Research Article - Journal of Clinical Respiratory Medicine (2018) Journal of Clinical Respiratory Medicine (Special Issue 1-2018)
Compare permissive underfeeding enteral nutrition with stand feeding under enough protein intakes on mortality in critically ill patients: a systematic review and meta-analysis planning councils in the United States.
Chang De Wu1, Jian Feng Xie2, Xi Wen Zhang2, Cong Shan Yang2, Ying Zi Huang2, Song Qiao Liu2, Hai Bo Qiu2, Yi Yang2*
1Department of Sociology and Anthropology, Program in Health, Medicine and Society, Lehigh University, USA
2Department of Critical Care Medicine, Nanjing ZhongDa Hospital, School of Medicine, Southeast University, China
- Corresponding Author:
- Yi Yang
Department of Critical Care Medicine
School of Medicine, Southeast University
Nanjing ZhongDa Hospital, China
Tel: +86-025-832-62550
E-mail: yiyiyang2004@163.com
Accepted date: October 30, 2017
Citation: Wu CD, Xie JF, Zhang XW, et al. Compare permissive underfeeding enteral nutrition with stand feeding under enough protein intakes on mortality in critically ill patients: a systematic review and meta-analysis planning councils in the United States. J Clin Respir Med. 2017;1(1):3-11.
Abstract
Background: Though many guidelines about nutritional support, there are still dispute about permissive underfeeding, especially when the protein supply was enough. We performed a systematic review and meta-analysis to compare underfeeding with standard feeding on the clinical outcome of ICU patients.
Methods: Data from the MEDLINE, EMBASE, Cochrane database, Web of Science and https://clinicaltrials.gov/ (until December, 31, 2016) were searched to identify relevant studies. Trial sequential analysis (TSA) was also calculated.
Results: A total of 6 studies with 1455 patients were included in the analysis. Overall, there was no significant difference in the risk of mortality [RR=0.82, 95% CI (0.59, 1.14)] and the length of ICU stay [Mean Difference=-0.94, 95% CI (-5.57, 3.69)] in the permissive underfeeding group with standard feeding group. The incidence of nosocomial acquired infections also had not significant difference in the two group [RR 0.95; 95% CI (0.83, 1.09); p=0.46].
Conclusion: This meta-analysis demonstrated there is no difference in the risk of hospital mortality, ICU length of stay (LOS) and nosocomial acquired infection between permissive underfeeding enteral nutrition and standard feeding under enough protein.
Keywords
Underfeeding, Hypo caloric nutrition, Hypo energetic nutrition, Critically ill.
Introduction
Patients with critical illness always demonstrate a systemic inflammatory response coupled with infectious complications, multiple organ dysfunction, prolonged hospitalization, and disproportionate mortality [1]. Nutritional therapy is very important to intensive care unit (ICU) patients, in which early enteral nutrition support as a therapeutic tool is thought to help modulate systemic immunity, attenuate disease severity and the physiologic stress response and favorably affect patient outcome [2,3].
Yet the best formulation and amount of enteral nutrition remain unknown. According to published guidelines from American Society for Enteral and Parenteral Nutrition (A.S.P.E.N.) indirect calorimetry (IC) or a simple weightbased equation (25-30 kcal/kg/day) could be used to determine energy requirements [1]. However, feeding intolerance and common care practices (e.g. gastric residual volume limits) often impede the achievement of recommended goals [4-6]. Doig et al. [6] suggested that evidence-based feeding guideline had promoted earlier feeding and greater nutritional adequacy. However, use of the guideline did not improve clinical outcomes. Guideline and control ICUs did not differ with regard to hospital discharge mortality (28.9% vs. 27.4%, p=0.75) or to hospital length of stay (24.2 vs. 24.3 days, p=0.97) or ICU length of stay (9.1 vs. 9.9 days, p=0.42). However, two trials suggested that EN should be supplemented with parenteral nutrition in critically ill patients [7,8]. They proposed that avoiding caloric debt could result in the reduction of infection. However, in both studies there have been the difference in protein delivery between the control group (not supplemented) (53-56 g/d) and the intervention group (76-79 g/d), which might influence the difference in mortality and nosocomial infection. Provision of protein is more closely linked to positive outcomes than provision of total energy.
In fact, several studies suggested that 50%-65% of goal energy may be enough to prevent increasing of intestinal permeability and systemic infection in burn and bone-marrow transplant patients, to promote faster return of cognitive function in head injury patients and to reduce mortality in high-risk hospitalized patients [1,2,9]. Moreover, studies suggest that hypocaloric feeding or permissive underfeeding may result in shorter duration of mechanical ventilation and the reduction of mortality [10-13]. While the Permit Trial assigned 894 critically ill adults to permissive underfeeding (40 to 60% of calculated caloric requirements) or standard enteral feeding (70 to 100%) for up to 14 days [14]. There was no significant between-group difference in feeding intolerance, diarrhea, infections acquired in the ICU, ICU or hospital length of stay and 90 days mortality [14]. However, provision of protein in some of the study was not the same.
Just for lack of clear evidence regarding the optimum amount of energy for critically ill patients, we conducted the meta-analysis to find the appropriate caloric feeding strategy with enough protein through reviewing published randomized clinical trials. In the present meta-analysis, we addressed the question whether permissive underfeeding has a positive effect on clinical outcomes in patients who were critically ill compared with standard calorie feeding under the condition of similar protein intake.
Methods
Data source
We performed this meta-analysis by using the methodology detailed in the Cochrane Handbook for Systematic Reviews of Interventions Study identification and following the PRISMA group statement [15]. The following databases were searched for published articles until December, 31, 2016: MEDLINE, EMBASE, Cochrane database, Web of Science and https://clinicaltrials.gov/ We used broad search terms containing “trophic feeding” or “hypocaloric nutrition” or “permissive underfeeding” or “gradual enteral nutrition” or “standard enteral nutrition” or “intensive enteral nutrition” or “concentrated enteral nutrition” or “normocaloric nutrition” or “full feeding” or “artificial underfeeding” or “hypocaloric feeding” or “hypoenergetic”, and “critical illness” or “critically ill” or “ICU” or “intensive care” or “acute lung injury” or “respiratory insufficiency” or “sepsis” or “septic shock”, and “randomized” or “clinical trial”, not “children” not “infants”. The search was conducted as described in the search strategy (Tables S1). Data publication details, details on study characteristics age, body mass index (BMI), critical illness severity (APACHE II score), nutrition therapy (including Caloric intake, the protein delivered) and results were extracted.
Study selection
We included original studies only if they met the following inclusion criteria:
1. Study design: Randomized clinical trials.
2. Study population: Critically ill adult patients (>18 years of age), defined as patients admitted to an ICU. Also all of the patients were given nutrition support beyond 48 h in ICU. We included trials with the features that have the same disease status such as age, APACHE II score and IBM.
3. Intervention: Permissive underfeeding or hypo-calorie feeding defined as below 60% of calculated caloric requirements according to the nutrition guideline calorie target (25-30 kcal/kg/d), protein intake beyond 0.8 g/kg/d or 40 g/d.
4. Control: Standard feeding defined as calorie consumption beyond 60% of daily energy expenditure target (25-30 kcal/kg/d), protein intake beyond 0.8 g/ kg/d or 40 g/d.
5. Study outcomes: a) Primary outcome: The hospital mortality or 28 days mortality or 90 days mortality; b) Secondary outcomes: ICU and hospital lengths of stay (LOSs), the risk of acquired infections, ventilator associated pneumonia (VAP), duration of mechanical ventilation or feeding intolerance.
Studies were excluded if they met either of the following criteria: 1) the main calorie supply was from parenteral nutrition; 2) EN was administered with less than 2 days; 3) the nutrition route is discriminative (such as nasogastric tube vs. nasointestinal tube); 4) studies for the timing of enteral nutrition initiate; 5) studies which did not reported hospital mortality, ICU mortality and 28 mortality.
Two reviewers independently screened titles and abstracts to determine whether a particular study met the inclusion criteria. The full texts of the articles were then reviewed according to the inclusion and exclusion criteria. Any disagreements were resolved by a consensus on the inclusion or exclusion of a particular study after a discussion with a third reviewer.
Methodological quality assessment
By using a scoring system of the Cochrane Handbook for Systematic Reviews of Interventions, we also assessed the internal validity of the identified trials about random sequence generation, allocation concealment, blinding of participants, personnel, and outcome assessment, missing data and selective reporting.
Statistical analysis
We analyzed data from the included studies using Review Manager (Review Manager, version 5.3). For dichotomous outcome variable, a risk ratio (RR) with 95% confidence interval (CI) was calculated. For continuous outcome variable, mean differences between permissive underfeeding and standard feeding were calculated and 95% CIs were derived. The heterogeneity among the included studies was assessed by the Mantel-Haenzel chi-square test and the I2 test. Any obvious heterogeneity was predefined as p<0.05 with the Mantel-Haenzel chi-square test or I2>40%, then the randomeffects model was applied. Otherwise, the fixed-effects model was applied. A publication bias was assessed using funnel plot. Due to type I errors which result from an increased risk of random error and repeated significance testing [16], we used trial sequential analysis (TSA; TSA software version 0.9 Beta; Copenhagen Trial Unit, Copenhagen, Denmark), which combines information size estimation with an adjusted threshold for statistical significance in the cumulative metaanalysis [16,17]. Information size was calculated as diversityadjusted information size (DIS) [18]. Sensitivity analyses were used to assess the impact of study quality on the overall effect. A subgroup meta-analysis was performed to determine the discriminative feeding strategy on the critically ill patients with different BMI (BMI<28, 28 ≤ BMI<32 and BMI ≥ 32), also different critical illness severity (APACHE II score ≥ 20 and APACHE II score<20). Moreover, we also performed meta-analysis to compare the effects between underfeeding and standard feeding enteral nutrition on the outcomes of ventilator associated pneumonia (VAP).
Results
Study location and selection
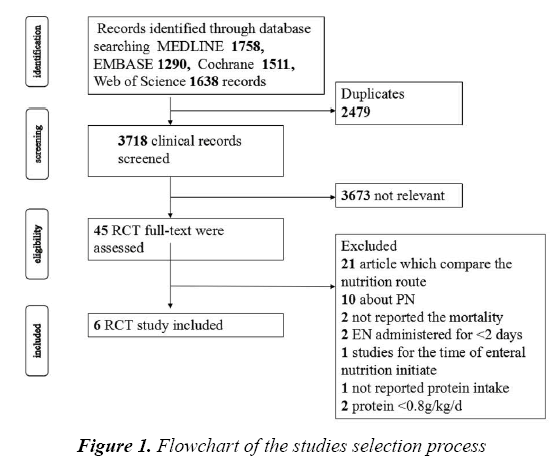
We identified a total of 3718 titles and abstracts after the primary search. After screening the abstracts, 3673 articles were determined to be non-relevant. The remaining 45 articles were retrieved for an eligibility assessment, of which, 39 were deemed to be excluded (Figure 1). Finally, 6 studies were included in this meta-analysis [10,14,19-22].
Summary of studies
Permissive underfeeding versus standard feeding was shown in 2 trials and the comparison between hypocaloric nutrition and nomalcaloric nutrition was also seen in 2 trials. While one trial compares the intensive medical nutrition intervention (IMNT) vs. the standard care, the percentage of estimated energy needs received per day is 84.7% vs. 55.4% [22]. As summarized below the overall description the characteristics of studies included respectively (Table 1).
| Study | Year | Setting | Population | Sample size | Nutrition interventions | Age | BMI (kg/m2) | APACHE II | Caloric intake (Kcal/kg/d) / percentage of caloric goal (%) | protein intake (g/kg/d or g/d) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| under* | st# | Under* | st# | Under* | St# | Under* | St# | Under* | St# | Under* | St# | |||||
| Dickerson et al [10]. | 2002 | Single Center |
Critically ill Obese, EN>7 d. | 40 | hypocaloric<20Kcal/kg/Day | Encaloric,≥ 20Kcal/kg/Day | 45.0 ± 16.6 | 43.3 ± 15.5 | 41.3 ± 13.7 | 36.0 ± 12.4 | 17.2 ± 6.7 | 18.1 ± 5.1 | 54% | 71.6% | 1.58 ± 0.52 | 1.81 ± 0.46 |
| Arabi et al [20]. | 2011 | Single Center |
ICU>2 days; MV |
240 | Permissive underfeeding, 60-70% | Target feeding, 25-30 Kcal/kg/Day,90-100% | 50 | 52 | 28.5 | 28.5 | 25 | 25 | 59% | 71.4% | 47.5 ± 21.2 | 43.6 ± 18.9 |
| Braunschweig et al [22]. | 2015 | Single Center |
Acute lung injury | 78 | Standard care, 55.4% of caloric goal | IMNT; 84.7% of caloric goal | 58.6 ± 16.2 | 52.5 ± 17.1 | 30.1 ± 8.9 | 29.8 ± 9.3 | 27.7 ± 7.9 | 23.4 ± 9.3 | 55.4% | 84.7% | 60.4 ± 24 | 82 ± 23 |
| Charles et al [19]. | 2014 | Single Center |
SICU>2 days | 83 | Hypocaloric, 50% | Eucaloric, 25-30 Kcal/kg/Day |
50 | 53 | 32.9 | 28.1 | 17 | 17 | 40.5% | 73% | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Arabi et al [14]. | 2015 | Multicenter | ICU>3 days, MV | 894 | Permissive underfeeding, 40-60% | Standard enteral feeding, 70-100% |
50 | 51 | 29 | 29.7 | 21 | 21 | 46% | 71% | 57 ± 24 | 59 ± 25 |
| Rugeles et al [21]. | 2016 | Single Center |
require en through nose tube > 96 h. | 120 | Hypocaloric, 15 Kcal/kg/Day | Normocaloric, 25-30 Kcal/kg/Day | 53.8 ± 19.0 | 51.8 ± 20.3 | 25 ± 2.5 | 25 ± 2.5 | 13.5 ± 6.4 | 13.7 ± 6.8 | 50.4% | 82% | 1.4 ± 0.4 | 1.4 ± 0.3 |
*under means permissive underfeeding enteral nutrition #st means standard feeding enteral nutrition
Table 1: Characteristics of studies included in meta-analysis, *under means permissive underfeeding enteral nutrition, #st means standard feeding enteral nutrition
Quality assessment
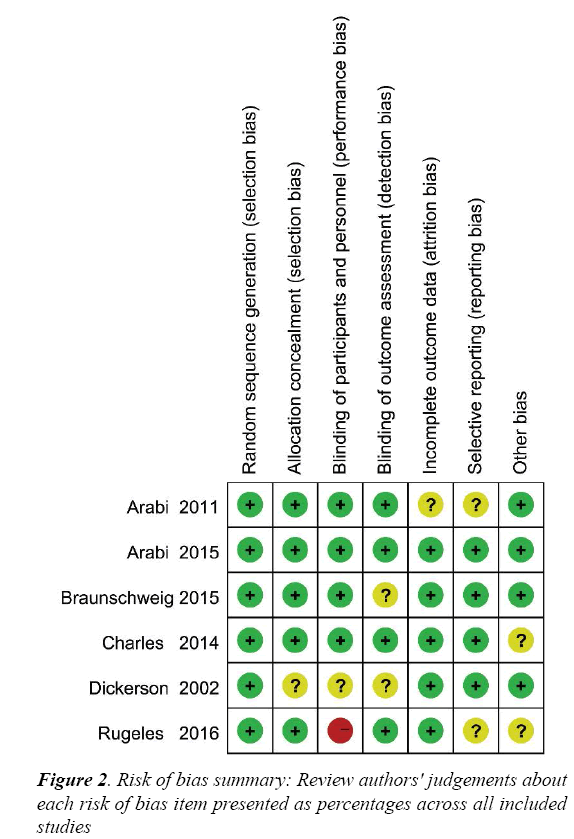
The risk of bias for each RCT is displayed in Figure 2 and the risk of bias across all RCTs is displayed in Figure S1. These indicated generally good methodological quality.
Random errors
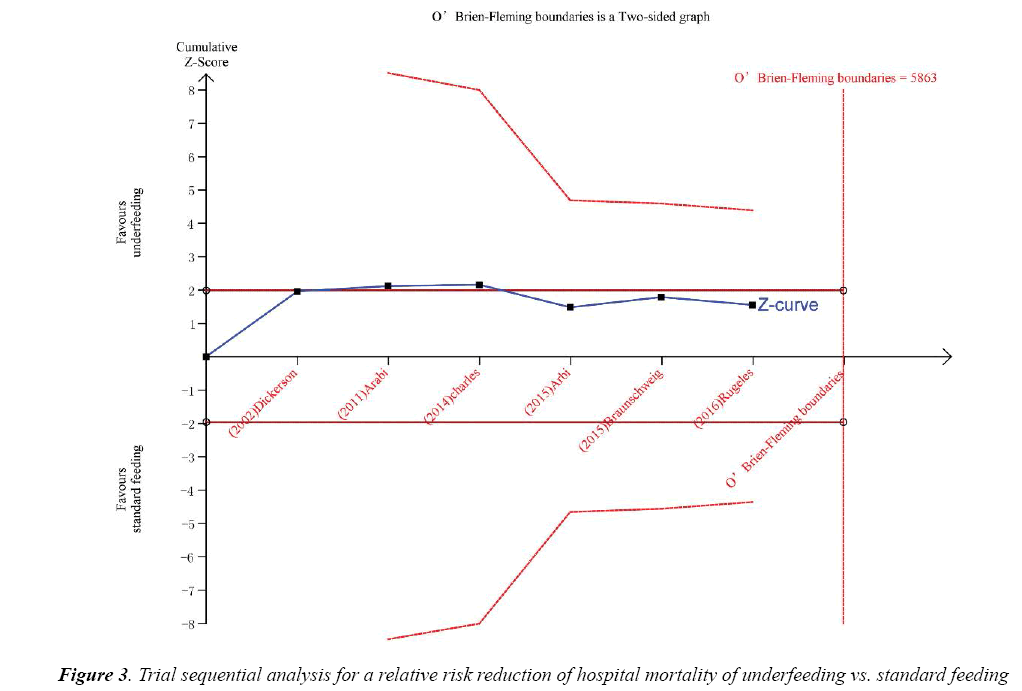
Trial sequential analysis (TSA) was calculated with α=0.05 and β=0.2 (power 80%) and a required diversityadjusted information size based on the intervention effect by using a random effects model (RRR of 17.89% regarding mortality and 5863 patients). TSA indicated that there was lack of reliable and conclusive evidence for a beneficial effect of underfeeding on hospital mortality (Figure 3), since the monitoring boundaries were not finally surpassed and the required size was not reached.
Sensitivity analysis
As shown in Table S2, we did not found significant heterogeneity.
The impact on mortality
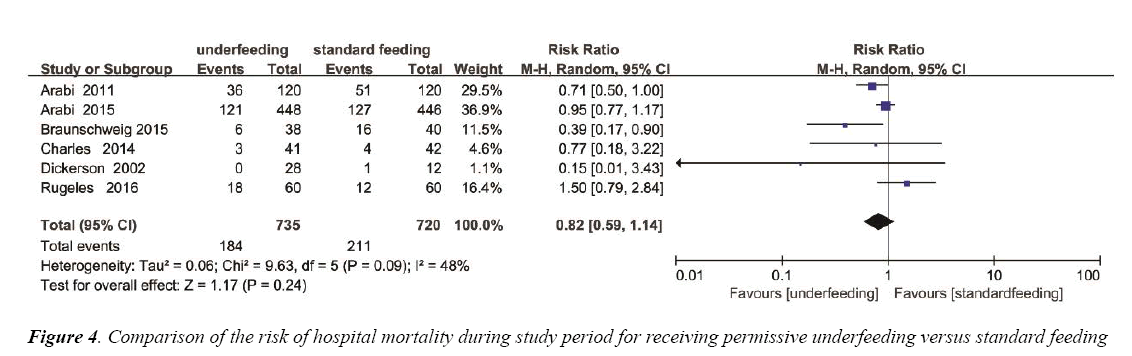
The overall effect of different feeding Strategies on the mortality rates was estimated from 6 trials, which included a total of 1455 patients (Figure 4). We detected no evidence of a publication bias after a funnel plot analysis (Figure S2) and the heterogeneity was determined to be significant (p=0.09, I2=48%). There was no significant difference in hospital mortality between permissive underfeeding group and standard feeding group (RR=0.82; 95% CI [0.59, 1.14], p=0.24) (Figure 4).
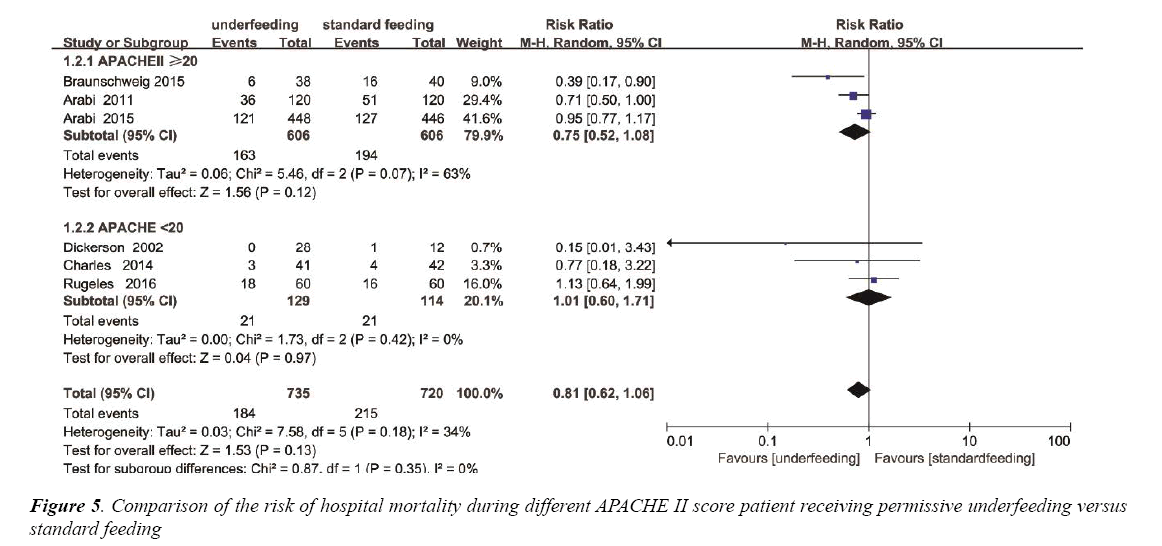
Based on the mean APACHE II score, Subgroup analysis was performed to assess the differences in mortality between underfeeding and standard feeding among the two subgroups. Mortality was not different in the APACHE II<20 subgroup (RR, 1.01 95% CI [0.60, 1.71]; P=0.97). In contrast, in APACHE II ≥ 20 subgroup, mortality was also not different (RR, 0.75; 95% CI, 052 to 1.08; P=0.12) (Figure 5). The heterogeneity was also determined to be not significant (p=0.18, I2=34%) (Figure S3).
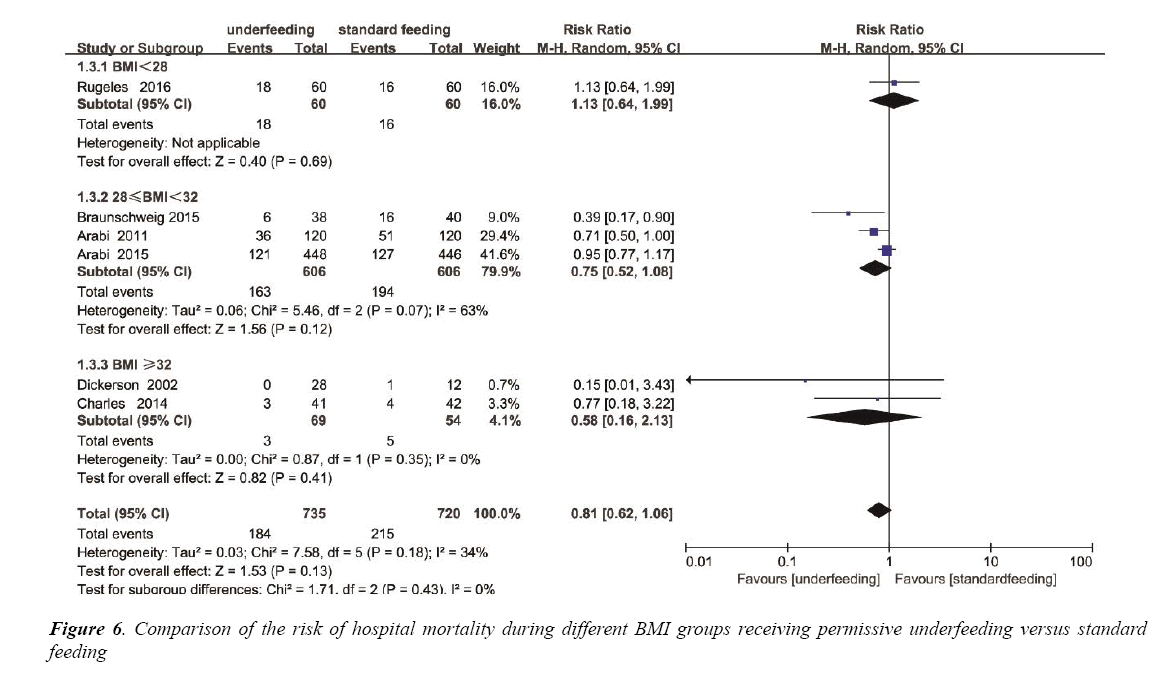
Among the different BMI groups (BMI<28, 28 ≤ BMI<32 and BMI ≥ 32), subgroup analysis was individually performed. Mortality was not different in the BMI<28 subgroup (RR, 1.13; 95% CI, 0.64 to 1.99; P=0.69), also not different in the 28 ≤ BMI<32 subgroup (RR, 0.75; 95% CI, 0.52 to 1.08; P=0.12), and not different in the BMI ≥ 32 subgroup (RR, 0.58; 95% CI, 0.16 to 2.13; P=0.35) (Figure 6). The heterogeneity was also determined to be not significant (p=0.18, I2=34%) (Figure S4).
The impact on ICU length of stay
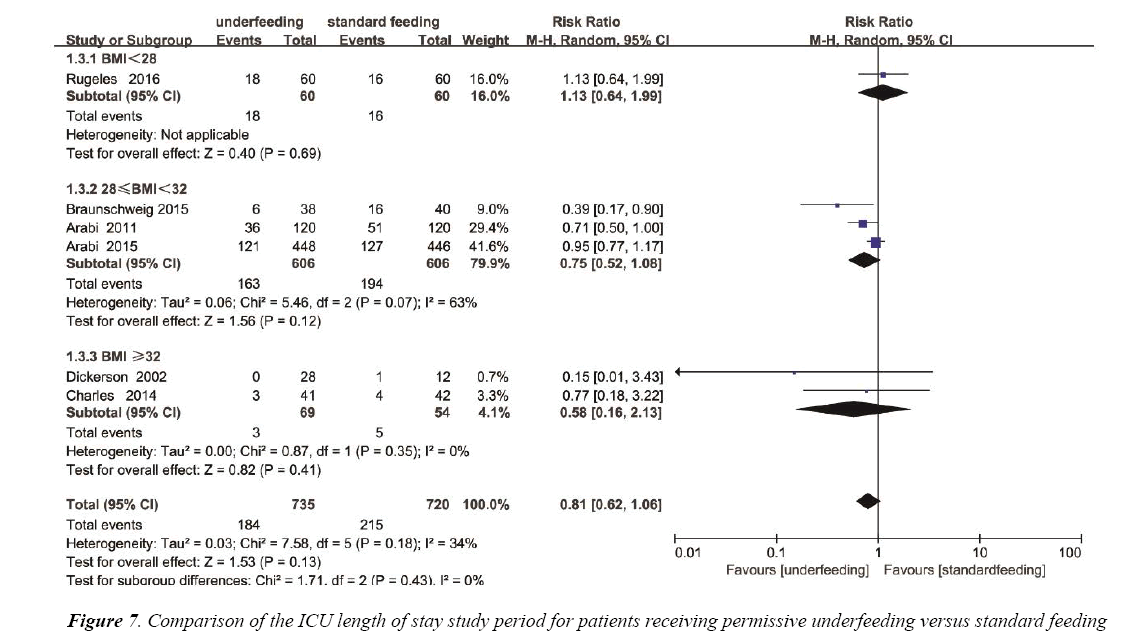
The ICU length of stay was reported in 4 trials with a total of 441 patients. We detected no evidence of a publication bias (Figure S5), but the heterogeneity was statistically significant (p=0.0002, I2=85%). There has no statistics difference between the underfeeding groups and standard feeding group with respect to the length of hospital stay (Mean Difference=-0.94, 95% CI (-5.57, 3.69), p=0.69) (Figure 7).
The impact on the incidence rate of nosocomial acquired infections
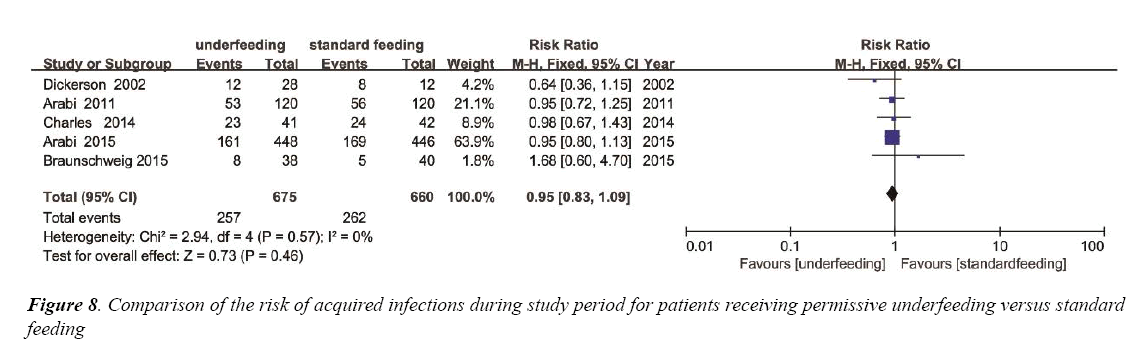
To estimate the overall impact of underfeeding and standard feeding on the risk of acquired infections, 5 trials were enrolled with 1335 patients (Figure 8). No evidence of a publication bias was observed by a funnel plot (Figure S6) and the heterogeneity (p=0.57, I2=0%). The incidence of nosocomial acquired infections had no significant difference between the two group (RR 0.95; 95% CI, 0.83 to 1.09; p=0.46) (Figure 8).
The impact on the incidence rate of pneumonia
To estimate the overall impact of underfeeding and standard feeding on the risk of Pneumonia, 3 trials were included with a total of 1017 patients (Figure S7). No evidence of a publication bias was observed following a funnel plot assessment (Figure S8), the heterogeneity (P=0.48; I²=0%). The incidence of nosocomial acquired Pneumonia had no significant difference between the two group (RR 0.89; 95% CI [0.72, 1.10]; p=0.28) (Figure S7).
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation as well as the experimental conclusions that can be drawn.
Discussion
This meta-analysis demonstrated there might be no benefit of standard feeding (targeting caloric requirements beyond 60% of daily energy expenditure) compared to permissive underfeeding under enough protein in the critical illness. We also found no significant difference between underfeeding and standard feeding with respect to ICU-acquired infections. These findings would challenge conventional view and current nutrition guidelines [1,12]. Several studies examining various doses of enteral feeding have yielded conflicting results [23-27].
Our results were consistent with the Permit Trial which assigned 894 critically ill adults to permissive underfeeding or standard enteral feeding [14]. Also, we analyzed subgroup meta- analysis of feeding strategy on the critically ill patients with different BMI (BMI<28, 28 ≤ BMI<32 and BMI ≥ 32) and different critical illness severity (APACHE II score ≥ 20 and APACHE II score<20), which were the important foundations of the Nutrition Risk in Critically ill (NUTRIC) score [28]. No difference between these feeding strategies was found.
It has been suggested that underfeeding nutrition may impair immune responsiveness and increase infectious complications [29,30]. Enteral nutrition supports the structural and functional integrity of the intestine, helping to prevent increased gut permeability and associated bacterial translocation [31]. A small amount of enter nutrition is enough to preserves tight junctions between epithelial cells, and promotes blood flow. With the improvement of the nurse skills, the infection rate may have no difference [32,33]. As to the overall impact of underfeeding and standard feeding on the risk of acquired infections, we found no difference. It indicates that the permissive underfeeding enteral nutrition support could meet the body need for support intestinal structure and function, and prevent bacterial translocation.
When referring to nutrition therapy, both the calorie goal and protein intake are main priority for critically ill patients. Rugeles et al. [21,34] focused on not only the calorie goals but also the protein intake. In one study of 2013 [34], the total amount of calories delivered was similarly low in both groups (12 kcal/kg in intervention group vs. 14 kcal/kg in controls), but protein delivery was significantly different (1.4 vs. 0.76 g/kg). The hyper-protein group showed an improvement in SOFA score at 48 h (delta SOFA1.7 ± 1.9 vs. 0.7 ± 2.8, P=0.04) and less hyperglycemic episodes per day. In other study of 2016 [21] both group hypocaloric (15 kcal/kg/day) and normocaloric (25 kcal/kg/day) with hyper-protein intake (1.7 g of protein/kg/day). They did not find a statistically significant difference in ΔSOFA at 48 h. This seemingly paradox further proved the important of protein intake. When exogenous calories are delivered beyond the tolerance of the body, then the endogenous production may result in metabolic disease [35-37]. The reasons for why standard calorie goal feeding did not have some advantage may be that standard calorie nutrition support did not alter the catabolic process or immune response associated with acute critical illness. As a therapy measure, nutrition support should be assigned along with the body metabolism state [35]. However, the duration of underfeeding nutrition support was different in the related trials, such as 7 days, 14 days and the duration of ICU stay [10,14,19-22,38,39]. As we all know, underfeeding may be detrimental for patients especially in the recovery phase, as the body need more nutrition support to repair past the acute phase. Therefore permissive underfeeding may be appropriate to the critically ill patients in the acute phase, not to all of the patients during the whole disease phase. In a large observational study, Heyland et al. [40] showed that for low nutrition risk patients, no correlation between the percent of goal energy delivered and mortality. From this meta-analysis, we can find the tendency to favour underfeeding EN for high APACHE II score patients. It is important to emphasize that this meta-analysis compared the provision of different levels of energy and protein on different severity of illness and metabolism phase.
Limitations
The main limitation of this meta-analysis is that the feeding protocols were not the same across studies. The patient sample sizes are also heterogenous. Although we analyzed subgroup of feeding strategy on the different BMI and APACHE II score which were the important foundations of the NUTRIC score, the patients enrolled were had not assessed the nutrition risk. The studies enrolled in this analysis may be high selectivity; it is possible that this metaanalysis do not have enough power to rule out the presence of a clinically meaningful effect.
Future Directions
According to the results of trial sequential analysis, additional prospective studies targeting this patient population would be required to resolve this issue. The main critical factor may include the quantity, quality and proportion of protein and calorie, but also the severity of illness and nutrition risk.
Conclusion
This meta-analysis demonstrated with enough protein intakes, there have been no difference in the risk of hospital mortality, ICU length of stay (LOS) and nosocomial acquired infection between permissive underfeeding enteral nutrition and standard feeding. To critically ill patients if we support enough protein in the early phase than the energy requirements may be not the key point of enteral nutrition than affect clinical prognosis.
Acknowledgement
This work was supported by grants YKK16259 from the Nanjing general research foundation.
Author’s Contribution
CDW and JFX conducted the literature searches, study selection, data extraction, assessed study quality and prepared initial drafts of the manuscript. XWZ and CSY reviewed abstracts, selected studies meeting inclusion criteria, extracted data, and assessed study quality. CDW, YZH and SQL input data and performed the statistical analyses. XWZ and HBQ helped synthesize data and gave methodological guidance on the use of RevMan 5.3 and TSA 0.9 Beta software. YY provided methodological guidance on drafting and revising the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taylor BE, McClave SA, Martindale R, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). J Parenter Enteral Nutr. 2016;40(2):159-211.
- Jabbar A, Chang WK, Dryden GW, et al. Gut immunology and the differential response to feeding and starvation. Nutr Clin Pract. 2003;18(6):461-82.
- Hiesmayr M, Schindler K, Pernicka E, et al. Decreased food intake is a risk factor for mortality in hospitalized patients: The nutrition day survey 2006. Clin Nutr. 2009;28(5):484-91.
- Heyland DK, Dhaliwal R, Day A, et al. Validation of the Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients: Results of a prospective observational study. Crit Care Med. 2004;32(11):2260-6.
- Rice TW, Swope T, Bozeman S, et al. Variation in enteral nutrition delivery in mechanically ventilated patients. Nutrition. 2005;21(7-8):786-92.
- Doig GS, Simpson F, Finfer S, et al. Effect of evidence-based feeding guidelines on mortality of critically ill adults: A cluster randomized controlled trial. JAMA. 2008;300(23):2731-41.
- Singer P, Anbar R, Cohen J, et al. The tight calorie control study (TICACOS): A prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011;37(4):601-9.
- Heidegger CP, Berger MM, Graf S, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet. 2013;381(98964):385-93.
- Taylor SJ, Fettes SB, Jewkes C, et al. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med. 1999;27:2525-31.
- Dickerson RN, Boschert KJ, Kudsk KA, et al. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition. 2002;18:241-6.
- Ibrahim EH, Mehringer L, Prentice D, et al. Early versus late enteral feeding of mechanically ventilated patients: Results of a clinical trial. J Parenter Enteral Nutr. 2002;26:174-81.
- Krishnan JA, Parce PB, Martinez A, et al. Caloric intake in medical ICU patients: Consistency of care with guidelines and relationship to clinical outcomes. Chest. 2003;124:297-305.
- Arabi YM, Haddad SH, Tamim HM, et al. Near-target caloric intake in critically ill medical-surgical patients is associated with adverse outcomes. J Parenter Enteral Nutr. 2010;34:280-8.
- Arabi YM, Aldawood AS, Haddad SH, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372:2398-408.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700:1-b2700:27.
- Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64-75.
- Thorlund K, Devereaux PJ, Wetterslev J. Can trial sequential monitoring boundaries reduce spurious inferences from meta- analyses? Int J Epidemiol. 2009;38:276-86.
- Wetterslev J, Thorlund K, Brok J, et al. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. 2009;9:1-86.
- Charles EJ, Petroze RT, Metzger R, et al. Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical intensive care unit: A randomized controlled trial. Am J Clin Nutr. 2014;100:1337-43.
- Arabi YM, Tamim HM, Dhar GS, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: A randomized controlled trial. Am J Clin Nutr. 2011;93:569-77.
- Rugeles S, Villarraga-Angulo LG, Ariza-Gutiérrez A, et al. A High-protein hypocaloric vs. normocaloric enteral nutrition in critically ill patients: A randomized clinical trial. J Crit Care. 2016;35:110-4.
- Braunschweig CA, Sheean PM, Peterson SJ, et al. Intensive nutrition in acute lung injury: A clinical trial (INTACT). J Parenter Enteral Nutr. 2015;39:13-20.
- Heyland DK, Murch, L, Cahill N, et al. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: Results of a cluster randomized trial. Crit Care Med. 2013;41:2743-53.
- Rice TW, Mogan, S, Hays MA, et al. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med. 2011;39:967-74.
- Rice TW, Wheeler AP, Thompson BT, et al. National Heart, Lung and Blood Institute acute respiratory distress syndrome (ARDS) clinical trials network, initial trophic vs. full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA. 2012;307:795-803.
- Petros S, Horbach M, Seidel et al. Hypocaloric vs. normocaloric nutrition in critically ill patients: A prospective randomized pilot trial. J Parenter Enteral Nutr. 2016;40:242-9.
- Peake SL, Davies AR, Deane AM, et al. TARGET investigators and the Australian and New Zealand intensive care society clinical trials group. Use of a concentrated enteral nutrition solution to increase calorie delivery to critically ill patients: A randomized, double-blind, clinical trial. Am J Clin Nutr. 2014;100:616-25.
- Heyland DK, Dhaliwal R, Jiang X, et al. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268.
- McCowen KC, Friel C, Sternberg J. Hypocaloric total parenteral nutrition: Effectiveness in prevention of hyperglycemia and infectious complications--a randomized clinical trial. Crit Care Med. 2000; 28:3606-11.
- Rubinson L, Diette GB, Song X, et al. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004;32:350-7.
- MacFie J, Reddy BS, Gatt M, et al. Bacterial translocation studied in 927 patients over 13 years. Br J Surg. 2006;93:87-93.
- Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506-17.
- Owais AE, Kabir SI, Mcnaught C, et al. A single-blinded randomised clinical trial of permissive underfeeding in patients requiring parenteral nutrition. Clin Nutr. 2014;33:997-1001.
- Rugeles SJ, Rueda JD, Díaz CE, et al. Hyperproteic hypocaloric enteral nutrition in the critically ill patient: A randomized controlled clinical trial. Indian J Crit Care Med. 2013;17:343-9.
- Stapleton RD, Jones N, Heyland DK. Feeding critically ill patients: What is the optimal amount of energy? Crit Care Med. 2007;35:s535-40.
- Ning YC, Cai GY, Zhuo L, et al. Beneficial effects of short-term calorie restriction against cisplatin-induced acute renal injury in aged rats. Nephron Exp Nephrol. 2013;124:19-27.
- Weijs PJ, Looijaard WG, Beishuizen A, et al. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care.2014;18(6):701.
- Desachy A, Clavel M, Vuagnat A, et al. Initial efficacy and tolerability of early enteral nutrition with immediate or gradual introduction in intubated patients. Intensive Care Med. 2008;34:1054-9.
- Martin CM, Doig GS, Heyland DK, et al. South-western Ontario critical care research network. Multicentre, cluster randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT). CMAJ. 2004;170:197-204.
- Heyland DK, Stephens KE, Day AG, et al. The success of enteral nutrition and ICU-acquired infections: A multicenter observational study. Clin Nutr. 2011;30:148-55.