Research Article - Journal of Biochemistry and Biotechnology (2023) Volume 6, Issue 4
Comprehensive Clinical Evaluations of the Aesthetic Enhancements on Skin, Hair, and Nails through the Administration of Marine Collagen
Maheshvari Patel1*,Samrat Warma2, Himanshi Warma2, Apeksha Merja1, Nayan Patel1
1Department of Clinical Trials, NovoBliss Research Private Limited, Ahmedabad, India
2Collagen Life sciences Private Limited, Nashik, Maharashtra, India
- *Corresponding Author:
- Maheshvari Patel
Department of Clinical Trials
NovoBliss Research Private Limited
Ahmedabad, India
E-mail: maheshvari@novobliss.in
Received: 31-Jul-2023, Manuscript No. AABB-23-111365; Editor assigned: 04-Aug-2023, PreQC No. AABB-23-111365(PQ); Reviewed:18-Aug-2023, QC No. AABB-23-111365; Revised: 24-Aug-2023, Manuscript No. AABB-23-1111365(R); Published: 27-Aug-2023, DOI:10.35841/aabb-6.4.152
Citation: Patel M. Comprehensive clinical evaluations of the aesthetic enhancements on skin, hair, and nails through the administration of marine collagen. J Biochem Biotech. 2023; 6(4):152
Abstract
Collagen is abundant, forming 70-80% of skin's dry weight in mammals. Studies highlight improved skin, hair, and nail conditions post collagen treatment. We aimed to assess the safety, tolerability and effectiveness of AQUACOLTM, a marine collagen derived from fish skin by proprietary process (enzyme hydrolysis), across various dosages (2.5g, 5g and 10g) in adult human participants. The study included 66 male and female participants, aged 30 to 50, who had mild to moderate wrinkles (crows' feet) around the eyes and experienced joint discomfort, swelling, stiffness, and limited motion. The study also included individuals concerned about hair loss, reduced hair growth, and brittle nails. AQUACOLTM usage led to statistical significant (p-value<0.0001) results with clinically significant improvement with 61.90% reduction in crow's feet wrinkles, 22.94% enhanced skin hydration, 16.52% higher hair density, 25.68% increased hair thickness, decreased pain by 47.32%, 15.45% improved skin elasticity, 39% (p-value<0.005) smoother skin, 53.72% (p-value<0.001) less hair fall and upper body muscle strength rose by 82.64%. Assessments showed significant improvements in sleep quality. All subjects perceived the product, highly effective and beneficial for skin, hair, nails and muscles strength. No subjects experienced serious adverse events or product-related adverse event. The study's findings indicated that a 2.5g dose improved skin health and elasticity, while a 10g dose alleviated joint pain and enhanced muscle strength. These results, supported by both measurements and participant input, emphasize AQUACOLTM's potential as a safe, well-tolerated, and effective contributor to overall physical well-being, particularly in the aspects of skin, hair, and nail quality
Keywords
Marine Collagen; Wrinkles and Fine lines, Hair Fall, Sleep Improvement, Brittle Nails, Hair Growth.
Introduction
About 30% of the total protein mass in humans is made of collagen [1]. Collagen is a fibrous protein that accounts for up to 70–80 percent of the dry weight of skin [2]. Collagen is composed of molecules that are packed together to form long, thin fibers which reinforce one another and are one of the causes of the skin's elasticity. Collagen is majorly found in skin, muscles, ligaments, bones and other fibrous tissue and supports the skin, making it strong and supple. It also helps in renewal of skin cells [3]. Collagen has abundant range of medical applications involving wound healing, regeneration of bone and tissue and drug delivery. Marine creatures like fish, sponges, jellyfish, and various other invertebrates serve as a notable reservoir of collagen protein.These organisms offer distinct advantages compared to alternative sources, as they possess good metabolic compatibility, absence of religious constraints, and freedom from animal pathogens [4]. The chief source of marine collagen is fish processing waste which is mainly obtained from the food industry and is used for food, cosmetics, pharmaceuticals and biotechnology applications. Marine invertebrates, including sponges, mollusks, jellyfish and sea cucumbers are widely used by biotechnological industries because of their richness in collagen and environmental availability. [5] Marine collagen has garnered significant scientific interest for biomaterial applications because of its safety, water solubility, biodegradability, easy extraction, and minimal immunogenicity [6]. Collagen derived from marine species could also be used in other tissue engineering areas such as dental, vascular, and corneal [7].
The aging process exerts a detrimental impact on the skin's connective tissue, causing a reduction in collagen fibers and consequently giving rise to the development of fine lines and wrinkles. Reduction in collagen causes reduced dermal thickness further leading to the progression of skin wrinkling [8]. Collagen makes the supportive layer beneath the skin, which also helps in promoting hair growth and lowering the hair fall by making the scalp strong to withhold the hair. Loss of skin collagen can be due to intrinsic ageing, consumption of diet which are non-balanced in nutrients, irradiation, micronutrients deficiency and stress [9-11]. Role of extracellular matrix in the skin is to retain water and to support skin, thereby making it strong.
Previous studies demonstrated that the reduction in the collagen due to ageing can be reversed by oral administration of various bioactive collagen peptide which are obtained by hydrolysis of natural collagen.After ingestion of bioactive collagen, it gets further metabolized to di-peptides and tripeptides in gastrointestinal tract. The metabolized peptide then enters into the circulatory system and gets deposited in the skin and forms collagen biomatrix. Various in-vitro and invivo analysis have confirmed that the marine collagen is safe for consumption. Matsumoto and colleagues conducted a double-blind placebo-controlled study on 214 healthy female volunteers who consumed 2.5 g, 5 g, or 10 g of fish hydrolyzed collagen and reported that after 6 months of supplementation with fish collagen peptide, subjects showed enhanced skin hydration [12-19].
Materials and Methods
Study design and ethical considerations
A prospective, post-approval, single-blinded, single-center, comparative, dose response clinical study of 60 days’ duration was conducted at NovoBliss Research Private Limited at Ahmedabad, Gujarat, India to evaluate the efficacy, tolerability and safety of AQUACOL™. The study was performed according to the declaration of Helsinki, ethical principles for medical research involving human subjects, statutory provisions prescribed under Drugs and Cosmetic Rules, 1945, ethical guidelines for biomedical research 2006 by the Indian Council of Medical Research (ICMR), and principles of Good Clinical Practice (GCP) as well as Indian GCP. Before initiating the study, the protocol underwent thorough review and approval by an Independent Ethics Committee (IEC). The study was registered in the Clinical Trial Registry India (CTRI) under the registration number CTRI/2022/11/047149 on November 9, 2022. Additionally, the clinical trial was also registered with ClinicalTrials.gov, and it was assigned the trial identifier number NCT05613660. These registrations ensure transparency and adherence to regulatory requirements, allowing for the proper documentation and tracking of the trial. Prior to any study-related procedures, all subjects who voluntarily agreed to participate in the study provided their informed consent by signing an acceptance form. This step ensured that the subjects were fully aware of the study's purpose, procedures, potential risks, and benefits, and they willingly chose to participate based on their understanding of the information provided.
Study population
In this study, 66 adult subjects were recruited and enrolled, with a target of subjects per dose/test treatment. Ultimately, a total of 63 subjects completed the study, with each treatment arm consisting of 21 subjects. The subjects were divided into three groups (2.5g, 5g and 10g), with each group receiving a different dose of AQUACOL™. The screening process for subject selection included assessing the presence of mild to moderate crow's feet area wrinkles, mild to moderate swelling, stiffness, and decreased range of motion. Additionally, subjects were evaluated for mild skin aging using the Physician Global Assessment (PGA) criteria, specifically Glogau Skin age II or III. Complaints of hair fall, decreased hair growth, and the presence of thin, dry, and brittle hair and nails were also taken into consideration during the screening process. Overall, this approach aimed to ensure a diverse and representative subject population for the study, allowing for a comprehensive evaluation of the safety, efficacy and tolerability of AQUACOL™ across different dose groups.
Study product
The test treatment AQUACOL™ a marine collagen is a Type 1 collagen which is derived from fish scales/skin as this sort of collagen has the highest, most bioavailable source of collagen available in the market. This marine collagen peptide is manufactured from Rohu Katla/Tilapia fish scales and contains 90%+ protein. AQUACOL™ is more bio-available than any other types of collagens because the Molecular weight Marine collagen is comparatively lesser hence resulting in good absorption. This is fast absorbable Low Molecular Weight Collagen Peptides which is prepared by proprietary process (enzyme hydrolysis) from fish scales/ skin. Throughout the study, participants will consume 1 scoop/ sachet daily with approx. 250 mL water, spanning of 8-week timeframe.
Inclusion criteria
The study enrolled healthy adult males and non-pregnant/ non-lactating females between the ages of 30 and 50 years. Female participants of childbearing potential were required to provide a negative pregnancy test during the screening visit. The inclusion criteria for subject selection were as follows: Subjects needed to have moderate crows' feet wrinkles and a score of at least "mild skin aging" based on Physician Global Assessment (PGA) and Glogau skin age II or III. They should also experience mild-to-moderate joint pain, swelling, stiffness, and decreased range of motion. Additionally, subjects should have complaints of hair fall and decreased hair growth, and self-declared nonpathological thin, dry, and brittle hair and nails. Participants were required to be willing to forgo cosmetic procedures for three months prior to and throughout the study. They were also expected to follow their normal skincare routines without introducing any new skincare products during the study. Subjects needed to be willing to abstain from changes in baseline medications, nutritional supplements, or any other collagen peptide powder during the study period. Female subjects of childbearing potential were allowed to participate if they practiced and agreed to maintain an established method of birth control, such as an IUD, hormonal implant device/injection, regular use of birth control pills or patch, diaphragm, condoms with spermicide or sponge with spermicidal jelly, cream or foam, partner vasectomy, or abstinence. Females who were considered non-childbearing potential were those who were surgically sterile, had been post-menopausal for at least one year, or had a tubal ligation. If subjects were using hormonal contraception, they needed to have been using it for at least six months and agree to continue using the same method for the duration of the study. Subjects were instructed not to introduce any new soaps, cleansers, lotions, creams, or any other face products throughout the study period. They were required to provide written informed consent and follow the study procedures. Subjects were committed to not using medicated/prescription shampoos, hair care products containing Minoxidil or anti-thinning agents, or any other hair growth treatments or products other than the test product for the entire duration of the study. Additionally, participants were expected to use the test treatment consistently throughout the study period. These inclusion criteria ensured that the selected subjects met the necessary requirements for the study and were willing to comply with the study procedures, product usage guidelines, and any necessary precautions to ensure the validity of the results.
Exclusion criteria
Subjects who had a known history of allergy or sensitivity to the ingredients of the test treatment or products containing fish were excluded from the study. Additionally, individuals with pre-existing or dormant dermatologic conditions such as psoriasis rashes, eczema, seborrheic dermatitis, acne, or any other condition that could potentially interfere with the study outcomes, as determined by the Investigator, were not included. Subjects who had been using systemic therapy with chronic antibiotic therapy, retinoids, and/or oral steroids within the four weeks prior to the study start or anticipated the need for such therapy during the study, were excluded. Similarly, individuals who had applied any topical retinoids within two weeks of the screening visit or anticipated the need for such application during the study were not eligible. Participants who were not willing to avoid the unprotected sun or other UV radiation exposure throughout the study period were excluded. Those with a history of prior use of hair growth treatments within the past three months, or prior hair growth procedures such as hair transplant or laser treatment, were also excluded. Subjects with a history of alcohol or drug addiction, plans to shave scalp hair during the study, or participation in or planning to start a weight loss program that may significantly change overall body weight were not included. Subjects with a history of prior use of skin radiance or wrinkle laser treatments within the past three months, as well as those with any other condition that, in the discretion of the dermatologist or investigator, could warrant exclusion from the study, were not eligible to participate. Pregnant or breastfeeding individuals, or those planning to become pregnant during the study period, were also excluded.
Subjects with a history of chronic illness that may influence the cutaneous state were not included. Furthermore, individuals who were participating in other similar nutraceuticals, food, supplemental, or therapeutic trials, or had used other skin care products within the last four weeks were excluded from the study. These exclusion criteria were put in place to ensure the safety and integrity of the study results, by avoiding potential confounding factors or risks to the participants.
Internal training and process validation
NovoBliss Research has conducted an In-house training and validation study to demonstrate the procedural steps, processes and clinical activities such as hair growth rate measurement, 60-s combing test, pull test, pluck test, subjective questionnaires, scoring of hair quality appearance and scalp condition - evaluations techniques that are used during the conduct of clinical safety and efficacy studies for hair care product evaluation on healthy adult human subjects maintains the consistency in readings [20].
There was an internal training conducted by Dermatologist on scoring activities such as Physician Global Assessment (PGA) using Griffiths scoring scale for skin dryness, redness, wrinkles, fine lines, coarse lines, laxity, roughness and sallowness, Glogau Skin Age, hair assessments, scoring for nails brittleness, roughness, ruggedness, peeling, Acne severity rating–evaluated with standardized manner. The Dermatologist discussed skin and hair anatomy with the help of a PowerPoint presentation followed by an explanation and discussion of each evaluation using photographic examples with each trainee evaluator. Test photographic evaluations were done between Dermatologists and Evaluators prior to conducting the study evaluations in a blinded fashion. The photographic evaluation methods involve using standard photographs to assess or evaluate a particular phenomenon or subject. During the training, the dermatologist selected 50 high- resolution photographs for each parameter. Dermatologist and all evaluators used the same LED screen to view and score digital photographs using a dell computer system with specification of HD Graphics with resolution as 1920 x 1080, refresh rate as 60p Hz. Display Scaling was maintained and only (Light Emitting Diode) LED Screen brightness with screen light level 90 was used for photographic evaluation. Dermatologist & Evaluators scoring was analyzed to establish inter-evaluator reliability that helped maintain consistency between Dermatologist and Evaluators scoring during clinical study.
Physiotherapist had conducted another internal training for scoring activities such Visual Analogue Scale (VAS) scoring and muscles strength tests including manual isometric muscle testing, hand grip strength and upper body muscle strength, low plank hold. The Physiotherapist discussed regarding the anatomy of knee and elbow joints, range of motion of knee and elbow, muscle strength measurements, upper body muscle strength (push up), low plank hold (techniques and advantages) through power point presentation. The physiotherapist performed push-up and plank to show the correct posture. The certified physical trainer provided the training on push up and low hold plank for correct posture, steps involved, things to take care while performing the activity. The study staff replicated the activities to ensure their understanding and the study participants’ adherence during study period.
Study procedure
Potential subjects were screened according to the inclusion and exclusion criteria. During screening visit, inclusion/ exclusion criteria were evaluated, and various aspects such as demographics, physical examination (including age, height, weight, body mass index), medical and surgical history, current treatment history, and assessment of concomitant medication were recorded. Study subjects provided their informed consent by signing a form that detailed the study's objectives, endpoints, methodologies, potential risks, and advantages. Their voluntary enrollment was grounded in a comprehensive grasp of the disclosed particulars.
There were three test treatment groups, each treatment group had 22 subjects, and each group received 2.5g, 5g and 10g test treatment per randomization code for consecutive 60 days. All the subjects were instructed to take 1 scoop/sachet daily with approx. 250 mL water. The study included six visits in which Visit 1 (Day 04) was the screening visit and baseline evaluation (within 04 days prior to Day 1), Visit 2 (Day 01) was for the enrolment, hair growth rate measurement and evaluation, Visit 3 (Day 10) was for the evaluation amid treatment phase, Visit 4 (Day 30) was for the evaluation amid treatment phase, Visit 5 (Day 57) was for Treatment Period, Evaluations, Tattoo, Hair growth measurement, Visit 6 (Day 60) was evaluation and end of the study day.
As the study was conducted in a real-life setting, the study treatment was distributed to the subjects per randomization code. Compliance was assessed using case report forms, subject diary, and source data was reviewed to ensure compliance. Participants were considered to have completed the study if they adhered to the 8-week treatment period, which was recorded in the case report form.
During the entire study duration, close monitoring and documentation of Adverse Events (AEs) and Treatment- Emergent Adverse Events (TEAEs) was done. No adverse events or treatment-emergent adverse events were detected or reported in any of the patients throughout the entire duration of the study
Primary endpoints
The primary endpoints of this study were as follows: a) to assess the efficacy of the AQUACOL™ at different dosages i.e., at 2.5g, 5g and 10g after 60 days of treatment from baseline (Day 01) to Day 10 (+2 Days), Day 30 (+2 Days) and Day 60 (+2 Days), within treatment, between three different dosages; b) to determine change in the facial wrinkles and fine lines of Crow’s feet area, skin texture – roughness, dryness, wrinkles, smoothness using Visioscan® VC 20 Plus; c) to assess the change in skin elasticity using DermaLab®Combo or equivalent (Right cheek), to check Change in skin hydration using MoitureMeterEPiD (Right cheek), d) to measure change in Hair Thickness and Density using CASLite Nova through Phototrichogram; e) to assess the change in hair fall using 60- second hair count (Hair Combing Method), f) to evaluate the change in weight and BMI; g) to measure change in joint pain using VAS scoring evaluated by trained study staff; h) to assess change in muscles strength - as assessed by the sum of handgrip, elbow flexion and extension and knee flexion and extension strength evaluated by trained study staff. The study assessed the effect of test treatment in terms of change in hair growth on the scalp from baseline visit i.e., during Visit 01 (04 days before Day 01) and Visit 02 (Day 01) before treatment to Visit 05 (Day 57) and Visit 06 (Day 60) after treatment by the dermatologist trained evaluator.
Secondary endpoints
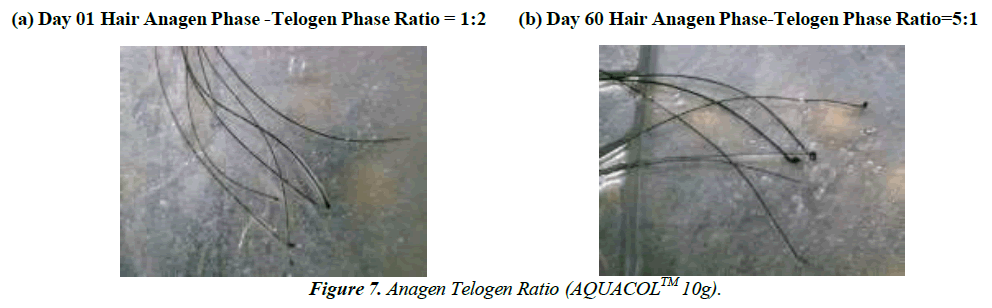
The secondary endpoint of this study were as follows: 1) to evaluate the efficacy of the AQUACOL™ at different dosages i.e., at 2.5g, 5g and 10g after 60 days of treatment from baseline (Day 01) to Day 10 (+2 Days), Day 30 (+2 Days) and Day 60 (+2 Days), between treatments, between three different dosages and within treatment for (i) change in PGA scoring using Griffiths scale - skin dryness, redness, fine wrinkling/lines, coarse wrinkling/lines, laxity, roughness and sallowness evaluated by dermatologist trained scorer or Dermatologist, (ii) change in Glogau Skin Age evaluated by dermatologist trained scorer or Dermatologist, (iii) change on hair strength by Pull test evaluated by dermatologist trained scorer or Dermatologist, (iv) change in PGA score for assessment of the signs of brittle nails, surface roughness, raggedness and peeling evaluated by dermatologist trained scorer or Dermatologist, (v) change in sleep quality using Leeds Sleep Evaluation Questionnaires (LSEQ) evaluated by trained study staff, (vi) about test treatment perception and consumer feedback on skin elasticity, suppleness, deep and sound sleep, digestion and gut health, joint health, nail and hair health using hedonic questionnaires evaluated by trained study staff. 2) To evaluate the effectiveness of the test treatments after 8 weeks of treatment in change in PGA score for assessment of acne severity from baseline (Day 01) to Day 60 (+2 Days) between three different dosages evaluated by dermatologist trained scorer in enrolled subjects with mild to moderate acne, to evaluate the effectiveness of the test treatments in Anagen Telogen (A:T) ratio using Pluck Test from baseline (Day01) to Day 60 (+2 Days), between treatments, between three different dosages and within treatment evaluated by dermatologist trained scorer to assess Hair growth cycle (Trichogram). 3) Change between “before” (Day 01) and “after” (Day 60) facial photographs (left/center/ right) and nails photographs of the subjects in three different dosages and between treatments.
Statistical analyses
Descriptive statistical methods were employed to summarize continuous variables, encompassing metrics such as the count of observations (N), mean, Standard Deviation (SD), median, minimum, and maximum values. Categorical variables were presented with frequencies and percentages, supplemented by appropriate visual aids when necessary. Given the absence of any reported adverse events, there was no necessity to summarize adverse event data. Comparisons between baseline and post-treatment measurements for continuous variables were conducted using paired t-tests, facilitating the identification of noteworthy alterations. The statistical analyses were executed utilizing R software (Version: 4.0) and GraphPad Prism (Version 9.5.1, build 733), with a significance threshold established at 5%. GraphPad Prism was specifically employed for analyzing IGA and VAS score data. To preserve data integrity, it's essential to underscore that individuals who withdrew from the study were excluded from the statistical assessment. These statistical methodologies were implemented to offer a comprehensive grasp of the data and to evaluate the significance of the observed pre- and posttreatment changes.
Results
A total of 66 subjects were included in the study, with each dosage (i.e. 2.5g, 5g and 10g) group consisting of 22 subjects. Out of the 66 subjects, 61 subjects (92.42%) successfully completed the study, while 5 subjects (7.58%) were discontinued due to loss to follow-up. Among the enrolled subjects, there were 34 females (51.52%) and 32 males (44.48%). The delta (Δ) age of the subjects at the time of screening visits was 38.2 years with detailed demographics (Mean, SD) presented in (Table 1). The study had a high completion rate, with the majority of enrolled subjects fulfilling the study requirements and successfully participating in the research.
| Parameters/ Statistics | AQUACOL(2.5g)(N=22) | AQUACOL(5g)(N=22) | AQUACOL(10g)(N=22) |
|---|---|---|---|
| Age | |||
| 36.50(4.74) | 39.91(5.46) | 38.41(5.37) | |
| Gender | |||
| F | 12(54.55%) | 13(59.10%) | 9(40.90%) |
| M | 10(45.45%) | 9(40.90%) | 13(59.10%) |
| Predominant Race | |||
| Asian | 22(100.00%) | 22(100.00%) | 22(100.00%) |
| Height(cm) | |||
| Mean(SD) | 162.56(9.12) | 159.21(11.10) | 165.13(9.91) |
| Weight(Kg) | |||
| Mean(SD) | 63.02(13.89) | 58.93(9.73) | 62.29(12.16) |
| BMI | |||
| Mean(SD) | 23.81(4.79) | 23.55(5.16) | 22.83(4.04) |
| Waist Circumference | |||
| Mean(SD) | 34.59(4.45) | 34.86(3.58) | 34.78(3.64) |
| Hip Circumference | |||
| Mean(SD) | 37.41(4.47) | 37.91(2.76) | 38.05(4.15) |
| Medical/ Concomitant Medication History(Yes/No) | |||
| No | 22(100.00%) | 22(100.00%) | 22(100.00%) |
| Type of Skin | |||
| Dry | 4(18.18%) | 7(31.82%) | 7(31.82%) |
| Mixed | 8(36.36%) | 4(18.18%) | 5(22.73%) |
| Normal | 10(45.45%) | 10(45.45%) | 10(45.45%) |
| Oily | 0(0.00%) | 1(4.54%) | 0(0.00%) |
| Sensitive | 0(0.00%) | 0(0.00%) | 0(0.00%) |
Table 1: Demographics of the study population
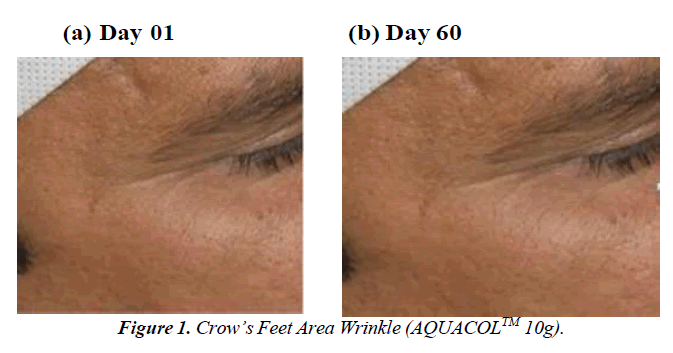
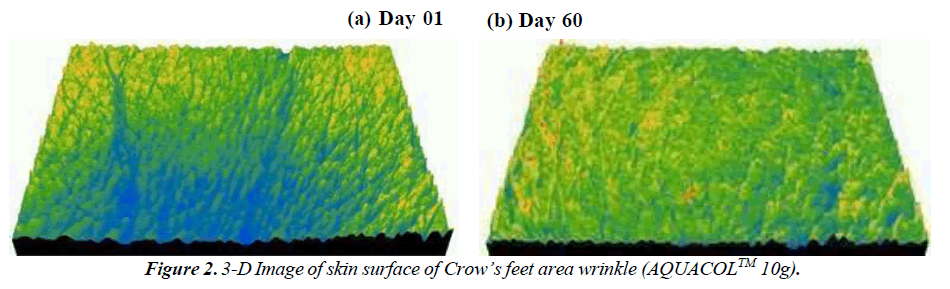
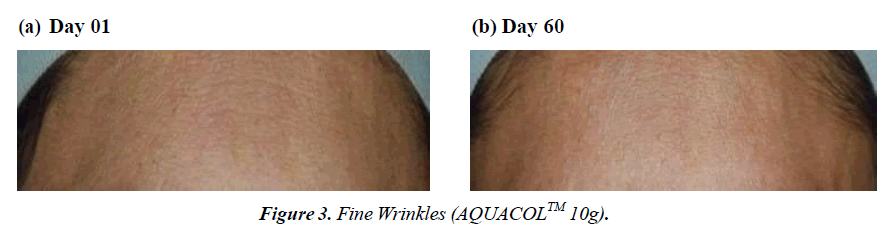
The study demonstrated significant reductions in crow's feet wrinkles over different time intervals. In the group taking 2.5g, 5g, and 10g dosages, wrinkles decreased by 36.08% (p<0.001), 33.84% (p<0.01), and 27.90% (p<0.0001) on Day 10, respectively, compared to baseline. By Day 30, the reduction was 45.21% (p<0.0001), 53.05% (p<0.0001), and 44.83% (p<0.0001), and on Day 60, it was 61.90% (p<0.0001), 42.19% (p<0.0001), and 57.34% for the respective dosages. This decline in wrinkles showcases the natural aging process due to collagen loss and skin elasticity reduction. The use of marine collagen supplements seems to counteract this by boosting collagen production, visibly reducing wrinkles (Figure 1-3). Skin roughness, a key indicator of cosmetic skin quality, was assessed in the study. The treatment effectively reduced skin roughness in the 2.5g, 5g, and 10g dosage groups at Day 30 (p<0.05, p<0.01, and p<0.05 respectively) and at Day 60 (p<0.01, p<0.0001, and p<0.05 respectively). This reduction led to visibly smoother and more supple skin, providing a noticeable improvement. The Dermatologist- assessed Physician Global Assessment (PGA) score showed consistent improvement across all dosages. By Day 30 and Day 60, there were significant reductions (p<0.01) in skin dryness, redness, fine and coarse wrinkling/lines, laxity, roughness, and sallowness . Notably, on Day 10, dosage groups 2.5g and 5g exhibited improvements (p<0.01 and p<0.05 respectively) in skin smoothness compared to baseline. Substantial enhancements (p<0.001, p<0.01, and p<0.05) in these aspects were observed on Day 30 and Day 60 for all dosages, reflecting consistent positive effects.
The test treatment significantly improved skin viscoelasticity, a sign of healthy skin elasticity. On Day 10, in the 2.5g group, there was a 12.57% increase (p<0.05) in viscoelasticity compared to baseline. By Day 60, the 5g and 10g groups showed improvements of 10.43% (p<0.01) and 6.77% (p<0.05) respectively. These improvements indicate enhanced skin shape maintenance, deformation resistance, and stress recovery. Skin retraction time, indicating elasticity, also improved. On Day 10, in the 2.5g, 5g, and 10g groups, skin retraction time decreased significantly by 17.96% (p<0.01), 15.13% (p<0.01), and 15.73% (p<0.05) respectively. On Day 30, attenuation was noted by 15.79% (p<0.05), 15.33% (p<0.01), and 15.67% (p<0.05) in the respective groups. Reduced retraction time suggests increased elasticity, associated with youthful appearance and skin health, possibly due to collagen presence. All doses of test treatment improved Young’s Modulus compared to baseline (Day 01), maintaining skin integrity. The 2.5g group saw 4.36% and 13.11% improvements on Day 10 and Day 60, while the 5g group showed 8.00% improvement on Day 60. The 10g group exhibited 1.46% and 5.83% improvements on Day 10 and Day 60 respectively.
Skin hydration
Skin hydration is pivotal for maintaining healthy skin as it supports cell plumping, structural integrity, and functional regulation. Our study results substantiate this notion. By Day 10, compared to baseline, there were significant perceived moisture content increases of 10.37% (p<0.05), 5.56% (p<0.05), and 14.07% (p<0.01) in the 2.5g, 5g, and 10g dosage groups respectively. By Day 30, these increases were 16.25% (p<0.01), 13.73% (p<0.001), and 16.21% (p<0.01), and by Day 60, they were 23.02% (p<0.0001), 20.25% (p<0.0001), and 22.94% (p<0.0001) in the respective dosage groups. Augmented skin hydration enhances cell plumping, structural maintenance, and functional regulation, promoting skin health. Post-treatment, Day 60 showcased statistically significant improvements in Hair Growth Rate: 48.81% for the 2.5g group, 56.11% (p<0.0001) for the 5g group, and 74.67% (p<0.001) for the 10g group, compared to baseline.
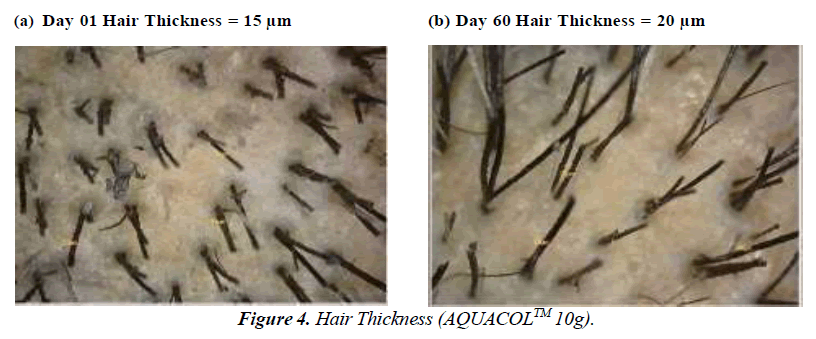
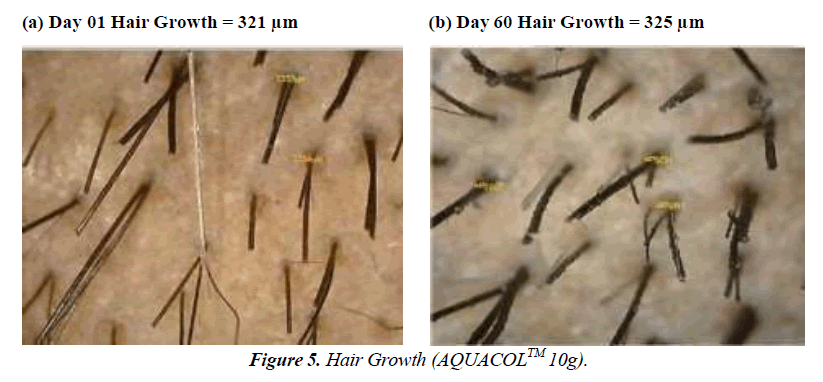
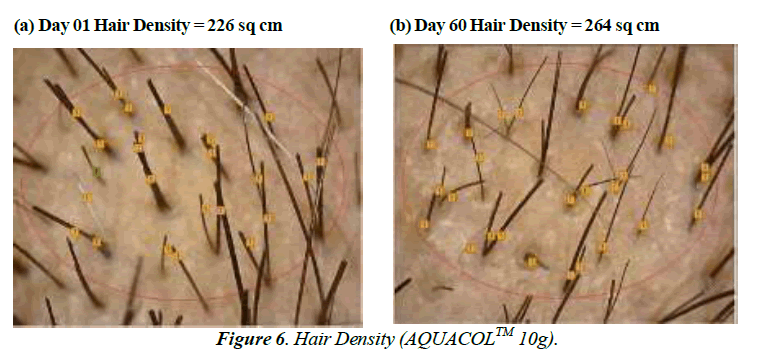
The test treatment exhibited significant improvements (p<0.0001) in Hair Growth Rate at Day 60 by 48.81%, 56.11% and 74.67% for groups treated with 2.5g, 5g and 10g of test treatment respectively. On Day 10, compared to baseline, there was a noteworthy increase in hair density of 7.91% (p<0.001) in the 2.5g group, 8.18% in the 5g group, and 4.55% in the 10g group. By Day 30, the 2.5g group saw a substantial 9.99% increase (p<0.01), while the 5g and 10g groups showed increases of 5.54% and 5.33% respectively. In comparison to baseline, the improvements in hair density on Day 60 were significant: 14.19% (p<0.0001) for the 2.5g group, 12.48% (p<0.0001) for the 5g group, and 16.52% (p<0.0001) for the 10g group. Regarding hair thickness, in comparison to baseline, the test treatment displayed substantial increases on Day 10: 13.08% (p<0.001) for the 2.5g group, 17.90% (p<0.0001) for the 5g group, and 10.06% (p<0.001) for the 10g group. By Day 30, the increases were significant as well: 17.65% (p<0.001) for the 2.5g group, 26.68% (p<0.0001) for the 5g group, and 16.35% (p<0.001) for the 10g group. On Day 60, there were further noteworthy increases: 25.20% (p<0.0001) for the 2.5g group, 25.68% (p<0.0001) for the 5g group and 25.16% (p<0.001) for the 10g group (Figure 4-6).
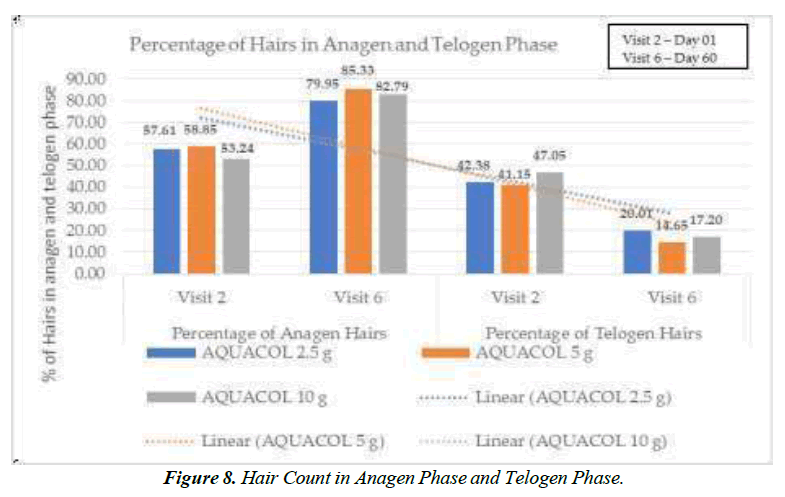
Following the treatment, a noteworthy decrease in hair fall was observed. On Day 10, compared to Day 01, there were reductions of 12.10% (p<0.05), 21.68% (p<0.01), and 21.09% (p<0.001) in the 2.5g, 5g, and 10g dosage groups respectively. On Day 30, these reductions were 25.10% (p<0.001), 34.41% (p<0.0001), and 39.89% (p<0.0001), and on Day 60, they were 48.28% (p<0.001), 53.27% (p<0.001), and 46.57% (p<0.0001) for the respective dosages. Collagen, known for enhancing hair follicle support and scalp blood circulation, can bolster hair strength and reduce breakage (Figure 7,8), [Table 2].
| Percentage Change from Baseline | ||||
|---|---|---|---|---|
| Variables | Visit days | Aquacol™(2.5g) | Aquacol™(5 g) | Aquaco™(10 g) |
| Crow’s Feet Area Wrinkles | Day 10 | 36.08%↓ | 33.84%↓ | 27.90%↓ |
| Day 30 | 45.21%↓ | 53.05%↓ | 44.83%↓ | |
| Day 60 | 61.90%↓ | 42.19%↓ | 57.34%↓ | |
| Roughness | Day 10 | 0.00%↑ | 53.65%↑ | 47.50%↑ |
| Day 30 | 122.81%↑ | 200.00%↑ | 95.00%↑ | |
| Day 60 | 119.30%↑ | 234.15%↑ | 170.00%↑ | |
| Scaliness | Day 10 | 32.67%↓ | 33.16%↓ | 26.51%↓ |
| Day 30 | 52.60%↓ | 54.53%↓ | 35.94%↓ | |
| Day 60 | 67.32%↓ | 68.72%↓ | 54.62%↓ | |
| Smoothness | Day 10 | 18.32%↓ | 25.44%↓ | 34.16%↓ |
| Day 30 | 18.13%↓ | 18.88%↓ | 39.01%↓ | |
| Day 60 | 4.32%↓ | 19.52%↓ | 28.39%↓ | |
| Skin viscoelasticity | Day 10 | 12.57%↑ | 6.13%↑ | 15.45%↑ |
| Day 30 | 0.28%↑ | 3.68%↑ | 1.89%↑ | |
| Day 60 | 11.17%↑ | 10.43%↑ | 6.77%↑ | |
| Skin Retraction time | Day 10 | 17.96%↓ | 15.13%↓ | 15.73%↓ |
| Day 30 | 15.79%↓ | 15.33%↓ | 15.67%↓ | |
| Day 60 | 16.42%↓ | 18.51%↓ | 11.30%↓ | |
| Skin Young’s module | Day 10 | 4.36%↑ | 5.50%↓ | 1.46%↑ |
| Day 30 | 8.25%↓ | 10.50%↓ | 5.83%↓ | |
| Day 60 | 13.11%↑ | 8.00%↑ | 5.83%↑ | |
| Skin hydration | Day 10 | 10.37%↑ | 5.56%↑ | 14.07%↑ |
| Day 30 | 16.25%↑ | 13.73%↑ | 16.21%↑ | |
| Day 60 | 23.02%↑ | 20.25%↑ | 22.94%↑ | |
| Hair density | Day 10 | 7.91%↑ | 8.18%↑ | 14.19%↑ |
| Day 30 | 9.99%↑ | 5.54%↑ | 12.48%↑ | |
| Day 60 | 14.19%↑ | 5.33%↑ | 16.52%↑ | |
| Hair thickness | Day 10 | 13.08%↑ | 17.90%↑ | 10. 06%↑ |
| Day 30 | 17.66%↑ | 26.68%↑ | 16.35%↑ | |
| Day 60 | 25.20%↑ | 25.68%↑ | 25.16%↑ | |
| Hair fall | Day 10 | 12.10%↓ | 21.68%↓ | 21.09%↓ |
| Day 30 | 25.10%↓ | 34.41%↓ | 39.89%↓ | |
| Day 60 | 48.28%↓ | 53.72%↓ | 46.57%↓ | |
Table 2: Percentage change from baseline in outcome variables
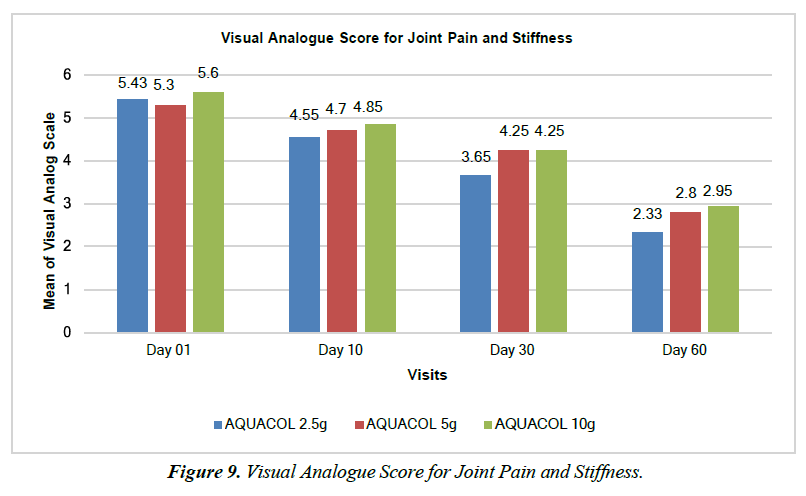
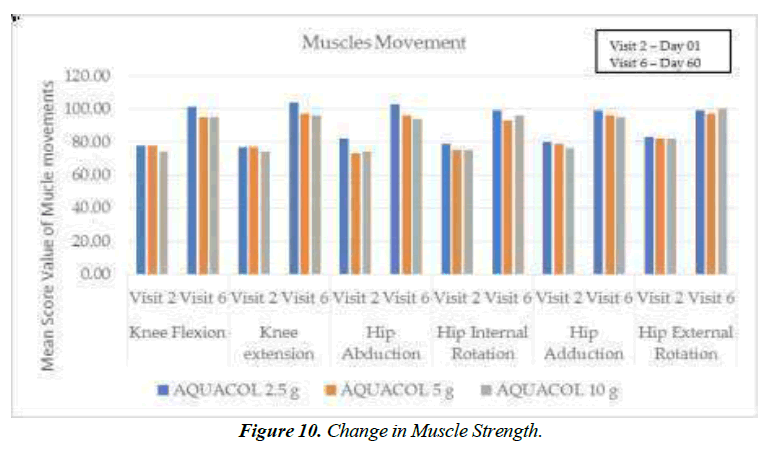
The test treatment led to significant reductions in Pain and Stiffness, evaluated using Visual Analogue Scale Scores. On Day 10, compared to baseline, reductions were observed: 16.21% (p<0.001) in the 2.5g group, 11.32% (p<0.01) in the 5g group and 13.39% (p<0.001) in the 10g group. By Day 30, these reductions were even more pronounced: 32.78% (p<0.0001), 19.81% (p<0.0001), and 24.11% (p<0.0001) respectively. On Day 60, substantial reductions were seen: 57.94% (p<0.0001), 47.17% (p<0.0001), and 47.32% (p<0.0001) for the respective dosages (Figure 9).The test treatment also significantly increased muscle strength. Hand Grip Strength showed significant improvement in the 5g and 10g groups on Day 10 by 7.56% (p<0.01) and 9.37% (p<0.005) respectively. On Day 30, Hand Grip Strength improved significantly (p<0.0001) in all dosage groups by 36.90%, 25.73% and 47.61% respectively. Similar improvements were observed for Low Plank Hold on Day 10 in the 2.5g and 5g groups by 26.11% (p<0.05) and 13.74% (p<0.001) respectively Similarly, on Day 30, significant improvement was observed across all three types of doses by 49.65% (p<0.0001), 28.02% (p<0.001), and 42.13% (p<0.05). For Day 60, all the three doses of test treatment showed significant improvement in Low Plank Hold by 68.42% (p<0.0001), 72.87% (p<0.0001), and 30.90% (p<0.05). Furthermore, muscle strength improvement was observed across various movements, including hand grip, knee flexion, knee extension, hip abduction, hip adduction, internal hip rotation, and external hip rotation. Upper body muscle strength assessed through push-ups showed significant improvement in muscle strength at Day 10 by 23.97% (p<0.001), 18.81% (p<0.0001), 21.74% (p<0.0001) for respective three doses. Similar improvements (p<0.0001) were observed at Day 30 across all three doses by 47.93%, 42.57%, and 45.22% respectively and at Day 60 by 82.64%, 74.26%, and 66.09% (p<0.0001) across all three respective doses, when compared to baseline.
Overall, muscle movement improvements were consistently observed from Day 1 to Day 10, Day 30, and Day 60 with the most significant improvement seen on Day 60 (Figure 10).
Secondary endpoint assessments
PGA scores were used to assess different parameters. PGA scoring for skin appearance was done for various skin parameters. These parameters were initially assessed using Griffith Scale. Post treatment with 2.5g, 5g, and 10g showed marked improvement in PGA score with every successive visit and ultimately in these skin parameters like fine wrinkles, coarse wrinkles, mottled hyperpigmentation, lentigines, tactile roughness, telangiectasia, pore size, elastosis and skin laxity on Day 30 and Day 60.
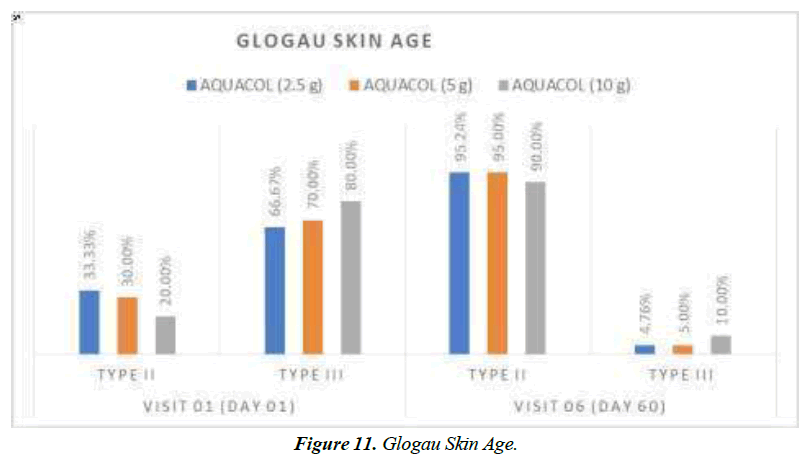
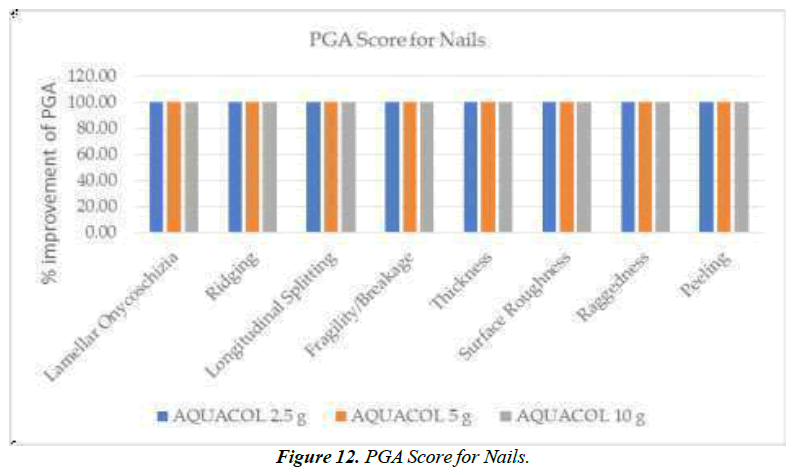
In group of patients treated with 2.5g, 5g, and 10g treatment, Glogau Skin Age Type III was present in 66.67%, 70.00%, and 80.00% respectively at baseline visit, which was decreased to 70.00% for group receiving 10g dose. Further decrease was observed in percentage of patients in Glogau Skin Age Type III at Day 30 to 50.00%, 40.00%, and 45.00% across all three respective doses. Day 60 also showed improvement in skin age across all three respective doses with only 4.76%, 5.00% and 10.00% of population having Type III Glogau Skin Age (Figure 11). As per Hair Pull Test assessment it was observed that there was 100% improvement in hair strength for all participants after treatment with 2.5g, 5g and 10g respectively on Day 60. With regards to Nail Appearance, PGA scoring showed improvement in lamellar onychoschizia on Day 60 with 95.24%, 80.00%, and 70% of patients across respective three doses as compared to baseline. Similarly, 2.5g and 10g of test treatment showed decrease in ridging of nails with improvement in 100% population at Day 60. For 5g dose, improvement was seen with 95% of population having PGA score 0 indicating no signs of nail ridging. Similarly, fragility or breakage of nails decreased with every successive visit with highest improvement observed at Day 60. 5g dosage group showed highest reductions having 100% patients with no signs of nail breakage at Day 60. Increase in nail thickness was seen in all the patients on every successive visit at Day 10, Day 30 and Day 60 as compared to baseline. Similarly, nail surface roughness also got reduced in study population with across all three doses at Day 60. Raggedness of nails showed reduction with no patients showing any signs of nail raggedness at Day 60 post treatment across all three treatment groups (Figure 12).
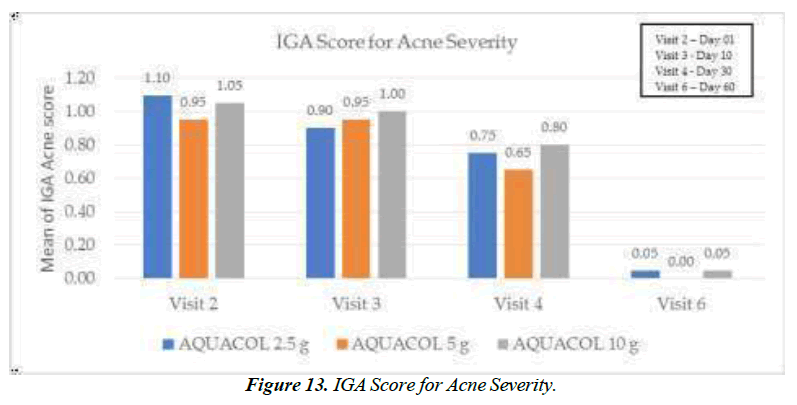
On Day 30, IGA score for Severity of Acne showed significant reduction (p<0.05) by 31.82% and 31.58% for doses 2.5g and 5g respectively. Highest reduction (p<0.0001) was observed at Day 60 by 95.45%, 100.00%, and 95.24% for doses 2.5g, 5g, and 10g respectively, as compared to baseline (Figure 13).
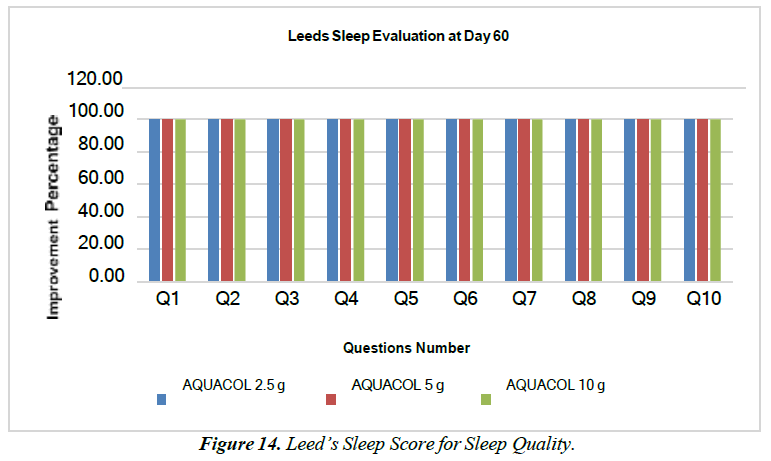
Leed’s Sleep Evaluation Test Questionnaires involving various questions which compare baseline and post treatment effects were used to evaluate quality of sleep. Question were asked regarding way to ease and quickness of falling asleep, sleepiness, restlessness, wakeful periods during sleep, difficulty/ease in awakening, balance/coordination during awakening, tiredness/alertness after waking up and during whole day. The results showed 100% of subjects observed improvement in overall sleep quality observed in all the treatment groups on Day 60 in comparison with baseline (Figure 14). Test treatment was also successful in improvement of general appearance of hair based on dermatological visual assessment. Overall increase was seen in hair volume. Hair shininess also showed good results at Day 60 after usage of 2.5g 5g, and 10g of doses. Similarly, hair smoothness increased with good results on Day 60 for the entire respective three treatment group. Density of hair increased with every visit and showed highest improvements on Day 60 across all treatment groups. Product perception assessment was done post treatment on Day 10, Day 30, and Day 60 for overall effect of test treatment on subjects. The results of assessment showed that majority of patients had positive effects of study treatment on face wrinkles, fine lines, smoothness and suppleness of skin, radiance/glow, skin moisturization, food digestion, gut health, bowel movement, quality and texture of skin, hair fall, hair growth, thickness, nail health, joint pain, stiffness, overall sleep, and taste of test treatment, and overall satisfaction. Hence, it can be said that the test treatment has positive impact on overall patient health. 100% of study subjects extremely liked the study product and were extremely satisfied with the same [Table 3].
| Subjective Perception Questionnaire (Percentage of Subjects in Agreement) | ||||||
|---|---|---|---|---|---|---|
| Question for Subject Perception post usage of Test Treatment | AQUACOL™2.5g | AQUACOL™5g | AQUACOL™10g | |||
| Day 30(N= 20) | Day 60(N=21) | Day 30(N=20) | Day 60(N=20) | Day 30(N=20) | Day 60(N=20) | |
| Improvement terms of reducing fine lines and wrinkles | 95.00% | 100.00% | 85.00% | 100.00% | 90.00% | 100.00% |
| Improvement in terms of smoothness and suppleness of skin | 90.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% |
| Effective in reducing crow’s feet area fine lines | 95.00% | 100.00% | 90.00% | 100.00% | 95.00% | 100.00% |
| Effective in terms of radiance/glow of skin | 90.00% | 100.00% | 95.00% | 100.00% | 95.00% | 100.00% |
| Improvement in skin moisturization | 90.00% | 100.00% | 100.00% | 100.00% | 90.00% | 100.00% |
| Improvement in food digestion capacity and gut health | 90.00% | 100.00% | 90.00% | 100.00% | 95.00% | 100.00% |
| Improvement in quality and texture of skin | 85.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% |
| Effective in reducing hair fall | 85.00% | 100.00% | 90.00% | 100.00% | 100.00% | 100.00% |
| Improvement in hair growth and thickness | 95.00% | 100.00% | 90.00% | 100.00% | 95.00% | 100.00% |
| Improvement in overall nails health | 100.00% | 100.00% | 85.00% | 100.00% | 85.00% | 100.00% |
| Effective in terms of reducing joint pain and stiffness | 85.00% | 100.00% | 85.00% | 100.00% | 90.00% | 100.00% |
| Improvement in terms of food digestion, bowel movement and gut health | 100.00% | 100.00% | 100.00% | 100.00% | 90.00% | 100.00% |
| Improvement in overall sleep | 95.00% | 100.00% | 85.00% | 100.00% | 90.00% | 100.00% |
| Likeness of test treatment | 95.00% | 100.00% | 100.00% | 100.00% | 90.00% | 100.00% |
| Overall Satisfaction with test treatment | 95.00% | 100.00% | 100.00% | 100.00% | 95.00% | 100.00% |
Table 3.Subjective perception questionnaire
Discussion
Over the past twenty years, marine collagen has garnered substantial attention from both the scientific and industrial communities. This marine-derived collagen holds significant potential across diverse health-related sectors including food, medicine, pharmaceuticals, and cosmetics. A notable catalyst in this exploration has been the abundant supply of waste byproducts stemming from the fish processing industry. These cost-effective remnants, such as fish skin and scales, have steered research efforts toward the transformation of these resources into collagen-based products of elevated value while minimizing environmental impact [21].
The beauty and wellness industry has witnessed a surge in interest regarding the use of collagen supplements for improving skin health, reducing joint stiffness and pain, promoting hair growth, and enhancing muscle strength. The current study effectively achieved its predetermined primary and secondary objectives and endpoints. Through the establishment of diverse evaluation criteria, it has demonstrated its success by significantly impacting human health. The test treatment has been proved to be efficient in the significant reduction of wrinkles, skin roughness, scaliness, hydration, smoothness and viscoelasticity. Additionally, it also reduced joint pain and stiffness with improved muscle strength when compared to baseline.
Human studies have also demonstrated that marine collagen possesses the ability to diminish wrinkles, enhance skin elasticity, and elevate the overall complexion and texture of the skin. Our investigation aligns with the findings of Ito N. et al, who reported a substantial increase in skin elasticity following the administration of collagen supplementation, with no observed instances of toxicity [22]. Evans et al. conducted a randomized clinical trial involving female aged 45 to 60 years as an aim to determine the effect of collagen on both wrinkled skin and elasticity. After three
References
- Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol Csh Perspect Biol. 2011 ;3(1):a004978.
- Couchman JR, Gibson WT. Expression of basement membrane components through morphological changes in the hair growth cycle. Dev Biol. 1985;108(2):290-8.
- Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol. 2006;12(3):145-54.
- Geahchan S, Baharlouei P, Rahman A. Marine collagen: a promising biomaterial for wound healing, skin anti-aging, and bone regeneration. Mar Drugs. 2022;20(1):61.
- Furtado M, Chen L, Chen Z, et al. Development of fish collagen in tissue regeneration and drug delivery. Eng Regen. 2022;3(3):217-31.
- Cho JK, Jin YG, Rha SJ, et al. Biochemical characteristics of four marine fish skins in Korea. Food Chem. 2014;159:200-7.
- Jafari H, Lista A, Siekapen MM, et al. . Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Poly. 2020;12(10):2230.
- Al-Atif H. Collagen supplements for aging and wrinkles: a paradigm shift in the fields of dermatology and cosmetics. Derma Practical & Conceptual. 2022;12(1).
- Blume-Peytavi U, Kottner J, Sterry W, et al. Age-associated skin conditions and diseases: current perspectives and future options. Geront. 2016;56(Suppl_2):S230-42.
- Pérez-Sánchez A, Barrajón-Catalán E, Herranz-López M, et al.. Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nut. 2018;10(4):403.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Trans Plan. 2018 ;27(5):729-38.
- Sato K. The presence of food-derived collagen peptides in human body-structure and biological activity. Food Funct . 2017;8(12):4325-30.
- Cole MA, Quan T, Voorhees JJ, et al. Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J Cell Commun Signal. 2018;12:35-43.
- Arseni L, Lombardi A, Orioli D. From structure to phenotype: impact of collagen alterations on human health. Int J Mol Sci. 2018;19(5):1407.
- Zague V, de Freitas V, Rosa MD, et al. Collagen hydrolysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J Med Food. 2011;14(6):618-24.
- Schlippe G, Bolke L, Voss W. Einfluss oraler Einnahme von Kollagen-Peptiden auf relevante Parameter der Hautalterung: Hautfeuchtigkeit, Hautelastizität und Hautrauigkeit. Aktuelle Dermatologie. 2015:529-34.
- Choi FD, Sung CT, Juhasz ML, et al. Oral collagen supplementation: a systematic review of dermatological applications. J Drugs Dermatol. 2019;18(1):9-16.
- Subhan F, Ikram M, Shehzad A, et al. Marine collagen: An emerging player in biomedical applications. J Food Sci Tech. 2015;52:4703-7.
- Czajka A, Kania EM, Genovese L, et al. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nut Res. 2018;57:97-108.
- Patel, M, Patel N, Nangha B, et al. A Novel Exploratory Approach of Validation and Standardization for Test Methods and Evaluators to conduct Safety and Efficacy Clinical Testing of Hair Growth Products. Paripex - Indian J Sci Res. 2023, 12(01), 33-39.
- Salvatore L, Gallo N, Natali ML, et al. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater Sci Eng.2020;113:110963.
- Ito N, Seki S, Ueda F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels—A randomized, double-blind, placebo-controlled trial. Mar Drugs. 2018;16(12):482.
- Evans M, Lewis ED, Zakaria N, et al. A randomized, triple?blind, placebo?controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J Cosmet Dermatol. 2021;20(3):825-34.
- Raabe O, Reich C, Wenisch S, et al. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histo Chem Cell Biol. 2010;134:545-54.
- Bae I, Osatomi K, Yoshida A, et al. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. Food Chem. 2008;108(1):49-54.
- Kim MM, Mendis E, Rajapakse N, et al. Effect of spongin derived from Hymeniacidon sinapium on bone mineralization. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2009 Aug;90(2):540-6.
- Hexsel D, Zague V, Schunck M, et al. Oral supplementation with specific bioactive collagen peptides improves nail growth and reduces symptoms of brittle nails. Food Chem. 2017;16(4):520-6.
- Paul C, Leser S, Oesser S. Significant amounts of functional collagen peptides can be incorporated in the diet while maintaining indispensable amino acid balance. Nut. 2019;11(5):1079.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref