Mini Review - Journal of Dermatology Research and Skin Care (2023) Volume 7, Issue 2
Clinical application of Nourkrin® as a pre and post−hair transplantation, growth stimulating treatment rationale and action mechanisms.
Jan Wadstein1*, Zaira Paulina Garcia Jimenez21Research and Development, Ostra Ronneholmsvagen Malmo, Sweden
2Fuera Calvicie Hair Restoration Clinic, Ciudad de Mexico, Mexico
- *Corresponding Author:
- Jan Wadstein
Research and Development
Ostra Ronneholmsvagen Malmo, Sweden
E-mail: dr.jan.wadstein@gmail.com, zairapgj@gmail.com
Received: 20-Feb-2023, Manuscript No. AADRSC-23-89660; Editor assigned: 21-Feb-2023, PreQC No. AADRSC-23-89660(PQ); Reviewed: 07-Mar-2023, QC No AADRSC-23-89660; Revised: 10-Mar-2023, Manuscript No. AADRSC-23-89660(R); Published: 17-Mar-2023, DOI:10.35841/aadrsc-7.2.137
Citation: Wadstein J, Jimenez Z P G. Clinical application of nourkrin® as a pre and post-hair transplantation, growth stimulating treatment rationale and action mechanisms. Dermatol Res Skin Care. 2023;7(2):137
Abstract
Pre and post-operative pharmacotherapy is often an essential adjunct to Hair Transplantation Surgery (HTS) to optimise its long-term clinical outcomes. Both systemic and topical hairstimulating therapies are often administered to minimise acute post-op hair shedding and loss/ miniaturisation of existing follicles at the recipient site. Emerging evidence revealed a progressive decline in proteoglycan expression in active parts of susceptible hair follicles, known as Follicular Hypoglycania (FHG). FHG contributes to the shortening of anagen and excessive hair shedding and can give rise to follicular miniaturisation due to Proteoglycan Follicular Atrophy (PFA). This unprecedented pathogenetic insight led to using Proteoglycan Replacement Therapy (PRT) with Nourkrin® as a novel adjunctive treatment. Nourkrin® Man and Woman contain Marilex-M and Marilex, rich in hair-specific natural proteoglycans, e.g., versican and decorin. These bioactive macromolecules are orally bioavailable and possess in-vivo anagen stimulating, catagen inhibitory and anti-miniaturisation properties. The positive effects of PRT with Nourkrin on hair growth and density are demonstrated through replicated clinical trials. This treatment can also improve some aspects of health-related quality of life in hair loss patients. Nourkrin® is safe and well tolerated for long-term use with no significant side effects in clinical studies. Accordingly, PRT can be recommended at least three months before the surgery, which may be resumed postop to ensure optimal survival and regrowth of the grafts and remaining follicles. Nourkrin® can be combined with conventional topical and systemic treatments and serve as a safe and effective part of a standard pre and post-HTS pharmacotherapy regimen.
Keywords
Proteoglycans, Nourkrin, Marilex, Proteoglycan Replacement Therapy, Hair Transplantation, Pattern Alopecia.
Introduction
Hair transplantation surgery: An ultimate solution to an unruly condition
Hair transplantation Surgery (HTS) that once was regarded an overly invasive and laborious procedure is now widely accepted as an effective, safe, and reliable hair restoration treatment. The development of micrografting techniques during the 1980s was a turning point in the evolution of surgical hair restoration and greatly improved its aesthetic outcomes. This revolutionary method, known as Follicular Unit Transplantation (FUT) using strip excision, has replaced the plug technique and continued to refine and evolve throughout the next few decades. The next leap forward was the introduction of Follicular Unit Extraction (FUE) technique in 2002 allowing individual hair follicles to be removed from the donor area. Both FUT and FUE techniques are in broad clinical use today, each having their own benefits and limitations.
In the past few decades, HTS has increasingly gained popularity across the globe helping thousands of men and women every day to regain a more youthful and natural appearance. Only in 2019, 735000 transplantation surgeries have been performed worldwide, which shows a 16% increase since 2016 [1]. The main target group for HTS is the individuals with advanced forms of pattern hair loss. This type of hair loss can occur in both men and women with distinctive presentation patterns, referred to as Male Pattern Hair Loss (MPHL) and Female Pattern Hair Loss (FPHL) respectively. Although their aetiologies have important disparities, both MPHL and FPHL are presented by an excessive and premature hair shedding and miniaturisation leading to significantly reduced hair density and coverage. There are two cardinal phenomena that underlie this clinical picture: first, the disturbance of hair growth cycle and multiplied anagento- telogen transition, and second, a stepwise terminal-tovellus hair transition. Both are important targets for the clinical treatment of pattern hair loss [2].
Currently, the standard medical treatment of diffuse pattern hair loss relies on topical minoxidil and oral finasteride. Despite the relative effectiveness of these medications in delaying the progress of MPHL and FPHL diffuse hair thinning, and patches of visible scalp will eventually appear in a considerable proportion of affected individuals [3]. HTS is the only available technique capable of restoring the lost hair and minimising its cosmetic and psychological complications. Follow-up studies show that HTS is an effective restorative method that significantly improves patients’ psychosocial wellbeing and satisfaction with appearance. Liu et al. reported that 6 months after the HTS, patients appeared on average 5.8 years younger and >80% experienced improvement in their social functioning [4].
In spite of its efficacy, HTS comes with few innate limitations/ complications that challenge patients and clinicians alike, including the following:
The progression of hair thinning and hair loss after the surgery: HTS is an aesthetic operation with no effect on the biological activity of existing hair follicles on the recipient site. Thus, the progressive anagen-shortening and miniaturisation of susceptible follicles will continue post-op undermining the operation’s long-term clinical outcomes.
Postoperative hair shedding (shock loss): Regardless of its technique, HTS is a traumatic procedure on the scalp and can trigger a diffuse hair thinning and shedding in the recipient and/or donor sites within a few weeks. This phenomenon is believed to be a localised telogen effluvium that affects both transplanted and existing hairs. Shock loss is a source of substantial concern and psychological stress for HTS candidates. This acute form of hair shedding may take up to 2 years to recover and tends to last longer in patients with high levels of psychological/physical stress [5].
Various modalities have been devised and utilised since the inception of HTS to prevent/minimise these limitations and maximise its long-term aesthetic benefits. Pre-/post-operative adjunctive pharmacotherapy is among the most popular of those modalities. A novel approach that is increasingly gaining attention is proteoglycan replacement therapy (PRT), which targets follicular proteoglycans.
The integral roles of bioactive proteoglycans in health and disease of the hair follicle
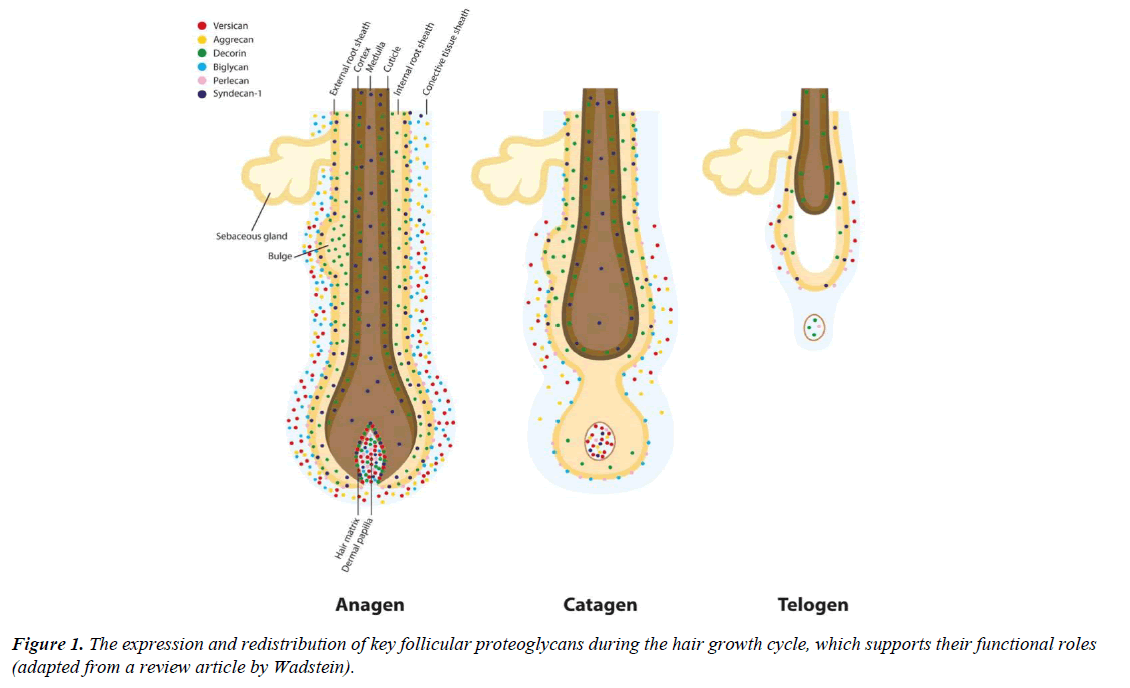
The essential regulators of hair growth and cycling: Proteoglycans are physiologically active macromolecules abundantly present in intracellular microenvironments, cell membrane and extracellular matrices. These macromolecules are closely involved in a wide range of cellular functions including signalling, growth, adhesion, migration, immune functions, and tissue repair [6]. Similar to all body organs, proteoglycans are also omnipresent in and around the hair follicle with a distinctive composition and tissue distribution. Specific proteoglycans such as versican, decorin syndecan and perlecan are highly expressed in the dermal papilla and other biologically important parts of the follicle. It is also discovered by histological studies that the expression of hair proteoglycans proactively changes as the hair follicle transits from anagen to telogen and vice versa (demonstrated in the Figure 1) [7,8].
Proteoglycans are known to play determining roles in the induction and maintenance of hair growth and preventing the active follicles from entering catagen. Through their multiple active domains, proteoglycans regulate and mediate the activity of growth factors and other anagen inducers, e.g., Wnt/β-catenin pathway. Versican and decorin are particularly potent anagen inducers [9,10]. During anagen, bioactive proteoglycans contribute to maintaining the anabolic homeostasis of the follicle and creating a loose and hydrated milieu necessary to support cell growth and migration. Besides, normal functioning and survival of follicular stem cells depends on the sufficient presence of proteoglycans. Therefore, induced underexpression/degradation of proteoglycans under the influence of androgens and other disruptive factors can lead to anagen shortening and miniaturisation of the hair follicles [8].
Proteoglycan dysmetabolism: An emerging aetiology of diffuse hair loss: Molecular research confirmed that the expression of certain proteoglycans is substantially reduced in androgen-sensitive hair follicles at both gene and protein levels [11]. Androgens are the suspected triggers of proteoglycan dysmetabolism in hair follicles due to their ability in suppressing the synthesise of extracellular matrix components by papillary fibroblasts [12,13]. This phenomenon leads to an induced decline in the concentration of proteoglycans in and around the hair follicle at anagen, termed ‘Follicular Hypoglycania (FHG)’ as proposed [8]. FHG leads to a significant decrease in the bioactivity of extracellular matrix and downregulation of anagen inducing factors (e.g., Wnt/β- catenin and IGF-1), which drives the follicle into entering catagen and staying in kenogen. Hence, long-standing FHG gives rise to progressively shorter anagen phases and hypotrophy and atrophy of susceptible hair follicles. This pathology is known as Proteoglycan Follicular Atrophy (PFA) presented clinically as follicular miniaturisation. Overall, regardless of the initial aetiology, FHG and PFA are suggested to underlie the main presentations of pattern hair loss in both men and women, which are anagen shortening and follicular miniaturisation [8].
Proteoglycan Replacement Therapy: An effective treatment to stop the progression of pattern hair loss
PRT is a therapeutic approach that utilises enzymaticextracted complexes of natural proteoglycans, branded as Marilex® and Marilex®-M by Pharma Medico Aps, to treat hair loss problems in women and men. These proteoglycanbased treatments are the main active ingredients of Nourkrin® Man and Woman (Pharma Medico Aps, Aarhus, Denmark). PRT with Nourkrin® is indicated for the treatment and prevention of androgenetic alopecia (MPHL and FPHL) and acute/chronic telogen effluvium in men and women. The clinical efficacy of this innovative method has been repeatedly tested objectively in controlled clinical trials [14-16] and subjectively in prospective cohorts [17,18] over the past three decades. The obtained evidence from clinical trials is summarised below. The reader is referred to a comprehensive review for more details on the pharmacology and clinical efficacy of Nourkrin® [8].
Anti-hair loss efficacy of Nourkrin® was evaluated in a longterm clinical trial on 60 patients of both sexes with diffuse hair loss. In this trial, 6 months of monotherapy with Nourkrin® significantly increased hair density by 32.4%, which was significantly higher than 0.9% in the placebo group (P<0.001). Continuing the treatment for another 6 months doubled the positive effect of Nourkrin® on hair density [14]. This trial was replicated in a group of 55 volunteers with diffuse hair loss. Nourkrin® was administered for a 6-month blinded period followed by an additional 6 months of open-label treatment. Intervention increased the average scalp hair count by 35.7% compared with 1.5% in the placebo group (P<0.001). The efficacy of Nourkrin® continued to increase over time; such that the hair density in affected areas increased by 60.8% after 12 months. Treatment satisfaction was considerably higher with Nourkrin® than with placebo (P< 0.001) [15]. Another interventional study was conducted on women with FPHL for 6 months to examine the effect of add-on therapy with Nourkrin® on quality of life. Various parameters of quality of life were assessed using Kingsley Alopecia Profile (KAP) score before and after adding Nourkrin® to a standard regimen of trichology treatments and lifestyle modifications. The final results led researchers to conclude that Nourkrin® therapy significantly improved the quality of life of patients with FPHL [16].
Using Nourkrin® as a Pre- and Post-Hair Transplantation Growth Stimulating Treatment
There is a consensus among skin surgeons on the necessity of hair stimulating pharmacotherapy before and after HTS. Maintenance treatment is particularly important in patients with ongoing hair thinning at the time of surgery [19]. Historically, a range of medications and nutri-cosmetic supplements have been tried as adjuncts to HTS with varying hair growth stimulatory efficacy and side effect profiles. The most common options are minoxidil, finasteride and vitamins/minerals.
Vitamin/mineral supplementation has no proven positive influence on hair growth and hair thinning of patients with pattern hair loss unless a significant clinical deficiency exists. As an example, biotin deficiency is a rare finding in healthy individuals on a regular diet and is mostly restricted to patients with e.g., malnutrition or biotinidase deficiency. Even in-vitro experiments failed to show any effect of biotin on the proliferation and differentiation capacity of follicular keratinocytes [20]. Based on this and the lack of interventional evidence for its therapeutic efficacy [21], biotin supplementation cannot be recommended as an adjunct to HTS in patients with pattern hair loss. On the contrary, topical minoxidil (prescribed one month pre- and three months postop) and per-oral finasteride (started one month before and continued for 12 months) were found to reduce postoperative shedding and improve the regrowth of transplanted hairs resulting in a higher hair density after transplantation [22,23]. However, according to our new understanding of the significant role of proteoglycan dysmetabolism in acute and chronic hair loss and miniaturisation [8], an extra targeted approach to address FHG and PFA is required for optimal hair growth stimulation and maintenance.
Nourkrin® Man and Woman are novel options for preand post-HTS pharmacotherapy with anagen-inducing and anti-miniaturisation effects. Natural proteoglycans in Marilex®/Marilex-M® are orally bioavailable [24] and work via promoting the synthetic activity of fibroblasts thus enhancing the concentration of extracellular proteoglycans. Mechanistic research revealed that this anabolic effect of marine proteoglycans is exerted via the activation of Erk1/2 signalling pathway by their EGF-like module [25]. Key proteoglycans, e.g., decorin and versican, in Marilex®/ Marilex-M® have direct, in-vivo, anagen-stimulating effects. Jing et al. showed that administration of decorin during telogen triggers a premature onset of anagen in dormant follicles. Decorin also delays catagen progression and significantly expands the bulbar volume of hair follicles (antiminiaturisation effect) [26]. Versican, another highly active proteoglycan, mediates the integral hair growth-inducing activities of Wnt/β-catenin pathway in dermal papilla cells [27]. This large matrix proteoglycan appears to induce anagen via its G1 domain, which stimulates cell proliferation by destabilising cell adhesion and binding to major cell-surface proteins [28]. Therefore, specific bioactive proteoglycans present in abundance in Marilex® and Marilex-M® impart anagen-inducing and hair-thickening properties to Nourkrin® making it a suitable option for Pre- and post- HTS pharmacotherapy. Another important feature of PRT with Nourkrin® is its excellent tolerability and benign side effect profile as none of the clinical trials using this treatment have reported any significant side effects. On the other hand, the oral route of administration together with having no known interaction with other medications or supplements ensures a high level of patient acceptability and compliance for Nourkrin®.
Conclusion
A developing understanding of the pervasive role of specific proteoglycans in normal growth and pathophysiology of the hair mini-organ led to the development of PRT. This innovative treatment involves the oral administration of Nourkrin® with Marilex®/Marilex-M®, which delivers a range of bioactive and bioavailable proteoglycans to the gastrointestinal system and peripheral tissues. Based on published evidence, Nourkrin® is a well-tolerated treatment that acts by stimulating anagen in dormant follicles, which can accelerate the recovery time from post-op shock loss. Nourkrin® also counteracts the progressive trend of telogenisation and miniaturisation in existing susceptible follicles by promoting the activity of anagen-inducing pathways and replenishing the depleted follicular proteoglycans, i.e., treating FHG and preventing PFA. It seems justified to start PRT three months before the surgery to augment the concentration of proteoglycans in the grafts to ensure their optimal survival and regrowth after transplantation. PRT with Nourkrin® can serve as a safe and effective adjunct to conventional hair loss medications. Combination therapy is likely to create synergistic effects considering the different action mechanism of Nourkrin® compared with conventional medications, e.g., finasteride and minoxidil. Further interventional research is warranted to compare the efficacy of monotherapy with Nourkrin® versus its combination with standard post-HTS treatments in optimising the outcomes of HTS. In conclusion, PRT with Nourkrin® is a promising novel option for pre- and post-HTS pharmacotherapy with potential to support the growth and delay the miniaturisation of remaining follicles at the recipient site and to shorten the duration of shock loss after the surgery.
Declarations
Acknowledgments
Not applicable.
Authors’ contributions
JW proposed the conception and preliminary design of this review. Both authors made substantial contributions to the literature search, drafting and final appraisal of this work.
Availability of data and materials
Supporting data for the conclusions of this review are published in peer-reviewed journals.
Financial support and sponsorship
The author has not received any funding or other support for preparing the current review.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Both authors approved the final version and consented to its publication.
References
- The International Society of Hair Restoration Surgery (ISHRS). ISHRS 2020.
- Sinclair RD, Dawber RP. Androgenetic alopecia in men and women. Clinics in Dermatol. 2001;19(2):167-78.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136-141.
- Liu Y, Liu F, Qu Q, et al. Evaluating the satisfaction of patients undergoing hair transplantation surgery using the face-q scales. Aesthetic Plast Surg. 2019;43(2):376-382.
- Unger RH. Female hair restoration. Facial Plast Surg Clin North Am. 2013;21(3):407-17.
- Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix biol. 2015;42:11-55.
- Malgouries S, Thibaut S, Bernard BA. Proteoglycan expression patterns in human hair follicle. Br J Dermatol. 2008;158(2):234-42.
- Wadstein J, Thom E, Gadzhigoroeva A. Integral roles of specific proteoglycans in hair growth and hair loss: mechanisms behind the bioactivity of proteoglycan replacement therapy with nourkrin® with marilex® in pattern hair loss and telogen effluvium. Dermatol Res Pract. 2020;2020:8125081.
- Yang Y, Li Y, Wang Y, et al. Versican gene: Regulation by the β-catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth. J Dermatoll Sci. 2012;68(3):157-63.
- Inui S, Itami S. A newly discovered linkage between proteoglycans and hair biology: decorin acts as an anagen inducer. Experim Dermatol. 2014;23(8):547-8.
- Soma T, Tajima M, Kishimoto J. Hair cycle-specific expression of versican in human hair follicles. J Dermatol Sci. 2005;39(3):147-54.
- Randall VA, Thornton MJ, Hamada K, et al. Mechanism of androgen action in cultured dermal papilla cells derived from human hair follicles with varying responses to androgens in vivo. J Invest Dermatol. 1992;98(6):S86-91.
- Randall VA, Thornton MJ, Hamada K, et al. Androgen action in cultured dermal papilla cells from human hair follicles. Skin Pharmacol Physiol. 1994;7(2):20-6.
- Thom E. Efficacy and tolerability of Hairgain in individuals with hair loss: A placebo-controlled, double-blind study. J Int Med Res. 2001;29(1):2-6.
- Thom E. Nourkrin®: Objective and subjective effects and tolerability in persons with hair loss. J Int Med Res. 2006;34:514-9.
- Kingsley D, Thom E. Cosmetic hair treatments improve quality of life in women with female pattern hair loss. J Appl Cosmetol. 2012;30:49-59.
- Wadstein J, Thom E. Treating female diffuse hair loss using nourkrin® woman (with marilex®)-an open-label, subjective, outcome study on hair growth and appearance, self-confidence and treatment satisfaction. J Clin Dermatol Ther. 2019;5:037.
- Mattos Simoes M, Thom E, Wadstein J. Woman with marilex® enhances hair growth and appearance and improves hair confidence in women with diffuse hair loss from brazil: An investigator-initiated clinical study. J Clin Investigat Dermatol. 2020;8(1):4.
- Avram MR, Cole JP, Chase C, et al. The potential role of minoxidil in the hair transplantation setting. Dermatol Surg. 2002;28(10):894-900.
- Limat A, Suormala T, Hunziker T, et al. Proliferation and differentiation of cultured human follicular keratinocytes are not influenced by biotin. Arch Dermatol Res. 1996;288(1):31-8.
- Patel DP, Swink SM, Castelo-Soccio L. A review of the use of biotin for hair loss. Skin Appendage Disord. 2017;3(3):166-9.
- Bouhanna P. Topical minoxidil used before and after hair transplantation. J Dermatol Surg Oncol. 1989;15(1):50-3.
- Leavitt M, David PM, Rao NA, et al. Effects of finasteride (1 mg) on hair transplant. Dermatol Surg. 2005;31(10):1268-76.
- Tsuchiya Y, Tomita M, Tsuboi M, et al. Absorption of proteoglycan via clathrin-mediated endocytosis in the small intestine of rats. Biosci Biotechnol Biochem. 2013:120773.
- Sano M, Shang Y, Nakane A, et al. Salmon nasal cartilage proteoglycan enhances growth of normal human dermal fibroblast through Erk1/2 phosphorylation. Biosci Biotechnol Biochem. 2017;81(7):1379-85.
- Jing J, Wu XJ, Li Yl, et al. Expression of decorin throughout the murine hair follicle cycle: hair cycle dependence and anagen phase prolongation. Exp Dermatol. 2014;23(7):486-91.
- Yang Y, Li Y, Wang Y, et al. Versican gene: regulation by the β-catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth. J Dermatol Sci. 2012;68(3):157-63.
- Theocharis AD, Skandalis SS, Tzanakakis GN, et al. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. The FEBS journal. 2010;277(19):3904-23.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref