomaly signal | Left occipital sharp and spine wave |
| 19 | Bilateral parieto-occipital atrophy | Hypsarrhythmia |
| 20 | Bilateral temporal-parietal lobe anomaly signal | Normal |
| 21 | Right parieto-occipital lobe osteomalacia | Hypsarrhythmia |
Table 2. Imaging and EEG results.
Treatment and prognosis of 21 infantile spasms children with history of hypoglycemic cerebral damage
In 21 infantile spasms children with history of hypoglycemic cerebral damage, the follow-up time for 20 patients was 6 months or more, with 3 months for 1 case. In all cases, only 1 case received combined oral topiramate and levetiracetam treatment after steroid pulse therapy, and obtained a long-term control of disease. The control rate of 21 cases was 4.76%. The remaining 20 patients were treated with 3-9 kinds of drug for 4-53 months. The drugs were mainly steroids and anti-epileptic drugs, including methylprednisolone, adrenocorticotropic hormone, prednisone, vigabatrin, prop sodium valproate, topiramate, levetiracetam, clonazepam, carbamazepine, lamotrigine. However, the disease did not achieve full control, and the cognitive, motion coordination and language development of patients were poor. Two patients showed cortical blindness (Table 3).
| No. | Therapeutic drugs | Development situation |
|---|---|---|
| 1 | GCS VPA TPM | Poor motion coordination, language lag |
| 2 | GCS VPA TPM CZP | Motion development lag |
| 3 | VPA GCS TPM | Coordination difficulty, language lag |
| 4 | GCS VPA LEV LTG CBZ KD | Coordination difficulty, language lag |
| 5 | GCS LEV TPM VPA | Motion development lag, dull eyes |
| 6 | GCS VPA CZP | Psychomotor retardation |
| 7 | GCS VPA LEV CZP | Normal motion development, poor intelligence |
| 8 | GCS VPA TPM CZP LEV VGB | Coordination difficulty, no language |
| 9 | GCS VPA TPM LTG CZP | Coordination difficulty, language lag |
| 10 | GCS VPA TPM LTG LEV KD PB | Coordination difficulty, poor intelligence |
| 11 | GCS VPA LEV | Normal motion development, language lag, cortical blindness |
| 12 | GCS VPA LEV | Motion and language development lag |
| 13 | GCS LEV VPA TPM | Motion and language development lag |
| 14 | GCS VPA | Motion and understanding lag |
| 15 | GCS TPM LEV | Normal motion development, language lag |
| 16 | GCS VPA TPM | Normal motion development, language lag |
| 17 | GCS CBZ PB LEV | Coordination difficulty, language lag, cortical blindness |
| 18 | GCS VPA TPM | Coordination difficulty, language lag |
| 19 | GCS CZP TPM VPA | Motion development lag |
| 20 | GCS VPA TPM VPA | Poor coordination |
| 21 | GCS CBZ LEV CZP | Motion and intelligence lag |
Table 3. Treatment and prognosis.
Results of logistic regression analysis on 212 children
In 106 children with infantile spasms, there were 21 cases with hypoglycemia and 85 cases without hypoglycemia. In 106 children patients without infantile spasms, there were 10 cases with hypoglycemia and 96 cases without hypoglycemia. In 212 children, the single variable logistic regression analysis showed the regression equation was as follows: logit P=-2.26+0.864X (X represented hypoglycemia). After the regression hypothesis test, the Wald statistic index χ2 was 4.393, with P value of 0.036, which indicating that the regression equation had statistical significance. Using non-hypoglycemia as control, the odds ratio of children with hypoglycemia for infantile spasms was 2.372 (1.058, 5.319). This indicated that the hypoglycemia was a risk factor of infantile spasms in children.
Discussion
Infantile spasms are a special type of epilepsy syndrome, of which the pathogenesis is unknown. Hamano et al. [7] have performed a retrospective study on epileptic patients and find that, the damage of occipital region may be related to the occurrence of infantile spasms, and EEG spike wave discharge of infantile spasms children is also easy to involve the occipital region. Endoh et al. [8] have analysed the EEG data of 25 cases of symptomatic infantile spasms before onset and find that, the early epileptic discharge is more common in the occipital region. This suggests that, the early damage of the occipital region is related to the occurrence of late infantile spasms, and may evolve to infantile spasms. The brain maturation process is from the occipital region to the forehead area. The myelination of occipital lobe (MRI) starts at 3 to 5 months, which is earlier than other parts of the brain. Three months after birth or so is a critical period of occipital mature, and the peak age of onset of infantile spasms is 4-7 months. Therefore, the occipital lobe epilepsy-like discharge may be related to the occipital cortex maturation. It is found that, the EEG of symptomatic infantile spasms, especially for infants with perinatal spasms, is easier to present discharge in occipital region than cryptogenic infantile spasms. Udani et al. [9] noted that, in children with epilepsy symptoms occurrence before the age of 3 years, the neonatal hypoglycemia is the most common cause of infantile spasms, and the infantile spasms is the most common seizure type. Kumaran et al. [10] have reported 5 cases of children with infantile spasms seizures caused by hyperinsulinemic hypoglycemia and suggest that, the hypoglycemia cerebral damage and infantile spasms may have correction to some degree.
In this study, we have conducted the etiology survey on 106 cases of infantile spasms in nearly two years, and there are 21 cases of children can be traced with a clear history of brain injury, accounting for 19.81%. This further confirms the relationship between hypoglycemia cerebral damage and infantile spasms. Occipital lobe is the visual center, and many cases of seizures have photosensitivity. The brain MRI of photosensitive epilepsy shows that, the occipital cortex sense has a high sensitivity to light [11]. Ferlazzo et al. [12] found that, the visual cortex has too high excitability when subjected to receiving photosensitive stimulation. Porciatti et al. [13] believe that, the visual center damage may have special reaction to photosensitive stimulation, leading to seizures. Therefore, the occipital injury induced by neonatal hypoglycemia prone to infantile spasms may be due to that, the occipital lobe of the brain in infancy is the most light-sensitive region to accept external stimulation [8]. The incubation period of infantile spasms caused by hypoglycemic cerebral damage in children is 0.5-30 months. It is difficult to predict the time of occurrence of infantile spasms for children with hypoglycemia brain injury. Therefore, the periodic EEG examination is necessary for children with hypoglycemic brain injury.
Glucose is the only energy source for a long time for the brain development in neonatal period. The long-term hypoglycemia may cause irreversible damage to nerve cells [14]. There are many causes for hypoglycemia. From 21 infantile spasms children with history of hypoglycemic cerebral damage, the main reason for hypoglycemia is late open milk, poor sucking and improper management. The traditional view suggests that, the gestational age birth through normal healthy full-term children do not need routine screening and monitoring of blood glucose. The new-borns with high risk factors should be monitored with blood glucose within the first 24 h after birth [15]. In these 21 cases, the time of hypoglycemia onset is 1-8 days after birth. There are 3 cases with risk factors. This prompts us to pay close attention to the blood glucose of children who have no obvious risk factor level or those who have 1 day age.
In this study, the MRI, CT and EEG of these 21 infantile spasms children with history of hypoglycemic cerebral damage show that, the head MRI and CT are mainly manifested with parietal occipital softening and glial scar, accounting for 76.19%, including 3 cases with neonatal period hypoxia asphyxia. However, the relevant literature finds that, the skull imaging of cerebral damage caused by lack of oxygen is mainly manifested with the lesion in watershed area and periventricular white matter, which generally does not cause characteristic occipital injury [16]. Therefore, these 3 cases are attributed to hypoglycemic cerebral damage. In EEG, the hypsarrhythmia and occipital dominated epilepsy wave distribution after treatment are the most common, which is consistent with previous literatures [7,17]. The reason of the special vulnerability of occipital region to hypoglycemia is unknown. It is generally believed that, this susceptibility is related to occipital lobe, which is the most productive part for neonatal axonal growth and synapse formation [18]. However, we believe that, this vulnerability may be related to that the occipital lobe is the earliest region for cerebral cortex development [8]. In this study, the treatment and 6 months of follow-up of 21 children show that, only 1 case obtains longterm control, with control rate of 4.76%. This is far less than the long-term (≥ 6 months) control rate (24.52%) of infantile spasms children with the same period of hospitalization.
In conclusion, the neonatal hypoglycemia is a risk factor of infantile spasms in children. The typical performance of hypoglycemic cerebral damage in imaging is occipital softening or glial scar. The occurrence of infantile spasms may be related to hypoglycemic cerebral damage. These children with infantile spasms due to hypoglycemic cerebral damage have difficult treatment and poor prognosis, compared with children with infantile spasms caused by other factors. That occipital injury induced by neonatal hypoglycemia is prone to infantile spasms. The reason may be that, the occipital lobe is the area in which in infancy the brain receives the most outside signal stimulation and light-sensitive stimulation. For children with hypoglycemic cerebral damage, the regular brain ECG examination should be performed, for early detection of abnormal discharge and timely intervention.
References
- Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol 2006; 33: 853-870.
- Nadeem M, Murray DM, Boylan GB, Dempsey ME, Cornelius A. Early blood glucose profile and neurodevelopmental outcome at two years in neonatal hypoxic-ischaemic encephalopathy. BMC Pediatr 2011; 11: 10.
- Harris L, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. Pediatr 2012; 161: 787-791.
- Kossoff EH. Infantile spasms. Neurologist 2010; 6: 69-75.
- Pellock JM, Hrachovy R, Shinnar S, Baram TZ, Bettis D, Dlugos DJ, Gaillard WD, Gibson PA, Holmes GL, Nordl DR, ODell C, Shields WD, Trevathan E, Wheless JW. Infantile spasms; a U. S. consensus report. Epilepsia 2010; 51: 2175-2189.
- Osborne JP, Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, Verity CM, OCallaghan FJ. The underlying etiology of infantile spasms (West syndrome); information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia 2010; 51: 2168-2174.
- Hamano S, Tanaka M, Kawasaki S. Regional specificity of localized cortical lesions in West syndrome. Pediatr Neurol 2000; 23: 219-224.
- Endoh F, Yoshinaga H, Kobayashi K, Ohtsuka Y. Electroencephalographic changes before the onset of symptomatic West syndrome. Brain Dev 2007; 29: 630-638.
- Udani V, Munot P, Ursekar M, Gupta S. Neonatal hypoglycemic brain: injury a common cause of infantile onset remote symptomatic epilepsy. Indian Pediatr 2009; 46: 127-132.
- Kumaran A, Kar S, Kapoor RR, Hussain K. The clinical problem of hyperinsulinemic hypoglycemia and resultant infantile spasms. Pediatrics 2010; 126: 1231-1236.
- Parra J, Kalitzin SN, Iriarte J, Blanes W, Velis DN, Lopes da Silva FH. Gamma-band phase clustering and photosensitivity; is there an underlying mechanism common to photosensitive epilepsy and visual perception? Brain 2003; 126: 1164-1172.
- FerIazzo E, zitkin BG, Anderman E, Andermann F. Cortical triggers in generalized reflex seizures and epilepsies. Brain 2005; 128: 700-710.
- Porciatti V, Bonanni P, Fiorentini A, Guerrini R. Lack of cortical contrast gain control in human photosensitive epilepsy. Nat Neurosci 2000; 3: 259-263.
- Heinz ER, Provenzale JM. Imaging findings in neonatal hypoxia: a practical review. AJR Am J Roentgenol 2009; 192: 41-47.
- Committee on Fetus and Newborn, Adamkin DH. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011; 127: 575-579.
- Spar JM, Lewine JD, Orrison WW. Neonatal hypoglycemia: CT and MR findings. Am J Neurol Radiol 1994; 15: 1477.
- Alkalay AL, Flores-Samat L, Samat HB, Moser FG, Simmons CF. Brain imaging findings in neonatal hypoglyceilia; case report and review of 23 cases. Clin Pediatr (Phila) 2005; 44: 783-790.
- Lim CC, Gan R, Chan CL, Tan AW, Khoo JJ, Chia SY. Severe hypoglycemia associated with an illegal sexual enhancement product adulterated with glihenclamide: MR imaging findings. Radiology 2009; 250: 193-201.