Keywords
|
| Enterococcus spp., MIC, Resistance, Aminoglycosides, Teicoplanin, Vancomycin. |
Introduction
|
| Enterococci are commensal and Gram positive cocci. These bacteria can cause infection in immunocompromised, long-stay hospital patients undergoing invasive procedures or receiving broad spectrum antibiotics, or elderly patients with serious underlying disorders [1]. In the disease process, enterococci have been found to have left the colonization site and to have produced pathologic changes through toxic effects and inflammatory responses. Enterococci were initially the cause of community-acquired infections, and from the 1990s, enterococci have become a common cause of hospital infections [1,2]. The organism is among the hospital-acquired urinary tract infections, wound infections, and bacteremia [3]. E. faecalis is the most frequently isolated strain and is found in 80-90% of the isolates. E. faecium accounts for 5-10% of the isolated strains. These rates, however, can vary according to regions and hospitals [4]. Antibiotic susceptibility profiles of the enterococci strains pose a significant challenge in the treatment. Antimicrobial resistance can be structural or acquired. Acquired resistance occurs through gene mutation or transmission of mobile resistance mobile elements such as plasmids or transposons. Structural resistance in enterococci essentially occurs against two groups of antibiotics: betalactams and aminoglycoside antibiotics. Considering the low efficacy of these antimicrobial agents, aminoglycosides are combined with betalactam and glycopeptide antibiotics in the treatment of severe infections. However, this treatment regimen has become restricted by increasing ampicillin and aminoglycoside resistance [1,5]. |
| The aim of the present study was to evaluate high aminoglycoside resistance in enterococcus isolates and the change in minimum inhibitor concentration (MIC) values of other antimicrobials over a four-year period, and finally to contribute to the rational antibiotic use policy in our hospital. |
Materials and Methods
|
| Enterococci strains isolated from the specimens obtained from patients who were admitted various services and outpatient clinics between 2008 and 2011 were included in the study. The specimens were inoculated onto 5% sheep blood agar and eosin methylene blue (EMB) agar and incubated at 37°C for 24 hours. Colony morphology, gram staining pattern, catalase test and the L-pyrrolidonyl- β-naphthylamide (PYR) test were used to identify the isolated strains. Automated BD Phoenix (Becton Dickinson Diagnostic Systems, Sparks, USA) was used to identify the strains and antibiotic susceptibility profiles of the identified enterococci spp. High-level gentamicin resistance (HLGR) and high-level streptomycin resistance (HLSR) were evaluated as susceptible or resistant according to CLSI; resistance to ampicillin, vancomycin, teicoplanin, and linezolid was determined by MIC values [6]. MIC50 and MIC90 values of the strains were determined in three consecutive years. E. faecalis ATCC 29212 was used as a quality control strain in the laboratory. The significance of the increases and decreases in MIC values according to years was determined by the Wilcoxon test. The level of significance was established as p<0.05. |
Results
|
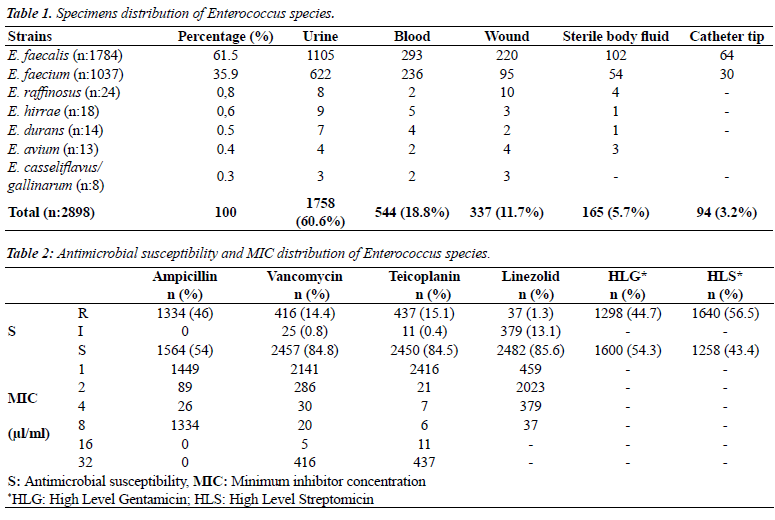
| E. faecalis was the most common strain among 2898 enterococci isolates, and seven different enterococci strains were identified. The strains were mostly isolated from urine (60.6%) and blood (18.8%). The distribution of strains and the specimens from which they were isolated are presented in Table 1. |
| The rates of ampicillin, vancomycin, teicoplanin, linezolid, high-level gentamicin and high-level streptomycin susceptibility were determines as 54%, 84.8%, 84.5%, 85.6%, 54.3%, and 43.4%. The distribution of MIC values and susceptibility rates are shown in Table 2. |
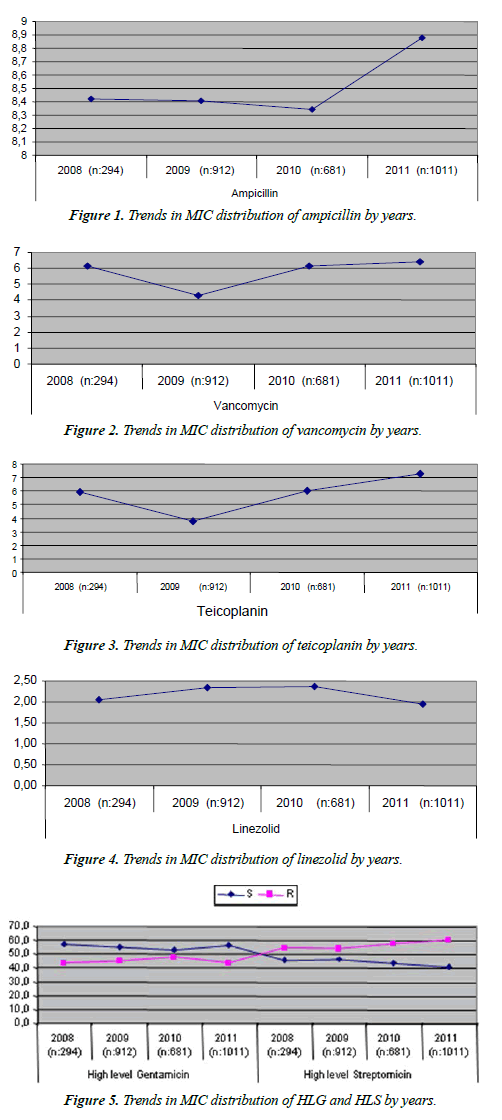
| Ampicillin MIC values did not show significant changes in the first three years (p>0.05) but the significant increase in the MIC value in 2011 was a striking finding (p<0.001) (Figure 1). Vancomycin MIC values showed a significant decrease in 2009 (p<0.001) and a significant increase thereafter (p<0.001) (Figure 2). Teicoplanin MIC values showed a significant decrease until 2009 (p<0.0.01) and a significant increase thereafter (p<0.001) (Figure 3). Linezolid MIC values showed a significant increase in 2009 and 2010, and significant decrease in subsequent years (p<0.001) (Figure 4). HLGR did not increase over the years, and HLSR showed a significant increase (p<0.005) (Figure 5). |
Discussion
|
| Enterococci are among the normal flora in the gastrointestinal tract. They mostly cause endogenous infections. In recent years, they have been increasingly reported as the causative agent of hospital-acquired infections [2]. Enterococci can cause urinary tract infections, endocarditis, bacteremia, wound infections and meningitis, and they mostly appear as urinary tract infections, wound infections, and bacteremia [7,8]. Enterococci show resistance to a significant proportion of the antibiotics that are effective against gram positive bacteria, which poses an important problem in the treatment of enterococcal infections [8]. Urinary tract infections and most wound infections caused by enterococci can be treated by a single drug regimen that involves ampicillin, penicillin G, or vancomycin [7]. The present study detected increases in ampicillin, vancomycin, and teicoplanin MIC values and streptomycin resistance in recent years. However, the combination of aminoglycosides with betalactam antibiotics or vancomycin is preferred in the treatment of severe life-threatening infections due to intrinsic and chromosomal resistance patterns [5]. It was a striking finding in the present study that ampicillin, vancomycin, and teicoplanin MIC values and streptomycin resistance rates increased in recent years. Linezolid has been also introduced into the practice in recent years, and it is also used in the treatment of infections caused by resistant gram positive bacteria particularly vancomycinresistant E. faecalis [9]. In a study conducted to evaluate antimicrobial susceptibility of enterococci isolates, Ç?nar et al. [10] reported 56% high-level gentamicin resistance and 46% high-level streptomycin resistance in hospital strains, and they did not report resistance to vancomycin, ampicillin, and teicoplanin. Another study reported 22%, 20%, and 33% resistance rates for ampicillin, high-level gentamicin, and high-level streptomycin, respectively; they did not report resistance to vancomycin [11]. In their study, Kaçmaz et al. [12] reported 26%, 22%, and 36% resistance rates for ampicillin, high-level gentamicin, and high-level streptomycin, respectively; they did not report resistance to vancomycin and teicoplanin. Berzeg et al. [13] reported 34%, 34%, and 16% resistance rates for penicillin, high-level gentamicin, and highlevel streptomycin, respectively; four strains showed moderate resistance to vancomycin and they did not report resistance to teicoplanin. In their study in 2011, Aktepe et al. [9] reported 81.5%, 46%, and 44.5% resistance rates for ampicillin, high-level gentamicin, and high-level streptomycin, respectively; they did not report resistance to vancomycin and teicoplanin. Aral et al. [14] reported 95%, 43%, 55%, 2.5%, 3%, and 4% resistance rates for ampicillin, high-level gentamicin, high-level streptomycin, linezolid, teicoplanin, and vancomycin, respectively. |
 |
 |
| The infections caused by E. faecalis were reported to be more common among other enterococcal infections. However, infections caused by E. faecium have shown an increase in recent years due to increase in vancomycinresistant enterococcal infections [7]. According to the results of the studies conducted in Turkey, E. faecalis accounts for 39-85.2%, and E. faecium accounts for 9-61% of enterococcal infections [9,11-15]. E. faecalis is the most commonly isolated strain in the reported studies; however, E. faecium was the most commonly isolated strain in the study by Aral et al. [14]. In the present study, E. faecalis constituted 61.5% and E. faecium constituted 35.9% of the isolated enterococci strains. |
| The studies conducted in other countries report varying resistance rates according to the regions. In an Indian study, Shah et al. [16] reported 40%, 53%, 68%, and 8% resistance rates for ampicillin, high-level gentamicin, high-level streptomycin, and vancomycin; they did not report resistance to teicoplanin. In a study by Misken and Deodhar [17] in 2002, 23%, 37%, and 34% resistance rates were reported for ampicillin, high-level gentamicin, and high-level streptomycin, respectively; they did not report resistance to vancomycin and teicoplanin. In a study from Iran, Farzaneh et al. [18] reported 57.7%, 69.2%, 3.8%, and 3.8% resistance rates for ampicillin, high-level gentamicin, high-level streptomycin, vancomycin, and teicoplanin, respectively. In a study in 2007, Gupta et al. [19] reported 34%, 75%, 69%, 2.1%, and 2.1% resistance rates for ampicillin, high-level gentamicin, high-level streptomycin, vancomycin, and teicoplanin, respectively. In a European study that evaluated the susceptibility of the gram positive bacteria isolated from various centers, 17.5%, 29%, 39%, 7%, and 5% resistance rates were reported for ampicillin, high-level gentamicin, high-level streptomycin, vancomycin, and teicoplanin, respectively [20]. In a multicenter study by Reinert et al. [21] no resistance was reported for penicillin and ampicillin in E. faecalis strains isolated in North America, and they reported 1% and 5% resistance rates for linezolid and vancomycin, respectively. In the same area, 86.8%, 88.3%, 3%, and 65.6% resistance rates were reported in E. faecium strains for penicillin, ampicillin, linezolid, and vancomycin, respectively. The present study revealed 46%, 14.4%, 15.1%, 1.3%, 44.7%, and 56.5% resistance rates for ampicillin, vancomycin, teicoplanin, linezolid, high-level gentamicin, and high-level streptomycin, respectively. The study reported higher resistance rates for vancomycin and teicoplanin as compared to other studies in Turkey. This finding is considered to be caused by the frequent isolation of VRE strains from clinical samples obtained from patients who were referred from other centers to our hospital. Resistance rates for ampicillin, HLG, and HLS are comparable to those reported in other studies in our country. Linezolid resistance was also comparable to that reported in the literature. |
| Various resistance rates were found against antibiotics that are frequently employed in the treatment of enterococcal infections. It is a striking finding that this study revealed a high resistance rate for aminoglycosides, which are usually combined with betalactams and glycopeptides. Likewise, observed increases in vancomycin and teicoplanin resistance require close monitoring by local, as well as country-wide data. |
|
|
References
- 1. Teixeria LM, Facklam RR. Enterococcus. In: Manuel of Clinical Microbiology. 10th Edn 2009; Washington DC: ASM press, pp: 430-443.
- 2. Musher DM. In: Mandell, Douglas and Bennett’s Principles and Practise of Infectious Diseases. 4th Edn 1995; Churchill Livingstone Inc, pp: 1811-1826.
- 3. Jett BD, Huyeke MM, Gilmore MS. Virulance of Enterococci. Clin Microb Rev 1994; 4: 462-478.
- 4. Murray BE. The life and times of Enterococci. Clin Microb Rev 1990; 1: 46-65.
- 5. Sümerkan B. In: Enfeksiyon Hastal?klar? ve Mikrobiyolojisi. 3rd Edn, Nobel T?p Kitabevi, 2008; pp: 2051-2057.
- 6. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 20th informational supplement, 2010; Wayne PA: CLSI, M100-S20.
- 7. Y?ld?r?m M. Enterokoklar ve enterokoklarla geli?en enfeksiyonlar. Düzce Üniversitesi T?p Dergisi 2007; 2: pp: 46-52.
- 8. Ar?kan Akan Ö. Enterokok türlerinin mikrobiyolojisi ve antimikrobiyal direnç. In: Yeni ve yeniden gündeme gelen infeksiyonlar. Ankara: Bilimsel T?p Yay?nevi, 1. Bask?, 2009; pp: 123-136.
- 9. Aktepe OC, A??k G, Çiftçi ?H, Çetinkaya Z. Klinik örneklerden izole edilen enterokok su?lar?n?n antibiyotik direnç oranlar?. Türk Mikrobiyol Cem Derg 2011; 41: 86-90.
- 10. Ç?nar T, Leblebicio?lu H, Ero?lu C, Sünbül M, Esen ?. Enterokokarda penisilin-aminoglikozid sinerjisinin ara?t?r?lmas?. Klimik Derg 1999; 12: 39-42.
- 11. Meriç M, Rüzgar M, Günde? S, Willke A. Hastanede yatan hastalardan izole edilen enterokok türleri ve antibiyotiklere direnç durumu. Ankem Derg 2004; 18: 141-144.
- 12. Kaçmaz B, Aksoy A. Antimicrobial resistance of enterococci in Turkey. Int J antimicrob Agents 2005; 25: 535-538.
- 13. Berzeg D, Kart Ya?ar K, ?engöz G, Bat? Kutlu S, Nazl?can Ö. Klinik örneklerden izole edieln enterokok su?lar?n?n antibiyotiklere duyarl?l?klar?. Türk Mikrobiyol Cem Derg 2005; 35: 279-283.
- 14. Aral M, Paköz N?E, Aral ?, Do?an S. Çe?itli klinik örneklerden izole edilen Enterococcus faecalis ve Enteococcus faecium su?lar?n?n antibiyotik direnci. Türk Hij Den Biyol Derg 2011; 68: 85-92.
- 15. Çaylan R, Üstünak?n M, Kad?mov V, Ayd?n K, Köksal ?. Fekal ve klinik örneklerden izole edilen enterokok su?lar?n?n antibiyotiklere duyarl?l?klar?. Türk Mikrobiyol Cem Derg 2004; 34: 24-28.
- 16. Shah L, Mulla S, Patel KG, Rewadiwala S. Prevelance of enterococci with higher resistance level in a tertiary care hospital: A matter of concern. Nat J Med Res 2012; 2: 25-27.
- 17. Miskeen PA, Deodhar L. Antimicrobial susceptibility pattern of Enterococcus species from urinary tract infections. J Ass Phys India 2002; 50: 378-381.
- 18. Farzaneh F, akhi MT, Mahvash O. Antibiotic resistance of enterococci isolated from clinical specimens. Med J Tabrez Uni Med Sci 2010; 32: 34-37.
- 19. Gupta V, Singla N. Speciation and Antimicrobial Susceptibility pattern of Enterococci from a Tertiary Health Care Center of North India. J Clin Diag Res 2007; 5: 385- 389.
- 20. Sader HS, Streit JM, Fritsche TR, Jones RN. Antimicrobial susceptibility of gram positive bacteria isolated from European medical centres: results of the daptomycin surveillance programme (2002-2004). Clin Microbiol Infect 2006; 12: 844-852.
- 21. Reinert RR, Low DE, Rossi F, Zhang X, Wattal C, Dowzincky MJ. Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecyccline. J Antimicrob Chemother 2007; 60: 1018-1029.
|

