Case Report - Ophthalmology Case Reports (2021) Volume 5, Issue 2
Congenital homonymous hemianopia and afferent pupillary defect due to concomitant dysplasia of the optic radiation and dorsal midbrain.
Kaori Ueda*, Akiyasu Kanamori, Yuko Yamada-Nakanishi, Makoto Nakamura
Division of Ophthalmology, Department of Surgery, Kobe University Graduate School of Medicine, Kobe, Japan
- Corresponding Author:
- Kaori Ueda
Division of Ophthalmology
Department of Surgery Kobe
University Graduate School of Medicine, Japan
E-mail: kueda@med.kobe-u.ac.jp
Telephone: +81-78-382-6048
Accepted date: February 16 2021
Citation: Ueda K, Kanamori K, Yamada-Nakanishi Y, Nakamura M. Congenital homonymous hemianopia and afferent pupillary defect due to concomitant dysplasia of the optic radiation and dorsal midbrain. Ophthalmol Case Rep. 2021;5(1):1-5
Abstract
Information regarding visual field and light reflex is transmitted through the same afferent pathway until the optic tract; thereafter, the former travels to the occipital lobe via the Lateral Geniculate Nucleus (LGN) and the latter to the efferent pathway through the pretectal area. Therefore, the combination of visual field defect and light reflex abnormalities depends on the locations of the lesion on the visual pathway. We present a rare case of congenital dysplasia of the left-sided optic radiation, dorsal midbrain, and cerebellar hemisphere, with other neurological symptoms. An 18-year-old female showed right-side homonymous hemianopia and Relative Afferent Pupillary Defect (RAPD). Optical Coherence Tomography (OCT) revealed a homonymous hemianopic thinning of the Ganglion Cell Layer (GCC) and the Circumpapillary Retinal Nerve Fiber Layer (cpRNFL) in both eyes. MRI indicated dysplasia of the left dorsal midbrain, optic radiation, and cerebellum. In this case, RAPD was detected in the right eye, but the amount of visual field defect was smaller than that in the left eye, indicating that these symptoms were independently caused by the lesions of the optic radiation and the dorsal midbrain, respectively. OCT findings appeared similar to those of congenital occipital hemianopia, suggesting trans-synaptic axonal degeneration of retinal ganglion cells.
Keywords
Congenital dysplasia, Relative afferent pupillary defect, Homonymous hemianopia, Trans-synaptic degeneration.
Introduction
A pathway that transmits visual fields is similar to an afferent pathway of light reflex from the optic nerve through the chiasm until the optic tract. Next, visual field information is transmitted via the Lateral Geniculate Nucleus (LGN) through the optic radiation to the occipital cortex, whereas the afferent fibers of the Light Reflex terminate at the pretectal nucleus in the midbrain. A brain lesion that damages the unilateral anterior visual pathway usually demonstrates both visual field defects and asymmetrical response of the pupils to the same intensity of light between the eyes, termed a Relative Afferent Pupillary Defect (RAPD). When the lesion is localized at the optic nerve, both the visual field defect and RAPD are present in the eye ipsilateral to the lesion. On the other hand, when the lesion is localized at the optic tract, contralateral homonymous hemianopia and RAPD in the eye with the temporal visual field defect are present. The optic tract damage also produces the characteristic combination of optic atrophy in both eyes, in which atrophy is predominant in the temporal and nasal sides in the contralateral eyes (band or bow-tie atrophy) and predominant in the superior and inferior sides in the ipsilateral eyes (hour-glass atrophy). This combination of optic atrophy patterns is named homonymous hemianopic atrophy.
In typical cases, if the lesion is localized at the posterior visual pathway, i.e., the optic radiation and the occipital cortex, only the contralateral homonymous hemianopia is exhibited. In that case, RAPD is not detected. On the other hand, if the lesion is localized at a unilateral pretectal area, only the RAPD in the eye contralateral to the lesion is exhibited. However, in this case, a visual field defect is not detected.
Congenital dysplasia of the unilateral occipital cortex is known to bear a unique combination of clinical symptoms and signs, known as congenital occipital hemianopia, which comprise the contralateral homonymous hemianopia, RAPD in the eye with the temporal visual field defect, and homonymous hemianopic patterns of optic atrophy in both eyes. These combinations of ocular manifestations are essentially the same as those seen in the case of optic tract damage as described [1]. However, optic atrophy shown in optic tract damage is due to the direct involvement of axons of the Retinal Ganglion Cells (RGCs), whereas the optic atrophy observed in congenital occipital hemianopia is presumed to be due to a trans-synaptic degeneration.
Here, we present a rare case of probable congenital dysplasia of the optic radiation and the dorsal midbrain, which independently produced contralateral-sided homonymous hemianopia, RAPD discordant to visual field defect asymmetry, and homonymous hemianopic optic atrophy in bilateral eyes due to trans-synaptic degeneration.
Case Report
An otherwise healthy 18-year-old female patient was referred to us for diplopia in near vision and rightward gaze for 4 years. She had no history of developmental problems and no other neurological complaints such as vertigo, gait disturbance, or sensory loss. Visual acuity was 20/20 in both eyes. The ocular position was Δ 14 exotropia with a near vision effort and Δ 8 exophoria with a far vision effort. The vertical misalignment was not present. The extent of eye movement was fully preserved with a small-amplitude, horizontal pendular nystagmus with a rightward gaze.
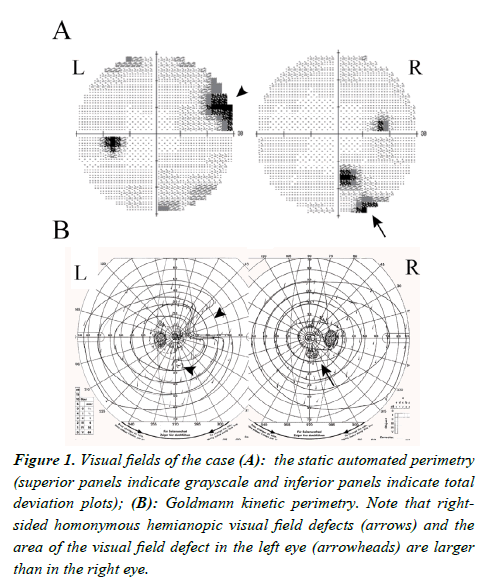
There were no abnormalities of anterior segments and retina. The optic discs were good color without enlargement of cupping. The right eye demonstrated RAPD. Both static and kinetic visual field tests presented a right-sided incongruous homonymous hemianopic decline of visual sensitivity, i.e., the inferotemporal visual field defect in the right eye (arrow) and the nasal upper and inferior to nasal lower areas of visual sensitivity decline in the left eye (arrowheads) (Figure 1). However, she was not aware of the visual field deficit. We noted that the area of visual field defect in the left eye, where the nasal field sensitivity reduction was manifest, was wider than in the right eye, where the temporal visual field defect was detected. Therefore, the RAPD was present in the eye with less damaged visual field defect.
Figure 1: Visual fields of the case (A): the static automated perimetry (superior panels indicate grayscale and inferior panels indicate total deviation plots); (B): Goldmann kinetic perimetry. Note that rightsided homonymous hemianopic visual field defects (arrows) and the area of the visual field defect in the left eye (arrowheads) are larger than in the right eye.
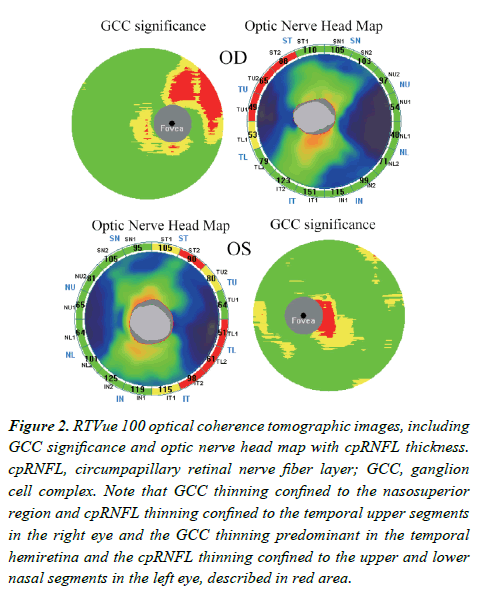
Despite normal-appearing optic discs and retina, RTVue-100 Optical Coherence Tomography (OCT; software version 4.0.5.39; Optovue, Inc., Fremont, CA, USA) demonstrated that both Ganglion Cell Complex (GCC), which comprises nerve fiber, ganglion cell, and inner plexiform layers, and circumpapillary Retinal Nerve Fiber Layer (cpRNFL) were bilaterally reduced compared with the inherent normative database (Figure 2). In other words, GCC thinning was confined to the nasosuperior region, and cpRNFL thinning was confined to the upper temporal segments in the right eye, while in the left eye, GCC thinning was predominant in the temporal hemiretina, and cpRNFL thinning was diverged and confined to the upper and lower temporal segments, all of which corresponded well to the areas of visual field defect.
Figure 2: RTVue 100 optical coherence tomographic images, including GCC significance and optic nerve head map with cpRNFL thickness. cpRNFL, circumpapillary retinal nerve fiber layer; GCC, ganglion cell complex. Note that GCC thinning confined to the nasosuperior region and cpRNFL thinning confined to the temporal upper segments in the right eye and the GCC thinning predominant in the temporal hemiretina and the cpRNFL thinning confined to the upper and lower nasal segments in the left eye, described in red area.
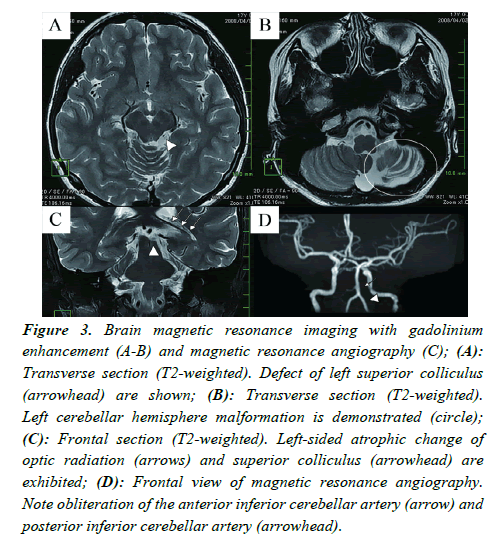
Brain Magnetic Resonance Imaging (MRI) with gadolinium enhancement demonstrated reduced volumes of the left-sided superior colliculus, optic radiation, and cerebellar hemisphere together with increased volumes of surrounding fluid-rich spaces, indicating the atrophic changes of these structures. MR Angiography (MRA) indicated obliteration of the Anterior Inferior Cerebellar Artery (AICA) and reduced delineation of the Posterior Inferior Cerebellar Artery (PICA) in the left side (Figure 3), suggesting the malformation or retardation of these vessels.
Figure 3: Brain magnetic resonance imaging with gadolinium enhancement (A-B) and magnetic resonance angiography (C); (A): Transverse section (T2-weighted). Defect of left superior colliculus (arrowhead) are shown; (B): Transverse section (T2-weighted). Left cerebellar hemisphere malformation is demonstrated (circle); (C): Frontal section (T2-weighted). Left-sided atrophic change of optic radiation (arrows) and superior colliculus (arrowhead) are exhibited; (D): Frontal view of magnetic resonance angiography. Note obliteration of the anterior inferior cerebellar artery (arrow) and posterior inferior cerebellar artery (arrowhead).
Additional neurological symptoms did not appear over 5 years of follow-up.
Discussion
Innate malformation of the Central Nervous System (CNS) that is involved in the visual pathway leads to various types of visual dysfunction and structural changes, among which congenital occipital hemianopia is one of the most investigated conditions. Patients with congenital occipital hemianopia literally exhibit homonymous hemianopia contralateral to the brain lesion, which usually accompanies macular splitting [2]. Hoyt et al. [1] described that patients with congenital occipital hemianopia also demonstrate characteristic patterns of homonymous hemianopia optic atrophy in bilateral eyes, which are similar to those in patients with optic tract syndrome. In other words, an eye contralateral to the lesion showed band or bow-tie atrophy and the other eye ipsilateral to the lesion showed hour-glass atrophy. However, optic atrophy in patients with optic tract syndrome occurs via direct involvement of the RGC axons, whereas atrophy in those with congenital occipital hemianopia is due to the trans-synaptic degeneration of these axons as mentioned earlier.
Patients both with congenital occipital hemianopia and optic tract syndrome usually manifest RAPD in the eye that shows a temporal visual field defect and is contralateral to the brain lesion. This means that the eye in which the nerve fibers originated from the nasal hemiretina and crossed at the chiasm is more influenced than the eye in which the nerve fibers originated from the temporal hemiretina and were uncrossed by those lesions because the crossed axons are reported to be more influenced than the uncrossed ones [3]. The asymmetry of fiber contents was estimated to be 53:47 for nerve fibers subserving the visual field while it was reported to be more (67:33) for those subserving the light reflex.
RAPD could be caused by retrogeniculate lesions possibly via cortical input into the light reflex. Wilhelm et al. detected an RAPD contralateral to a retrogeniculate lesion in 16 of 43 patients. They claimed that lesions closer than 10 mm to the LGN induced the RAPD with about 50% probability, whereas those farther than 18 mm from the LGN did not induce the RAPD [4]. Papageorgiou et al. also reported that a region in the early course of the temporal white matter, close to the LGN, was associated with RAPD [5]. In the present case, the optic radiation region was located further than 20 mm from the LGN and thus was unlikely to be responsible for RAPD production in the right eye ipsilateral to the region.
The present case exhibited unique clinical and radiological findings. The homonymous hemianopia, presence of RAPD in the eye with the temporal visual field defect, and homonymous hemianopic pattern of cpRNFL and GCC thinning on OCT appear to coincide with left-sided optic tract syndrome [6]. However, in the present case, the area of nasal visual field defect in the left eye was wider than that of the temporal visual field defect in the right eye. There is a rule that the amount of RAPD correlates with the degree of asymmetry of visual field defects in bilateral eyes. Previous studies demonstrated that RAPD was detected in eyes with at least 0.3 log units more visual field damage than in the contralateral eye [7]. In other words, RAPD should exist in the eye that has a wider area of visual field damage, i.e., the left instead of the right eye in the present case, if the single locus of the legion is responsible for both the presence of RAPD and the homonymous hemianopia.
This dilemmatic discrepancy between the visual field amount and the side of RAPD can be accounted for by considering the visual field pathway and light reflex pathway as independently damaged in the present case. This idea is supported by the MRI findings that clearly disclosed the left-sided atrophy of superior colliculus, optic radiation, and cerebellar hemisphere.
The superior colliculus comprises 7 alternate layers of nerve fibers and nerve cells and serves as the relay center of the light reflex. The superficial region (from the first to third layers) contains fibers from RGCs and the visual cortex called the retinotectal tract and corticotectal tract. Nerve fibers subserving light reflex pass through the superficial layer of the superior colliculus. Like the optic tract, the unilateral superior colliculus contains more crossed than uncrossed fibers of RGCs. Therefore, the presence of RAPD in the right eye with less visual field defect can be accounted for by atrophy of the left-sided superior colliculus, which does not contain nerve fibers transmitting the visual field information; there are reports of such an isolated RAPD free from visual field defects due to the midbrain lesion. Moreover, the intermediate and deep layers (from the fourth to seventh layers) of the superior colliculus contain other types of neuronal tracts related to the pursuit and saccadic as well as auditory and somatic sense [8]. Thus, the atrophy of the superior colliculus in addition to the cerebellum may be responsible for the pendular nystagmus with rightward gaze, although the detailed nature of nystagmus was not scrutinized in the present case.
Given that RAPD is presumed to be responsible for visual field pathway dissociation caused by the dorsal midbrain syndrome, the visual field defects in the present study are more likely to be attributed to the lesion of the optic radiation, which is in good agreement with the MRI findings. However, if this is true, we come to the conclusion that the characteristic patterns of cpRNFL and GCC thinning in both eyes, which are compatible with homonymous hemianopic atrophy, occurred as a result of the trans-synaptic degeneration of RGC axons as in the case with congenital occipital hemianopia. Until recently, transsynaptic degeneration has not been clinically observed in adult cases. However, several reports demonstrate that an occipital lobe lesion, even in patients with adult-onset diseases, can lead to homonymous hemianopic patterns of GCC and cpRNFL thinning due to the mechanism of trans-synaptic degeneration [9,10]. In addition, given that the present case is presumed to be a congenital anomaly of the Central Nervous System (CNS), it may be not surprising to think that trans-synaptic degeneration did occur.
Due to the complicated histogenesis of the CNS, congenital brain malformation is reportedly not uncommon (about 0.3%) [11]. Midbrain or hindbrain dysplasia causes various types and severities of mental retardation, cerebellar ataxic symptoms (e.g., seizures, nystagmus, vertigo), or other disorders (e.g., hemihypoesthesia, hemiparesis, facial dysplasia, chorioretinal lacunae) [12].
There are several reports on congenital dysplasia of optic radiation. De Morsier syndrome is known to cause congenital blindness and comorbid epilepsy or cerebral paralysis, characterized by optic nerve hypoplasia, septum pellucidum defect, and hypopituitarism. In the case with De Morsier syndrome, optic radiation hypoplasia is developed by anterograde trans-synaptic degeneration due to optic nerve hypoplasia. A very rare pediatric case of septum pellucidum defect has been also reported, which comprised ipsilateral optic nerve hypoplasia and contralateral hypoplasia of the optic tract and optic radiation [13]. As such, malformation of multiple areas of the CNS usually shows a variety of neurological symptoms. However, the present case is surprisingly unique as the patient did not manifest symptomatic neurological or developmental deficits other than visual dysfunction and mild ocular motility dysfunction.
MRA demonstrated the obliteration of AICA and PICA. AICA arises from the basilar artery, supplies many varieties of branching vessels to the middle cerebellar peduncle, inferolateral pons, cerebellar flocculus, and anteroinferior surface of the cerebellum [14]. Conversely, the PICA arises from the vertebral artery and supplies the dorsal and lateral medulla and inferior cerebellum [15]. Given that both arteries supply the cerebellar peduncle, the obliteration of AICA and PICA may be responsible for the atrophic change of the left cerebellum, resulting in pendular nystagmus as mentioned previously. But the dorsal midbrain and optic radiation are supplied by the posterior cerebral artery and anterior choroidal artery, respectively. Unfortunately, MRA could not delineate the abnormalities of these 2 arteries.
Conclusion
We present a rare case with simultaneous involvement of the optic radiation, superior colliculus, and cerebellar hemisphere as congenital malformations of the CNS, which independently led to homonymous hemianopia with trans-synaptic degeneration of RGCs, RAPD discordant to the number of the visual field defects, and gaze-dependent nystagmus, respectively.
Acknowledgments
This manuscript does not include any non-author contributors to acknowledge.
Conflicts of Interest
No authors have financial disclosures.
Funding Sources
This manuscript did not receive any funding.
Author Contributions
AK, YN, MN contributed to the patient management. KU and MN collected experimental data and drafted the manuscript. All authors read and approved the final manuscript for publication.
References
- Hoyt WF, Rios-Montenegro EN, Behrens MM, et al. Homonymous hemioptic hypoplasia. Fundoscopic features in standard and red-free illumination in three patients with congenital hemiplegia. Br J Ophthalmol. 1972;56:537-45.
- Skorkovská K. Homonymous Visual Field Defects. 1 ed. Springer; 2017.
- Kardon R, Kawasaki A, Miller NR. Origin of the relative afferent pupillary defect in optic tract lesions. Ophthalmology. 2006;113:1345-53.
- Wilhelm H WB, Petersen D, Schmidt U, et al. Relative afferent pupillary defects in patienst with geniculate and retrogeniculate lesions. Neuroophthalmology. 1996;16:219-24.
- Papageorgiou E, Ticini LF, Hardiess G, et al. The pupillary light reflex pathway: cytoarchitectonic probabilistic maps in hemianopic patients. Neurol. 2008;70:956-63.
- Kanamori A, Nakamura M, Yamada Y, et al. Spectral-domain optical coherence tomography detects optic atrophy due to optic tract syndrome. Grafes Arch Clin Exp Ophthalmol. 2013;251:591-5.
- Chew SS, Cunnningham WJ, Gamble GD, et al. Retinal nerve fiber layer loss in glaucoma patients with a relative afferent pupillary defect. Invest Ophthalmol Vis Sci. 2010;51:5049-53.
- Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165-82.
- Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132:628-34.
- Yamashita T, Miki A, Goto K, et al. Retinal ganglion cell atrophy in homonymous hemianopia due to acquired occipital lesions observed using cirrus high-definition-OCT. J Ophthalmol. 2016;2016:2394957.
- Keith L. Moore TVNP. The developing human: clinically oriented embryology. philadelphia. Saunders.2003.
- Alkan O, Kizilkilic O, Yildirim T. Malformations of the midbrain and hindbrain: a retrospective study and review of the literature. Cerebellum. 2009;8:355-65.
- Nishi T, Yukawa E, Taoka T, et al. Unilateral optic nerve hypoplasia with contralateral optic pathway hypoplasia: a case report. Neuroophthalmology. 2013;37:116-19.
- Hayashi Y, Nakau H, Shima H, et al. Infarction in anterior inferior cerebellar artery territory caused by occlusion of vertebral artery. Clin Radiol Extra. 2003;58:57-59.
- Macchi V, Porzionato A, Parenti A, et al. The course of the posterior inferior cerebellar artery may be related to its level of origin. Surg Radiol Anat. 2004 26:60-5.