Review Article - Journal of Pharmaceutical Chemistry & Chemical Science (2022) Volume 6, Issue 1
Click chemistry: A novel tool in pharmaceutical research.
Ashish Arun Karle1*, Ashish Jain1, Gangotri Yadav1, Bhushan Rane1, Sanket Mulgavkar2, Omkar Bagal21Department of Pharmaceutics, Shri D.D.Vispute College of Pharmacy and Research Centre Panvel, India
2Department of Pharmaceutics, Gahlot Institute of Pharmacy Navi Mumbai, India
- Corresponding Author:
- Ashish Arun Karle
Department of Pharmaceutics
Shri D.D.Vispute College of Pharmacy and Research Centre Panvel
India
E-mail: karleashisharun@gmail.com
Received: 29-Jul-2021, Manuscript No. AAPCCS-22-38196; Editor assigned: 31-Jul-2021, PreQC No. AAPCCS-22-38196 (PQ); Reviewed: 14-Aug-2021, QC No. AAPCCS-22-38196; Revised: 25-Jan-2022, Manuscript No. AAPCCS-22-38196 (R); Published: 01-Feb-2022, DOI:10.35841/aapccs-6.1.101
Citation: Karle AA, Jain A, Yadav G, et al. Click Chemistry: A Novel tool in pharmaceutical research. J Pharm Chem Chem Sci. 2022;6(1):101
Abstract
The term click chemistry has been introduced with a goal to develop an expanding set of powerful, selective, and modular blocks that work reliably in both small- and large-scale applications includes reactions that are high yielding, wide in scope, create only by-products that can be removed without chromatography, are stereospecific, simple to perform, and can be conducted in easily removable or benign solvents. Click reactions usually join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions. Click chemistry covers wide applications like drug design and discovery, bioconjugation, oligonucleotide and carbohydrate chemistry, peptide chemistry, radiochemistry, single chain nanoparticle construction, dendrimer synthesis, glycobiology, in synthesis of block copolymer, polymeric micelles etc. It also participates in modification of natural products and pharmaceuticals. It is a tool for drug target in several life threatening diseases or disorders like, Anticancer, antiviral, anti HIV, anti-tubercular or in diabetes mellitus. Oligonucleotides, proteins, polysaccharides, viruses and bacteria such as E. coli have all been successfully conjugated to various substances using this approach. Covalent and rapid linkage of two dissimilar components together under friendly, nontoxic conditions are properties that are completely complementary to the creation of novel agents for the development of both imaging and therapeutic products
Keywords
Click chemistry, Bioconjugation, Oligonucleotide.
Introduction
Click Chemistry includes reactions that are high yielding, wide in scope, create only byproducts that can be removed without chromatography, are stereospecific, simple to perform, and can be conducted in easily removable or benign solvents. Click Chemistry term was introduced by K. B. Sharpless in 2001.
In chemical synthesis, "click" chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In general, click reactions usually join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in pharmacological and various biomimetic applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules.
Advantages of Click Chemistry over Conventional method:
1) These are fast reaction than the normal reaction.
2) Reactions are simple one than conventional reaction.
3) Highly purified product with maximum yield
4) Minimum by-product and easy to separate it from product without chromatography.
5) Product formed is stereospecific
6) These reactions are simple and easy to perform.
Methodology
Click chemistry is a chemical concept introduced by K Barry Sharpless of The Scripps Research Institute, in 2001 it describes chemistry that generate substances quickly and reliably by joining small units together. This is inspired by the nature which also generates substances by joining small modular units. It is a concept that mimics nature [1]. Click chemistry is defined as generating substances by joining small units together with heteroatom links(C-X-C). The aim of this method is to form an expanding set of powerful, selective, and modular units that work reliably in both small- and large- scale applications. The reactions in click chemistry must be meet several criteria that are:
• The reaction should be quick, wide in scope, modular, stereospecific and provide a high yield. A high thermodynamic driving force greater than 20 kcal mol-1, which turns the process faster and more selective.
• Reaction should produce minimum amounts of byproducts, which are non-toxic, inoffensive and readily removed by non-chromatographic methods. The reaction must be simple to perform.
• Reaction conditions must be insensitive to oxygen and water, starting material and reagents have to be easily accessible.
• Product isolation and purification should be carried out by distillation or crystallization, without requiring chromatographic methods.
• Products have to be stable under physiological conditions.
• The importance of water in click chemistry reactions: The most successful click reactions require water as the reaction medium. Reaction without water can be dangerous on a large scale since click reactions are highly exothermic and water is the best heat-sink for processing the enormous heat output. Another function of water is the prevention of interference with protic functional groups (alcohols, amides). Reactions will not damage biological and fragile structures in the body, because they are performed under mild conditions. Water in the human body provides good conditions for applications of click reactions [2].
Major classifications of click chemistry reactions are:
Cycloaddition click reactions
Cycloaddition are reactions in which two π bonded molecules come together to make a new cyclic molecule with the formation of two new σ bonds. The Cycloaddition reaction of click chemistry is follows:
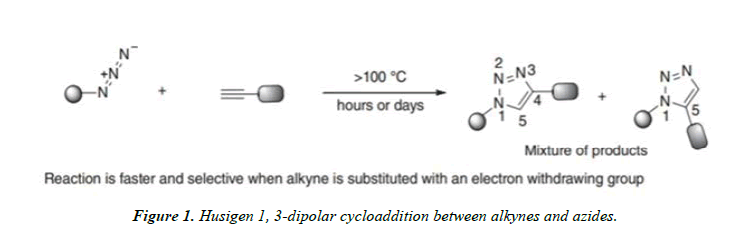
Azide–alkyne Huisgen 1, 3-dipolar cycloaddition: The azide-alkyne Huisgen Cycloaddition is a 1, 3-dipolar Cycloaddition between an azide and a terminal or internal alkyne to give a 1, 2, 3-triazole [3].
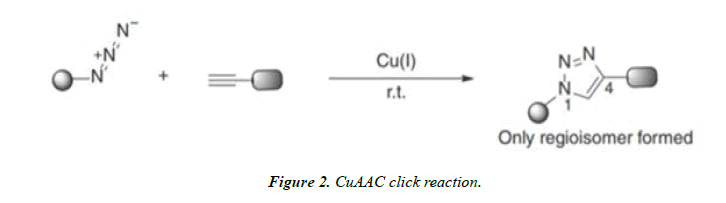
Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) Click Reaction: Sharpless and Meldal gives a Cu (I)- catalyzed version of the Cycloaddition reaction between azides and terminal alkynes, which is 10^7 times faster than the uncatalyzed reaction. The interaction between Cu (I) and terminal alkynes enhancing its reaction with azides.
The Cu (I)-catalyzed reaction is highly regioselective and only the 1,4-adducts are formed. The Cu (I)-catalyzed reactions can be carried out at room temperature and at a much faster rate [4].
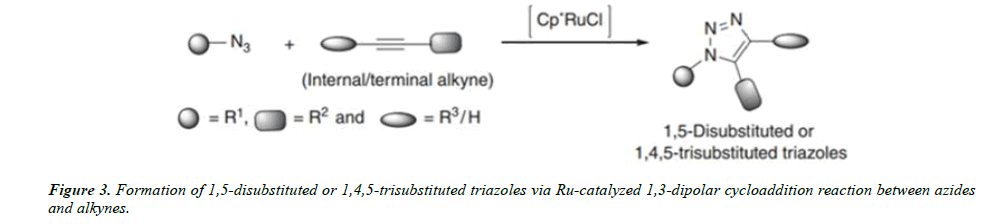
Ruthenium-Catalyzed Azide–Alkyne Cycloaddition (RuAAC) click reactions: Azide–alkyne Cycloaddition reactions catalyzed by ruthenium complexes showed a preference for the formation of 1,5-Disubstituted triazoles or 1,4,5-trisubstituted triazoles Out of the various ruthenium complexes studied for catalysis of this Cycloaddition reaction, the most successful catalysts are Cp*RuCl, Cp*RuCl(PPh3)2, Cp*RuCl(COD), and Cp*RuCl(NBD).
The reactions are performed with 1–2 mol% of the catalyst in THF/dioxane or in any nonprotic solvent at temperatures ranging from ambient to 80℃ [5].
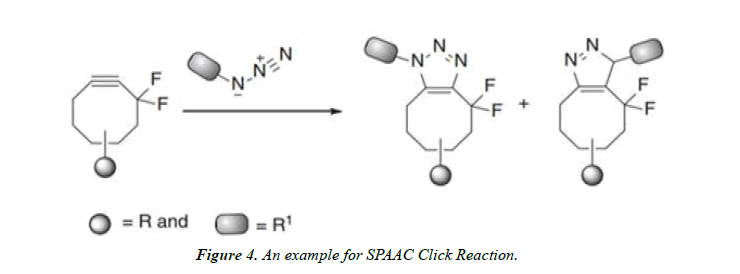
Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC) reactions: The release of substantial ring strain of nearly 18 kcal/mol in the cyclooctyne is the driving force for this reaction. Bertozzi and coworkers explain the possibility of the reaction using this strain-promoted azide–alkyne cycloaddition (SPAAC) reaction as a click reaction for bioconjugation.
The electron-withdrawing groups (EWGs) present on to the cyclooctyne system to increase its reactivity toward cycloaddition reactions. Mono- and difluorinated cyclooctyne derivatives are prepared [6].
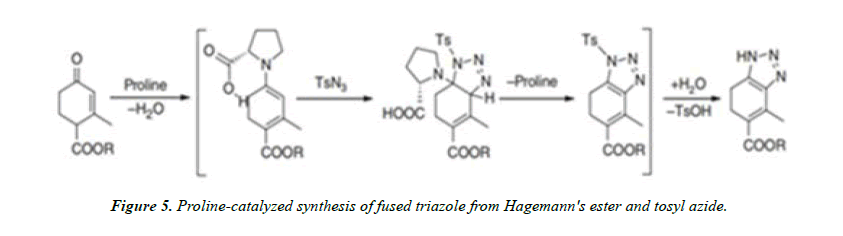
Organocatalytic triazole formation: Organocatalysts are eco-friendly, insensitive to oxygen and water, and they are easily available. The examples of organocatalysts are enolates and enamines which can be easily produced by condensation of amine and aldehyde, so many researchers exploring organocatalyst as dipolarophiles. An example of organocatalytic triazole formation is the proline-catalyzed reaction between Hagemann’s ester and tosyl azide to give fused triazoles performed by Ramachary et al [7].
Thiol-based click reactions
Thiols are easily available conjugation tools. The reactions of thiols can broadly classified as radical reactions and nucleophilic reactions. Radical reactions sometimes make them selective toward certain groups which are under specific reactions conditions [8].
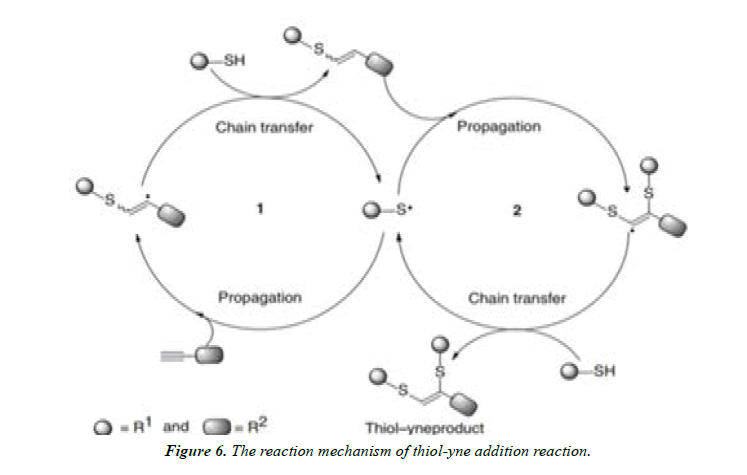
Radical click reactions of thiols: The reactions of thiols toward alkenes and alkynes precede the presence of light or a radical initiator. There is no need of catalysts like transition metal for this reaction and are mostly uses as a conjugation method for the formation of functional molecules for biological applications (Figure 1-4).
Thiol–Ene radical click reaction: The reaction between thiols and alkenes is first explained by Posner in 1905. The reactions can be initiated by using a radical initiator or irradiating thiols with a UV source, mostly at 254 nm. Irradiation of thiols initiates bhomolysis of the S–H bond resulting in the formation of a thiyl radical.
Thiol–Yne radical click reaction: The thiol–yne radical click reaction is the formation of a 1, 2-dithioether through double addition of thiols on to an alkyne. The reaction is used to generate multifunctional polymer structures. Repetitive thiol– yne reactions are used to form multifunctional molecules, which are used to maken dendrimer or hyperbranched polymers.
Nucleophilic addition click reactions of thiols: Thiols and thiolate anions are nucleophiles in nature. Many click reactions are developed based on the nucleophilic attack of thiols on to the electrophilic substrates such as isocyanates, epoxides, halides. The rates of reactions are dependent on the substrates and their inherent ability to attack by thiols and thiolate ions. This below discusses the various click reactions developed based on this concept.
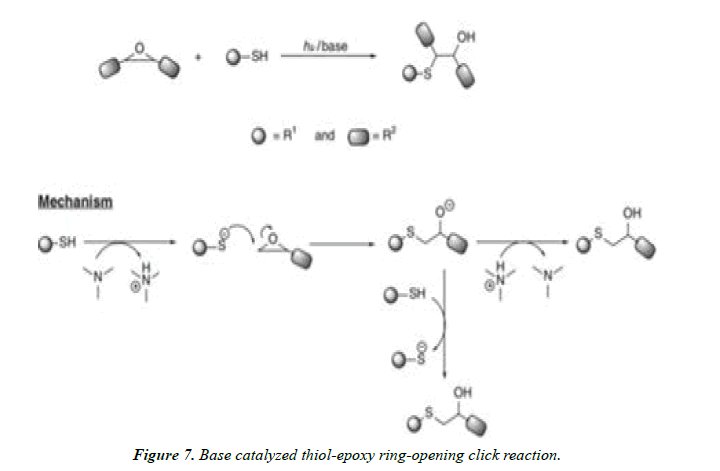
Thiol–epoxide click reactions: Epoxides are undergoing ring opening reactions in the presence of nucleophiles. These reactions are proceeding in acidic medium, which make epoxide more electrophilic by protonation. Thiols are more acidic than water and alcohols lare readily deprotonated to give thiolate ions in the presence of very dilute basic solutions.
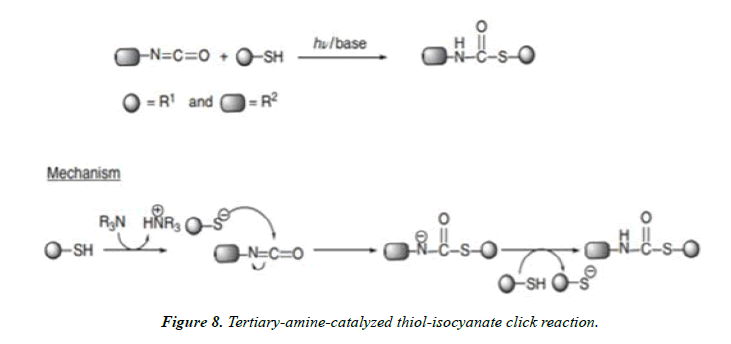
Thiol–isocyanate click reactions: Isocyanates are very reactive compounds, which react readily with alcohols, amines, water, and thiols or thiolate ions. Reaction of isocyanates with thiols gives thiocarbamates in very large quantity. The reaction has a very good potential to be used in surface engineering.
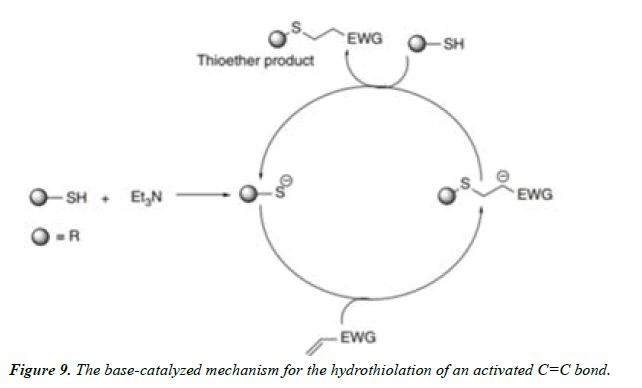
Thiol–Michael addition click reactions: Thiol Michael addition reactions require activated carbon–carbon double bonds, which are in conjugation with a EWG. In the presence of trialkylamine bases such as NEt3, the reaction proceeds smoothly to give the addition products in very high yields. On deprotonation of thiols by base such as NEt3, thiolate anions are formed along with triethylammonium cations.
Thiol–Halogen nucleophilic substitution reaction: Displacement of bromine by various thiols by SN2 nucleophilic substitution is definitely one of the best examples of click reaction. These reactions proceed well even in the presence of other nucleophiles such as alcohols and amines, owing to the increased nucleophilicity of thiols and thiolates.
Miscellaneous click reactions Other than the click reactions mentioned earlier, there are few others that can be included in this list [9]:
1. Nucleophilic ring-opening reactions of epoxides, aziridines, aziridinium ions, episulfonium ions, and cyclic sulfates have been included by Sharpless under the category of click reactions they are termed as springloaded reactions.
2. Nonaldol carbonyl-type reactions such as formation of hydrazones, oximes, amides, ureas, and isoureas are also effective click reactions.
3. Addition to carbon–carbon multiple bonds leading to the formation of three-membered rings (epoxidations, aziridinations), dihydroxylations, nitrosyl–halide addition, sulfenyl–halide addition, and a few Michael additions are also grouped under click reactions (Figure 5-9).
Applications of click chemistry
Click chemistry in drug discovery: Drug discovery based on natural products can be hampered by slow, complex synthesis. Click chemistry, on the other hand, simplifies and optimizes syntheses, providing faster, efficient reactions. Click chemistry was employed by Zhang et al. to produce the peroxisome proliferator-activated receptor γ (PPAR-γ) agonists for the treatment of type II diabetes. Copper-catalyzed coupling of acetylene derivatives of carbohydrates and azides was used in the preparation of a series of multivalent triazolelinked neoglycoconjugates. This approach mimics nature's preparation of multivalent carbohydrates to increase the interaction between carbohydrates and receptors or enzymes [10].
Lewis et al. extended the Huisgen 1, 3-dipolar cycloaddition to prepare an enzyme-bound inhibitor. They performed the click reaction in the presence of the enzyme, acetylcholinesterase (AChE), so that the association of the triazole click product with AChE would produce an inhibitor. The enzyme, AChE, is known to be involved, to a high degree, in the hydrolysis of neurotransmitter in the central and peripheral nervous systems. The results showed that AChE catalyzed the 1, 3-dipolar cycloaddition reaction of one of the azide-alkyne combinations tested to form a triazole product. When the active site of the enzyme was blocked, the triazole product was not detected, indicating that AChE was acting as a reaction template.
Click chemistry in receptor−ligand binding studies
Several research groups used the click chemistry reaction for the development of selective agonist, antagonist, and efficient binding ligands for various GPCR receptors. These developments of the new agonist, antagonist, and efficient binding ligands are very useful in the new drug discovery for uncured disease [11].
Selective agonists:
1) Calenbergh and his workers explored click chemistry to synthesize two series of 2-(1, 2, 3-triazolyl) adenosine derivatives. Some triazol-1-yl analogues showed A3AR affinity in the low nanomolar range, with a high ratio of A3/ A2A selectivity, and a moderate-to-high A3/A1 ratio. The 5′-OH ethyluronamide analogues showed increased A3AR affinity and behaved as full agonists [12].
2) Jacobson et al. employed click chemistry for the synthesis of 2-dialkynyl derivatives of N-methanocarba trizole nucleosides for A3 adenosine receptor-selective agonists’ development. He most potent and selective novel compound was a 1-adamantyl derivative [13].
3) Hausch and his co-workers employed click chemistry or the development of peptide-peptide conjugates ligand synthesis for full activator of CRF1R using a high-throughput approach. An acetylene-tagged peptide library was synthesized and conjugated to an azide-modified high-affinity carrier peptide derived from the CRF C-terminus using copper-catalyzed dipolar cycloaddition. The resulting conjugates reconstituted potent agonists and were tested in situ for activation of the CRF1 receptor in a cell-based assay. Showed good activation and binding affinity toward CRF1 receptor [14].
Selective antagonists:
1) Jacobson and his co-workers prepared GPCR ligand− dendrimer (GLiDe) conjugates from a potent adenosine receptor (AR) antagonist for treating Parkinson’s disease, asthma, and other diseases. Xanthine amine congener (XAC) was appended with an alkyne group on an extended C8 substituent for coupling by Cu (I)-catalyzed click chemistry with azide-derivatized G4 (fourth-generation) PAMAM dendrimers to form triazoles [15].
2) “Click chemistry” was explored to synthesize two series of 2-(1, 2, 3-triazolyl) adenosine derivatives. Binding affinity at the human A1, A2, and A3ARs (adenosine receptors) and relative efficacy at the A3AR were determined. 2-Triazole analogues with an unmodified ribose moietyshowed good antagonist activity at the A3AR [16].
3) Chaiken and his co-workers explored click chemistry for the synthesis of high-affinity dual antagonist for HIV-1 envelope glycoprotein gp120 [17].
4) A series of peptide conjugates was constructed via click reaction of both aryl and alkyl acetylenes with an internally incorporated azidoproline derived from the parent peptide. These conjugates were found to exhibit several orders of magnitude increase in both affinity forHIV-1 gp120 and inhibition potencies at both the CD4 and the coreceptor binding sites of gp120 with Kd = 9 nM [18].
5) Gmeiner and his co-workers employed click chemistry for the synthesis of 1, 1′-substituted ferrocene trizole derivatives for the development of novel antagonist for dopamine receptor [19].
6) Tagged biologically active molecules represent powerful pharmacological tools to study and characterize ligand receptor interactions. Click chemistry was used as a tool to facilitate the access to labelled novel pirenzepine derivatives [20].
7) The expression and function of endothelin (ET) receptors are abnormal in cardiovascular diseases, tumor progression, and tumor metastasis. Michel and his co-workers explored click chemistry for the synthesis of new high affinity ETA selectivereceptor ligands [21].
8) Wang and his co-workers employed click chemistry for the synthesis of cyclopeptidic Smac (second mitochondriaderived activator of caspase) mimetics. These two compounds (133, R=Ph, Bz) bind to XIAP (X-linked inhibitor of apoptosis protein) and cIAP-1/2 (Cellular Inhibitor of Apoptosis-2) with low nanomolar affinities, and restore the activities of caspase-9 and caspase-3/-7 inhibited by inhibitors of apoptosis proteins (XIAP). (IC50=0.43 nM antagonist effect with XIAP4 (R=phenyl), IC50=1.3 nM antagonist effect with XIAP4 (R=benzyl)) potently inhibits cancer cell growth and is 5−8 times more potent than the initial lead compound [22].
Selective binding ligands:
1) The histamine H3 (H3R) and H4 (H4R) receptors playing an important role in inflammation and immune responses with possible applications in diseases such as inflammatory bowel disease, allergic asthma, and pruritis. Interrogated H4R/H3R selectivities use ligands with a triazole core.
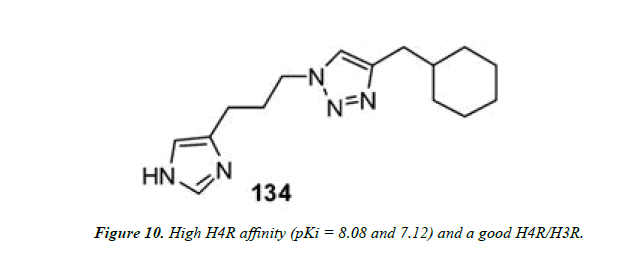
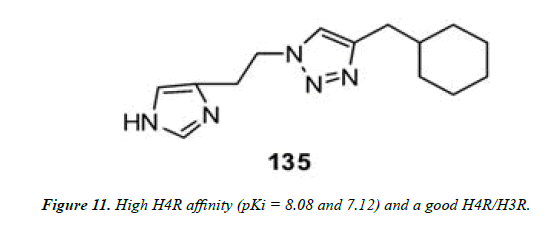
Wijtmans and co-workers employed Cu (I)-assisted “click chemistry” to assemble diverse triazole compounds containing a peripheral imidazole group. Were obtained, which boosted high H4R affinity (pKi = 8.08 and 7.12) and a good H4R/H3R selectivity of 15 and 10 noteworthy for a monosubstituted imidazole compound (Figure 10 and Figure 11) [23].
2) Human histamine H3 receptors (hH3R) in the central nervous system (CNS) are auto- and heteroreceptors modulating the synthesis and the release of histamine as well as the liberation of various other neurotransmitters [24].
Stark and his co-worker employed click chemistry for the synthesis of human histamine H3 receptor (hH3R) binding ligands using ω-piperidinylalkayl azide derivatives. Showed the most binding affinity toward hH3R within this series, showing receptor binding affinity in the nano- and subnanomolar concentration range (pKi = 6.6).
3) Dopamine (DA) D3 receptor belongs to the D2-like subfamily of dopamine receptors, including D2, D3, and D4, functionally distinct from the D1-like subfamily (D1 and D5). D3 receptor is implicated in a variety of brain functions and proposed as a promising therapeutic target for a number of neurological and psychiatric disorders, including schizophrenia, depression, drug abuse, Parkinson’s disease, and restless leg syndrome. Zhang and co-workers explored click chemistry for the synthesis of a series of new aporphine analogues (aporlogues) prepared from appropriate precursors and aryl piperazines displayed good to high affinity at the D3 receptor [25].
Click chemistry in drug development using fragmentbased drug discovery
Click chemistry is one of the powerful tools to synthesize many drugs using fragment-based drug screening methods. This click-FBDD-based screening allows for more efficient lead identification and lead optimization procedures in medicinal chemistry.
Hence, we are describing some potential applications of the click chemistry reaction found in the literature toward novel drug development for many incurable diseases such as anticancer, anti-TB, etc.
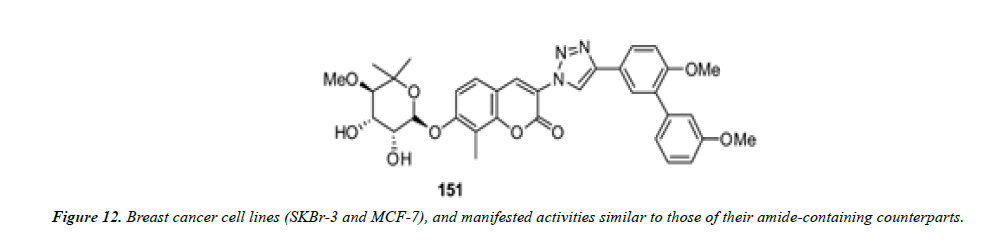
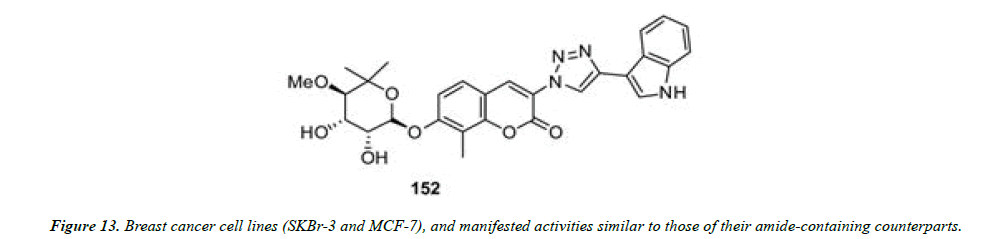
1) Blagg and his co-workers employed click chemistry for the synthesis of a series of triazole-containing novobiocin analogues. These compounds contain a triazole ring in lieu of the amide moiety present in the natural product. The antiproliferative effects of these compounds were evaluated against two breast cancer cell lines (SKBr-3 and MCF-7), and manifested activities similar to those of their amide-containing counterparts (Figure 12 and Figure 13) [26].
2) A series of benzo-macrolactone derivatives were prepared by click chemistry reaction and evaluated as inhibitors of heat shock protein 90 (Hsp90), an emerging attractive target for novel cancer therapeutic agents [27].
3) Chang and his co-workers employed click chemistry for the synthesis of a library of carbohydrate−cyclopamine conjugates. The synthetic protocol is suitable for generating cyclopamine derivatives with various structural motifs for exploring the desired activity. From this initial library, we have observed one derivative that exhibits improved activity against lung cancer cell as compared to cyclopamine [28].
4) Danishefsky et al. employed click chemistry for the synthesis of novel heat shock protein 90 (Hsp90)-based anticancer agent, triazole-cycloproparadicicol derivative. The key step involved an efficient cross coupling reaction using a “click” type construction. Preliminary biological evaluations revealed triazole-cycloproparadicicol [29].
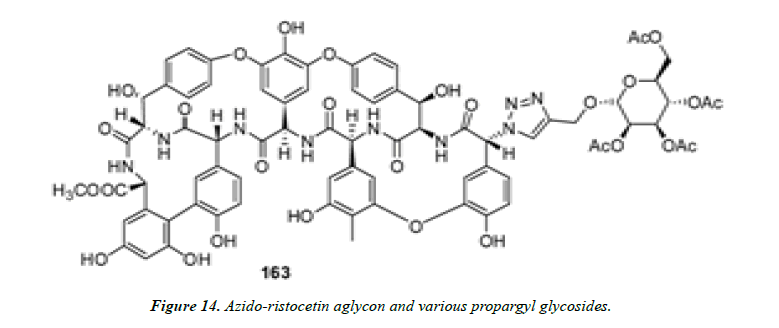
5) Herczegh and his co-workers employed click chemistry for the synthesis of sugar derivatives of ristocetin from azidoristocetin aglycon and various propargyl glycosides. Some of the sugar derivatives were found active against Gram-positive bacteria and showed favorable antiviral activity against the H1N1 subtype of influenza a virus. Figure 14; showed good inhibition activity against H1N1 subtype of influenza a virus with an EC50 value of 4.0 μM [30].
6) DNA intercalators have found great utility as antitumor agents, from natural products such as doxorubicin and actinomycin D to synthetic agents such as mitoxantrone and amsacrine. Amsacrine is an acridine that binds to DNA and inhibits the enzyme topoisomerase. A small set of 9-aminoacridine-3- and 4-carboxamides were synthesized efficiently using benzyne/azide click chemistry. The synthesis of threading intercalators was bound to DNA with reasonable affinity and had up to low micromolar antitumor activity. This compound revealed good antitumor activitywith HL60 cell line with IC50 = 5.4 μM.191 [31].
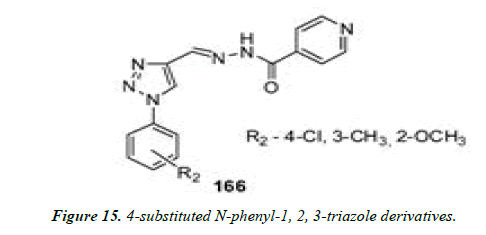
7) Boechat and co-workers reported the synthesis of 4-substituted N-phenyl-1, 2, 3-triazole derivatives using click chemistry. The derivatives were screened in vitro for antimicrobial activity against Mycobacterium tuberculosis strain H37Rv (ATCC 27294) using the Alamar Blue susceptibility test [32].
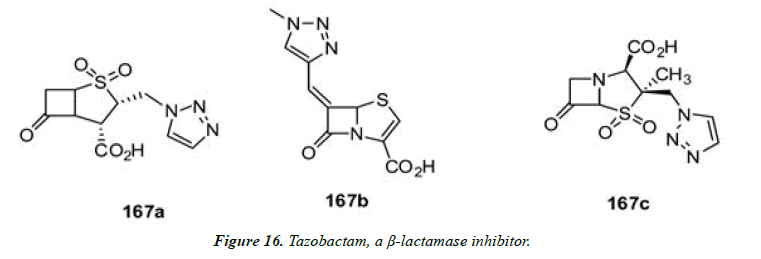
8) Among the best-known examples of triazole-containing structures is tazobactam, a β-lactamase inhibitor that is marketed in combination with the broad spectrum antibiotic piperacillin. Tazobactam (167a) and related triazolecontaining (Figure 15 and Figure 16) turned out to be potent β-lactamase inhibitors with higher potency than clavulanic acid and sulbactam, and the triazole ring appears to play a pivotal role for its potency [33].
9) Epilepsy is a neurological disorder characterized by unprovoked seizures affecting at least 50 million people worldwide. There is a continuing demand for new anticonvulsant agents as it has not been possible to control every kind of seizure with the currently available antiepileptic drugs exhibiting antiepileptic activity was generated via click chemistry [34].
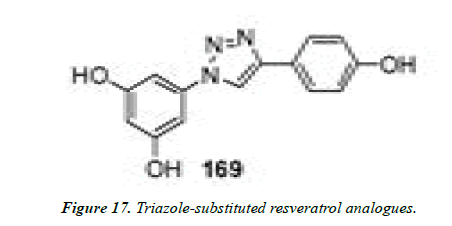
10) Genazzani et al. employed click chemistry to generate triazole-substituted resveratrol analogues. Resveratrol possesses numerous therapeutic actions including cytotoxic activity, and therefore the rapid synthesis of these triazolecontaining resveratrol analogues was utilized to generate an enormous chemical database for preliminary screening of analogues with an antitumoral potential. Some of the compounds screened were found to be more potent (Figure 17) than resveratrol as cytotoxic/antiproliferative agents [35].
Synthetic utility of click chemistry in the development of enzyme inhibitors
Enzyme inhibitors play a significant role in curing diseases, and they are drug targets for many diseases like cancer, tuberculosis, and many uncurable diseases within this context, click chemistry, due to its highly modular and efficient reaction nature, has been identified as one of the most practical methods toward fragment-based enzyme inhibitor development.
Protein tyrosine phosphatase inhibitors: Protein tyrosine phosphatases (PTPs, protein tyrosine phosphatase class enzyme) constitute an important class of signaling enzymes that catalyzes the dephosphorylation of phosphotyrosine residues in a protein substrate. PTP1B (protein tyrosine phosphatase 1B, nonreceptor phosphotyrosine PTP) has been identified as the major regulator of both insulin and leptin signaling pathways [36].
1) Zhang and co-workers prepared a highly potent and selective mPTPB inhibitor using a novel, double click chemistry strategy. The most potent mPTPB inhibitor from this approach possesses a Ki value of 160 nM and a >25- fold selectivity for mPTPB over 19 other protein tyrosine phosphatase inhibitors [37].
2) Xie et al. reported new PTP inhibitor entities by simply “clicking” alkynyl amino acids onto diverse azido sugar templates. Triazolyl glucosyl, galactosyl, and mannosyl serine and threonine derivatives were efficiently synthesized via click reaction, one of which was identified as a potent and selective PTP1B inhibitor against a panel of homologous PTPs [38].
3) Tang and co-workers recently reported a library of benzyl 6- triazolo (hydroxy) benzoic glucosides via the Cu(I)- catalyzed azide−alkyne 1,3-dipolar cycloaddition. These glycoconjugates bearing alkyl chain, sugar, and (hydroxy)- benzoic derivatives were identified as new PTP1B inhibitors with selectivity over T-Cell PTP (TCPTP), SH2- containing PTP-1 (SHP-1), SHP-2, and leukocyte antigen related tyrosine phosphatase (LAR) [39].
4) Zhou and co-workers recently reported a potent and selective mPTPB inhibitor with highly efficacious cellular activity, from a combinatorial library of bidentate benzofuran salicylic acid derivatives assembled by click chemistry.
Protein kinase inhibitors: Protein kinases are key regulators of cell function that constitute one of the largest and most functionally diverse gene families. Protein kinase phosphorylating enzymes play a pivotal role in cellular signal transduction; PK enzymes play important roles in several signal transduction cascades. Hence, a significant portion of drug discovery efforts has made protein kinases as primary targets [40].
1) Liskamp and co-workers employed bisubstrate-based kinase inhibitors that target the more selective peptide-binding site in addition to the ATP-binding site. These active binding peptides were linked with chemo-selective click chemistry to an ATP-binding site kinase inhibitor, and this led to novel bisubstrate structures. The most promising potent inhibitors had nanomolar affinity and selectivity toward PKC alpha and PKC teta among three isozymes [41].
Transferase inhibitors: Transferase inhibitors are potential drug targets for many diseases like cancer, and many viral and bacterial infection diseases.
Glycosyltransferase inhibitors: The biosynthesis of carbohydrates catalyzed by fucosyltransferases involves the transfer of an L-fucose moiety from guanosine diphosphate β-L-fucose (GDP-fucose) to a specific hydroxyl group of sialyl N-acetyl-lactosamine. Selective inhibitors of these enzymes might provide drugs by blocking the synthesis of glycosyl and fucosyl end-products, and the pathology they trigger.
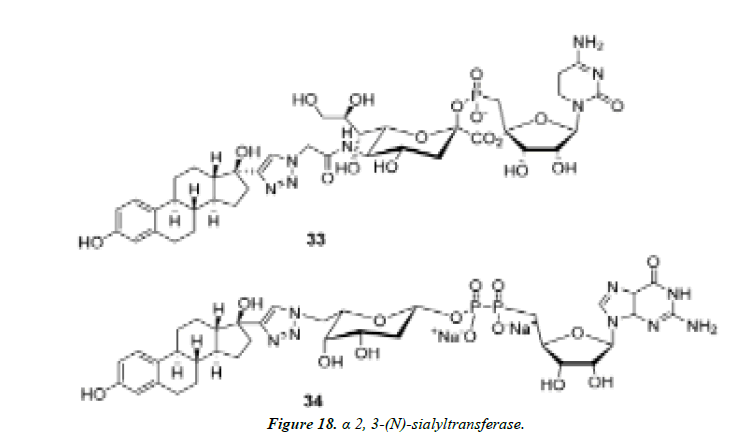
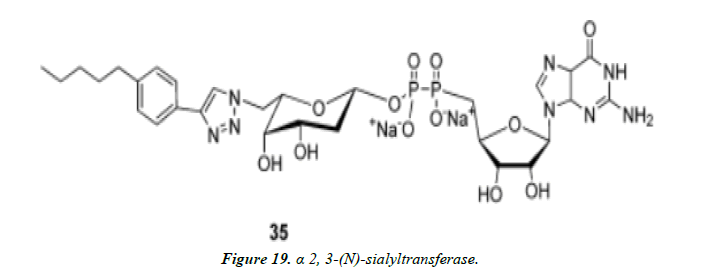
1) Nishimura and co-workers identified potent and selective inhibitors of glycosyltransferases by high-throughput quantitative MALDI-TOFMS-based screening of focused compound libraries constructed by 1,3-dipolar cycloaddition from the desired azidosugar nucleotides with various alkynes. A non-natural synthetic sugar nucleotide was identified to be the first highly specific inhibitor for rat recombinant α 2, 3-(N)-sialyltransferase. Versatility of this strategy was further demonstrated by identification of two selective inhibitors (Figure 18 and Figure 19) for human recombinant α 1, 3-fucosyltransferase V [42, 43].
Glycogen phosphorylase inhibitors: It catalyzes the phosphorolysis of glycogen main-chain, linked through an a-1, 4-glucosidic bond, to glucose-1-phosphate (Glc-1-P) that is subsequently converted to a-D glucose.
1) Cheng et al. synthesized several dimers of oleanolic acid by using amide, ester, or triazole linkage with click chemistry. Among the synthesized homologous series of compounds, as found to be the most potent inhibitor against rabbit muscle glycogen phosphorylase (RMGPa) [44].
2) Xie et al. reported the synthesis and biological evaluation of glucoconjugates of oleanolic acid, linked by either a triazole moiety or an ester function, as novel inhibitors of glycogen phosphorylase. Several synthesized triterpene−glycoside conjugates exhibited modest inhibitory activity against RMGPa [45].
Serine hydrolase inhibitors: Because of the biological importance of serine hydrolases, clinically approved drugs target members of this enzyme class to treat diseases such as obesity, diabetes, microbial infections, and Alzheimer’s disease.
1) Cravatt et al. recently developed a click chemistry that generated triazole urea derivatives with ultra-potent inhibition for serine hydrolase with in vivo activity. Rapid lead optimization by click chemistry-enabled synthesis and comepetitive activity-based profiling identified 1, 2, 3-triazole ureas that selectively inhibit enzymes from diverse branches of the serine hydrolase class. The triazole urea inhibitors were highly potent inhibitors against their respective serine hydrolase targets in mouse T-cell proteomes [46].
Cysteine and serine protease inhibitors: Many cysteine proteases are essential in regulation of physiological processes and disease propagation, and the proteases play important roles in treatment of cardiovascular diseases, oncology, osteoporosis, and arthritis.
1) Ellman and his co-workers recently employed click chemistry for the synthesis of 1, 4-disubstituted-1, 2, 3-triazole cruzain inhibitor analogues from aryloxymethyl ketone azide and enantiomerically pure propargyl amine. It screened against T. cruzi-infected C3H mice, and tetrafluorophenoxymethyl ketone inhibitor was found to be the most potent inhibition against T. cruzi cysteine protease [47].
2) Yao et al. successfully developed a click chemistry strategy for the synthesis of small molecule inhibitor−peptide conjugates, which were subsequently used for organellespecific delivery of inhibitors into cancer cells. Biological testing showed that was successfully delivered to the lysosomes of HepG2 cells, where a greater inhibitory profile against cysteine cathepsins [48].
Metalloproteinase inhibitors: Matrix metalloproteinase (MMPs) are proteolytic enzymes that are involved in many physiological and pathological processes over activation of these enzymes results in tissue degradation, producing a wide array of disease processes such as rheumatoid arthritis, osteoarthritis, tumor growth and metastasis, multiple sclerosis, congestive heart failure, and others.
1) Ramos and his co-workers recently employed click chemistry for the synthesis of a new series of MMP2 (matrix metalloproteinase-2) inhibitors using fragment-based drug design approach. A click chemistry reaction was used to connect the azide to lipophilic alkynes selected to interact selectively with the S1′ subunit of MMP2 [49].
2) Yao and co-workers reported a panel of 96 metalloprotease inhibitors assembled using click chemistry by reacting eight zinc-binding hydroxamate warheads with 12 azide building blocks; inhibited MMP7 and MMP13 (matrix metalloproteinase-13) [50].
3) Hodder and his co-workers reported a compound library screening approach used to identify and characterize VIM- 2 inhibitors from a library of pharmacologically active compounds, as well as a focused “click” chemistry library; was found to be most potent inhibitor for VIM-2 [51].
4) Kolb and his co-workers employed click chemistry for the synthesis of carbonic anhydrase inhibitors using sulfonamide derivatives. Novel sulfonamide compounds were particularly active in inhibiting carbonic anhydrase (CA), and was identified as the most potent inhibitor of hCA-II and hCA-IX (h = human) [52].
Aspartic protease inhibitors: Aspartic proteases play important roles in several diseases such as AIDS (HIV protease), neoplastic disorders (cathepsin D and E), malaria (plasmepsins), and Alzheimer disease (β and γ secretase).
1) Carlier and his co-workers recently employed click chemistry for the preparation of β-site APP-cleaving enzyme 1 (BACE1) inhibitors from 120 reduced amide isostere inhibitors using a high-throughput in situ screening protocol [53].
2) Fischer and his co-workers recently reported the synthesis, SAR, and evaluation of aryl triazoles as novel gamma secretase modulators (GSMs). 1,2,3- Caryl-triazoles were identified as a suitable replacement for gamma secretase modulators [54].
Oxidoreductase inhibitors:
1) Zhu and his co-workers developed an efficient strategy for the fast construction of 108 compounds using click chemistry for monoamine oxidases-A/B inhibition. The fingerprint of inhibitory activity toward monoamine oxidases-A/B against this library was obtained, and four hit compounds were identified as selective inhibitors toward monoamine oxidases-A (MAO-A) [55].
2) Novel cinnamoyl and caffeoyl clusters were synthesized by multiple Cu (I)-catalyzed [1, 3]-dipolar cycloadditions, and their anti-5-lipoxygenase inhibitory activity was tested. Caffeoyl cluster showed an improved 5-lipoxygenase (LO-5) inhibitoryactivity as compared to caffeic acid, with caffeoyl tetramer [56].
3) Moses et al. reported the synthesis of a small library of bifunctional compounds using a fragment-based click chemistry approach, specifically designed to target hLDH-5. Promising lead structure.
Glycosidase inhibitors:
1) Ferreira and his coworkers reported the synthesis of a series of 4-substituted 1, 2, 3-triazoles conjugated with sugars, including D-xylose, D-galactose, D-allose, and D-ribose. Methyl-2, 3-Oisopropylidene-β-D-ribofuranosides, such as the 4-(1-cyclo hexenyl)-1, 2, 3-triazole derivative.
2) N-alkylaminocyclitols bearing a 1, 2, 3-triazole system at different positions of the alkyl chain were prepared as potential glucocerebrosidase pharmacological chaperones using click chemistry approaches.
In situ click chemistry
In principle, the “click chemistry” approach to drug design is applicable to almost any target. If it is possible to use a pure enzyme, the function of which can be measured easily, then the techniques are easier to apply. Further development work with enzyme inhibitors is now in progress in many research groups, on a variety of diseases including AIDS, cancer, anthrax, and Huntington’s disease.
Mycobacterial transcriptional regulator: Deprez and his co-workers postulated that synthetic inhibitors of EthR would increase transcription of ethA and thus improve ethionamide bioactivation and efficacy. The first inhibitor of EthR, BDM14500, was identified through the screening of compounds designed on the basis of the crystal structure of EthR.
Deprez and his co-workers successfully applied in situ click chemistry to probe the ligand binding domain of EthR, mycobacterial transcriptional regulator known to control the sensitivity of Mycobacterium tuberculosis to several antibiotics. The formation of compounds via in situ click chemistry was confirmed and showed most potent inhibition against EthR. From the inhibition results, it is clear that 1.4-substituted trizole was the most potent inhibitor of EthR.
Histone deacetylase inhibitors: Histone deacetylase is a class of enzymes that remove acetyl groups from an ε-N-acetyl lysine amino acid on a histone. Its action is opposite of that of histone acetyl transferase. HDAC inhibitors are attractive drug candidates for cancer, inflammation, and neurodegenerative disorders. HDAC inhibitors consist of a Zn-Binding Group (ZBG) that coordinates with the Zn ion in the active site, a capping region that interacts with residues on the rim of the active site, and a linker that connects the cap and ZBG at an appropriate distance.
Finn and his co-workers prepared two alkynes with hydroxamic acid and 15 alkyl azides as building blocks for in situ assembly screening. In conventional in situ click chemistry, a mixture of an alkyne and an azide is incubated in the presence of the target. The convenient assay of HDAC was chosen to see if enough of an inhibitor could be generated by in situ assembly to measurably affect enzyme function. The experiment revealed that the syn-triazole isomer proved to be a better HDAC8 inhibitor compound.
Protein−protein interaction modulators: Protein−Protein Interactions (PPIs) are central to a large number of vital biological processes and thus represent attractive targets for the development of novel therapies for a variety of diseases. Protein− protein interfaces are large and flat and lack deep cavities that might serve as good binding sites for small molecule.
Manetsch and his workers recently employed kinetic Target- Guided Synthesis (TGS) and in situ sulfo-click chemistry for the development of protein−protein interaction modulators. In kinetic TGS and in situ click chemistry, the target is directly involved in the assembly of its own potent, bidentate ligand from a pool of reactive fragments. Use and validation of kinetic TGS based on the sulfo-click reaction between thio acids and sulfonyl azides was screened. These results validate kinetic TGS using the sulfo-click reaction as a valuable tool for the straightforward identification of highquality PPIMs.
Antibody-like protein-capture agents: Heath and his co-workers recently reported accurate MALDI-TOF/TOF sequencing of one-bead-one-compound peptide libraries to the identification of multiligand protein affinity agents using in situ click chemistry screening. The researchers used the in situ click chemistry approach toward the construction of multiligand protein-capture agents and triligand capture agent evaluated against human bovine carbonic anhydrase II (h(b) CAII) as a model system.
Heath and his co-workers reported in situ click chemistry for the development of Akt-specific branched peptide triligand that was a drop-in replacement for monoclonal antibodies in multiple biochemical assays.
Acetylcholinesterase inhibitors: AChE catalyzes the hydrolysis of neurotransmitter acetylcholine at the synaptic cleft, facilitating nerve impulse transmission across the synaptic gap. The first target used for in situ click chemistry was the enzyme acetylcholinesterase (AChE), selected for its biological importance as a key component of neurological function and therefore a drug target, and for the structure of its active site.
1) Sharpless and coworkers applied in situ click chemistry, for the first time, via mixing 49 building block combinations of tacrine molecules equipped with alkyl azides and phenanthridinium molecules equipped with alkyl acetylenes in the presence of the enzyme.
2) Kolb and co-workers further optimized this approach with the help of liquid chromatography−mass spectrometry in selected ion mode. They employed a tacrine azide as an anchor molecule at concentrations sufficient to saturate the active site, and this enzyme−inhibitor complex was found to recruit the most preferred peripheral binding group from a mixture of alkynes, leading to the identification of four compounds 106a− 107b which were the most potent noncovalent inhibitors of AChE. Compounds 106a and 106b of the syn-trizole derivatives showed good binding affinity to AchE. Compound 107b showed moderate binding affinity toward AChE.
3) Fokin et al. reported successfully the combination of CuAAC and in situ click chemistry for the synthesis of a highly potent and selective ligand for Lymnaea stagnalis acetylcholine binding protein (AChBPs).
HIV protease inhibitors: The aspartic protease of HIV-1 is a major therapeutic target in the fight against AIDS.
Sharpless et al. prepared two focused libraries of 5 compounds each, based on hydroxyethylamine peptide isosteres. Azidebearing scaffolds were united via the new copper-catalyzed process with acetylenes, for library production; showed most potent inhibition against HIV protease.
Chitinase inhibitors: Chitinases catalyze the hydrolysis of chitin, a linear homopolymer of N-acetyl-D-glucosamine (GlcNAc). Chitinase inhibitors identified as potential antiinflammatory agents against asthma and allergic diseases, including atopic dermatitis and allergic rhinitis.
Omura and co-workers reported a chitinase templated formation of a potent triazole inhibitor marrying a biologically active azide-containing compound with structurally unrelated alkyne fragments. Compound based on a motif excised from a natural cyclic peptide binder of blowfly and Serratia marcescens chitinases (SmChi) was used as an anchor molecule for in situ click reactions with a library of alkynes, each in the presence of a mixture of three SmChi subtypes. Syn-trizole displayed high inhibitory activity against SmChiB.
Carbonic anhydrase inhibitors: Carbonic anhydrases (CA) are a family of metalloenzymes that catalyze the interconversion of HCO3−and CO2. The majority of inhibitors for this class of enzymes are aromatic or heteroaromatic sulfonamides, which act by coordinating to the zinc ion.
1) Kolb and his co-workers employed the in situ click chemistry with acetylenic benzenesulfonamide as the reactive scaffold and 24 different azides to identify potent inhibitors of CA.
Cell imaging: Distefano and his co-workers recently reported evaluation of a cell-penetrating prenylated peptide using fluorophore via in situ click reaction for cell imaging applications. The cell penetrating prenylated peptide was synthesized using standard Fmoc coupling conditions on an automated synthesizer with rink-amide resin to afford the C-terminal amide peptides. The N-terminal lysine side chain was acetylated, followed by acylation with an alkynecontaining acid (4-pentynoic acid) on the amino group to give the resulting N-terminal alkyne contains an alkyne but no fluorophore, whereas peptide 112b contains both an alkyne and a fluorophore.
Click chemistry and bioorthogonal chemistry
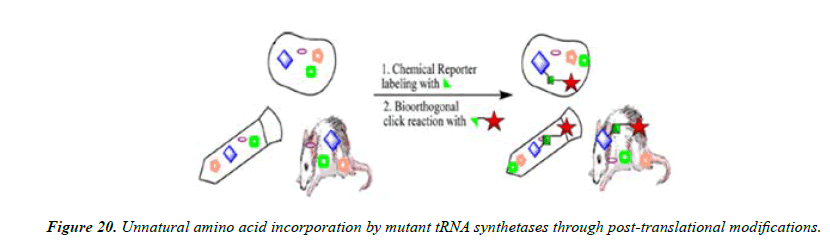
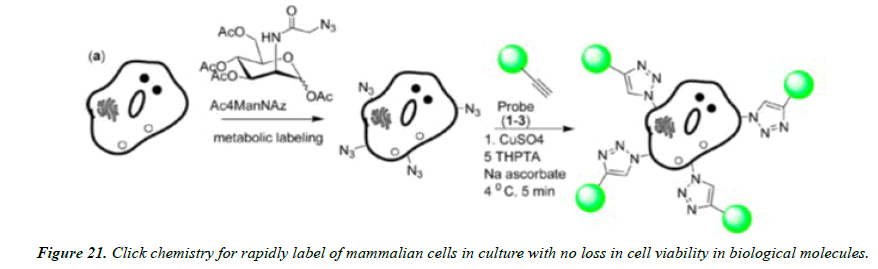
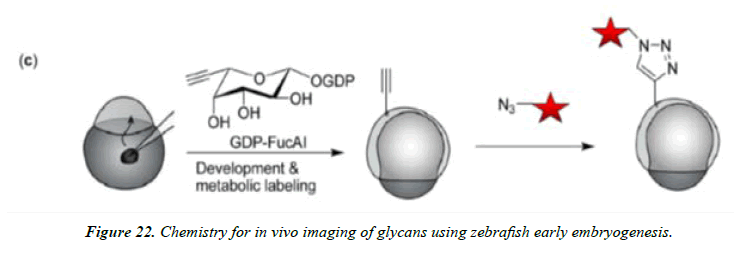
Understanding many biological structures and functions in the living system is essential for uncovering the secrets of biology, but it remains extremely challenging because of the high complexity of biological processes networks and their wiring. The bioorthogonal nature of click chemistry components lends these reactions as valuable tools for the selective labeling and detection of biological molecules in complex samples such as cellular extracts and ultimately in vivo. Such studies are necessary to understand the complex nuances of spatial and temporal aspects of biomolecule localization and function within the cell. The Staudinger ligation and azide− alkyne cycloadditions have both proven to be effective for these challenging tasks and have now been employed for selective derivatization of a wide range of biomolecules, including proteins, viruses, sugars, DNA, RNA, and lipids (Figures 20-22).
Click in peptide chemistry
Recently, Gauthier and Klok published an article compiling the existing strategies, including Huisgen cycloaddition and Staudinger ligation, for the preparation of peptide/ protein-polymer conjugates. The click reaction has proven to be very useful for modifying functional biomolecules because of its high chemoselectivity. Biologic oligomers and polymers, such as peptides, nucleic acids, and carbohydrates, have been modified by using the copper-catalyzed azidealkyne cycloaddition click reaction. Functionalization of an oligopeptide was demonstrated by Tornoe et al. in early 2002. They performed solid-phase syntheses of peptide-peptide conjugates by reacting a peptide-containing alkyne group with its counterpart peptide-containing azide group to yield peptidotriazoles (>95%).
Click in radiochemistry
The use of click chemistry is receiving more interest in the field of radiopharmacy, and there have been several reports recently based on the click-to-chelate approach for radiopharmaceutical applications. Since noninvasive nuclearimaging techniques, such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT), with high sensitivity have become available, radiolabeling of biologically active molecules has become an important tool to assess novel drug candidates.
Click chemistry in pharmaceutical science
Since its debut in 1999, click chemistry has stimulated enormous amount of interests in many different research fields, being utilized in everything from microelectronics to virus labeling to treatments for cancer. In the following sections, an in-depth look will be taken at some of its applications pertaining to the field of pharmaceutical sciences. The area of drug discovery will be excluded since multiple review papers on this subject already exist.
Green approach in click chemistr
Indexed at, Google Scholar, Cross RefReferences
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref