Case Report - Journal of Primary Care and General Practice (2021) Volume 4, Issue 5
Case report: Alogliptin induced bullous pemphigoid
Syed Jalali1*, Francesc Valenti21Ringmead Medical Practice, Bracknell, United Kingdom
2Consultant Dermatologist, Frimley Park Hospital, Portsmouth Road, Frimley, Camberley
- *Corresponding Author:
- Syed Jalali
Ringmead Medical Practice
Bracknell, United Kingdom
E-mail: Syed.jalali@nhs.net
Accepted date: September 13, 2021
Citation: Jalali S, Valenti F. Case report: Alogliptin induced bullous pemphigoid. J Prim Care Gen Pract 2021;4(5):104–106.
Abstract
Evidence has been growing over the significant association between treatment with Dipeptidyl Peptidase 4 inhibitors (DPP-4i) and the development of Bullous Pemphigoid (BP). At present, the mechanism is not clearly understood; however, several possible theories have been proposed, including inhibition of cell surface Plasminogen receptors resulting in over-expression of antigens within the skin basement membrane and increased activity of Caspase-1 pathways in the skin. Variability of reporting of BP between different DPP-4i has been attributed to differences in volume distribution and selectivity of sub-classes of structurally similar DPP. In this case report, we present a case of a diabetic patient seen in a community dermatology clinic with Alogliptin induced BP following a recent change from their previous long term treatment with an alternative DPP-4i. This particular case also deviated from previous reports of DPP-4i induced BP of a noninflammatory variant with greater mucosal involvement.
Keywords
Immunobullous disease, Diabetes, Drug reaction.
Introduction
Bullous Pemphigoid is an autoimmune blistering disorder in which autoantibodies target components of hemidesmosomes within the skin's basement membrane, particularly antigens BP180 and BP230 [1]. The result is an inflammatory disorder with characteristic tense stable blisters due to pathology affecting the dermo-epidermal junction.
A growing body of evidence is emerging over recent years implicating DPP-4i in the development of BP. Variation in the frequency of drug-induced BP between different DPP-4i has been observed but is yet to be fully understood. In this case, we present an elderly gentleman with a history of uncomplicated long term treatment with DPP-4i that presented with BP after a recent change to an alternative, more cost-effective DPP-4i. This case report supports the theory that this adverse reaction may not be a class effect with all DPP-4i and confirms this with two different observed outcomes with separate DPP-4is used in the same patient.
Case report
A 77-year-old diabetic gentleman with a background of Atrial Fibrillation and CKD stage 3b was seen in the clinic with a persistent rash he first noticed 3 months earlier.
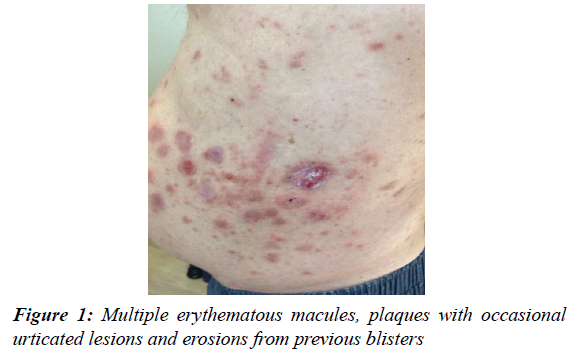
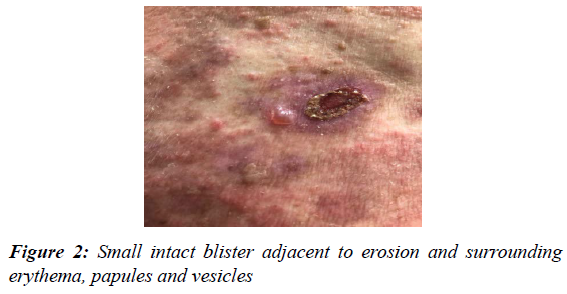
Initially, the lesions appeared clustered around his torso, appearing as itchy erythematous macules and plaque lesions (Figure 1). With time lesions took more of an urticated appearance, with subsequent blistering reported (Figure 2). Pruritic blisters would last for days before deroofing into erosions. He denied any oral, mucous membrane, genital or scalp involvement and no other symptoms on systemic enquiry. No medication changes were reported before the onset of rash; however, there had been changes to his Diabetes medication on review of his records. He was first managed with Saxagliptin for 8 years before this DPP-4i was changed to Alogliptin 6.25mg as part of the local CCG's prescribing audit activity to reduce diabetic drug expenditure through replacement with a cost-effective formulary alternative. His symptoms began 10 months after this change with no other changes to his Edoxaban, Felodipine, Atorvastatin and Perindopril.
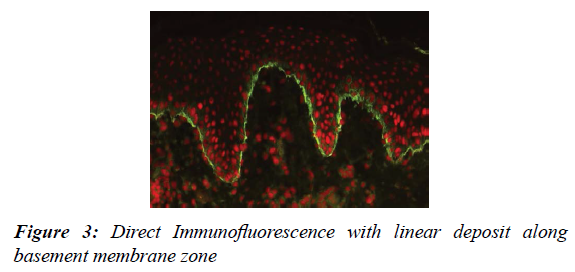
A diagnosis of BP was suspected. An excision biopsy of a whole blister was performed with an additional punch biopsy of peri-lesional skin taken within 2 cm of a blister and sent for direct Immunofluorescence. The diagnosis confirmed BP, with IgG and complement C3 demonstrated in a linear arrangement along the basement membrane (Figure 3), with eosinophilic infiltrates. After discussion with secondary care, given his most recent change in DPP-4i and stable dose of Ramipril for the last 7 years, a drug-induced BP was suspected to Alogliptin. His Alogliptin was stopped and started on Clobetasol Propionate 0.05% cream with Doxycycline 100 mg twice daily. Despite the previous suboptimal response to potent steroids, there was a noticeable improvement with no new blisters forming over the following days, and the skin continued to clear up over the following month.
Discussion
The annual incidence of BP is estimated to be between 2.5-21.7 cases per million, with incidence growing over the last decades alongside rising life expectancy.
It is most commonly seen in the elderly, with a mean age at onset between 69-83 years old and incidence rising exponentially with age [2]. There is also an increased incidence in those with Neurological diseases such as cerebrovascular disease, Epilepsy, Parkinson's disease, Multiple Sclerosis, and Dementia [3]. It is also associated with an increased 1-year mortality rate of 20.36% on recent retrospective cohort analysis, compared to the general population even after age and sex adjustment [4].
As many as 60 drugs, including Spironolactone, Furosemide, and Neuroleptics, have been associated with BP development; however, growing evidence is emerging of DPP-4i carrying statistically significant risk [5-7].
DPP-4i is a class of oral hypoglycemic agent that increase levels of Incretin peptides such as glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP). Through inhibition of enzymatic destruction of Incretin, there is reduced glucagon production and increased insulin release when required post prandially [8].
One large population-based study looked at the incidence of BP induced by DPP-4i compared with all other oral hypoglycemic agents in non-insulin-dependent type 2 diabetics [9]. It observed a doubling of incidence rates of BP from 20 to 47.3 per 100,000 person-years with an overall hazard ratio (HR) of 2.21 although highest in Linagliptin (HR 4.90) and Vildagliptin (HR 4.56). In addition, HR also increased up to 20 months of continuous use before falling thereafter [9]. One case-control study showed a latency ranging 1-26.5 months from initiation of the drug to onset of BP [10], and some suggesting this long latency to imply an underlying drug aggravated disease process. Although the mechanism of DPP-4i induced BP has yet been established, DPP-4 is known to be a cell-surface plasminogen receptor that, once activated, leads to plasmin formation; a serine protease that cleaves BP180 [11]. Inhibition of this process by DPP-4i may lead to the accumulation of BP180 and potential antigenicity [12]. Expression of DPP-4 is increased in several other skin conditions, including Cutaneous T-cell Lymphoma, Psoriasis, Lichen Planus, and Atopic Dermatitis. Except for Linagliptin, which is metabolized in the liver, all other DPP-4i is renally excreted [13]. Whether acute kidney injury or interaction with other nephrotoxic or renally excreted drugs has a role in instigating BP is unknown.
This case was interesting as the patient had previously been treated with Saxagliptin for 8 years without incident until switching to Alogliptin, reinforcing the variability of risk between different drugs within the same DPP-4i class. Earlier, Japanese Pharmacovigilance Database Analysis has demonstrated a significant association between DPP-4i and BP, particularly Vildagliptin (ROR 12.09), Teneligliptin (ROR 5.52) and Linigliptin(ROR 2.67), conclusions further supported by systemic review and Meta-analysis7. A more recent analysis of the American FDA database has shown a disproportionately higher reporting of BP in patients treated with Alogliptin (ROR 94.118) followed in decreasing order of reports, in Linagliptin (ROR 15.172), Sitagliptin (ROR 12.874)and lowest in Saxagliptin (ROR 6.213).
Variability in risk between different DPP-4i has been partly attributed to volume distribution differences, proposed selectivity of individual inhibitors and cross-reaction with structurally similar DPP-2, DPP-8, DPP-9 and Fibroblast Activation Protein [14]. One theory is that inhibition of DPP-8 & 9 results in activation of the Caspase-1 pathway. Upregulation of the Nucleotide-binding domain involving Caspase-1 has been observed in BP and associated with increased disease activity [15]. However, in one ex-vivo study, both Vildagliptin and Saxagliptin have been associated with significant inhibition of DPP-8/9 [16], which alone does not explain the variability in risk seen between these two drugs on Pharmacovigilance Database Analysis. Most DPP-4 is are once-daily preparations except Vildagliptin, which has a shorter half-life requiring twice daily administration. A shorter half-life results in a more significant fluctuation of plasma concentration across dosage intervals, which may contribute to Vildagliptin's higher risk than other DPP-4i and possible drug aggrevation.
It has been reported that DPP-4i induced BP is associated with a non-inflammatory reaction with reduced lesional eosinophilic infiltration and more frequent mucosal involvement [17]; however, neither of these findings were observed in this case. DPP-4 is expressed in many organs and modulates inflammation through enzymatic cleavage of immunoregulatory peptides and through its soluble form that directly engages immune cells. However, the role of DPP-4i is less clear with contradictory reports of reduced pro-inflammatory cytokine production and immune cell infiltration and studies that failed to demonstrate any real anti-inflammatory effect of DPP-4i [18]. Increased genetic susceptibility of DPP-4i induced BP has also been seen in those carrying the HLA-DQB1*03:01 gene and are more likely to present with a non-inflammatory BP variant [19].
Conclusion
Bullous pemphigoid is the most common autoimmune subepidermal blistering disorder in the elderly. Whilst the mechanism in triggering BP is unknown, there are differences in the frequency of observed adverse reactions with different DPP-4i and are notably higher in Vildagliptin, Linagliptin and Alogliptin. Therefore this complication may not be a class effect and opens up a broader debate with commissioners carrying out primary care prescribing audit activities, especially surrounding the appropriateness of drug switches based on cost alone. Primary care clinicians manage most Diabetics. More awareness is needed of this complication of BP from DPP-4i use, including the variable evolving appearance with differing reports of mucosal involvement and inflammatory appearance. It is essential that diagnosis is suspected and appropriate management is started as soon as possible to avoid unnecessary delay in managing this condition, given its marker of poorer prognosis.
Conflict of Interests
None.
References
- Liu Y, Li L, Xia Y. BP180 is critical in the autoimmunity of bullous pemphigoid. Front Immunol. 2017;8:1752.
- Kridin k, Ludwig R. The Growing Incidence of Bullous Pemphigoid: Overview and Potential Explanations. Front Med. 2018;5:220.
- Kaisa T, Outi V, Watary N. Dipeptidyl Peptidase-4 Inhibitor- Associated Bullous Pemphigoid. Front Immunol. 2019;10:1238.
- Persson MSM, Harman KE, Langan VSM, et al. Incidence, prevalence and mortality of bullous pemphigoid in England 1998-2017: population-based cohort study. Br J Dermatol. 2021;184(1):68-77.
- Masanori A, Jun S, Hiromi K, et al. Bullous Pemphigoid and Dipeptidyl Peptidase 4 Inhibitors: A Disproportionality Analysis Based on the Japanese Adverse Drug Event Report Database. Diabetes Care. 2018;41(9):e130-2.
- Huang L, Liu Y, Li H, et al. Bullous Pemphigoid and Diabetes medications: A disproportionality analysis based on the FDA Adverse Event Reporting System. Int J Med Sci. 2021;18(9):1946-52.
- Kirdin K, Cohen AD. Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid: A systematic review and meta-analysis. J Am Acad Dermatol. 2018;S0190-9622(18):32660-4.
- Mulvihill E, Drucker D. Pharmcology, physiology and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;6:992-1019.
- Douros A, Rouette J, Yin H, et al. Dipeptidyl Peptidase 4 Inhibitors and the Risk of Bullous Pemphigoid Among Patients With Type 2 Diabetes. Diabetes Care. 2019;42(8):1496-1503.
- Kridin K, Bergman R. Association of Bullous Pemphigoid with Dipeptidyl- Peptidase 4 inhibitors in patients with Diabetes: Estimating the Risk of the New Agents and Characterizing the Patients. JAMA Dermatology. 2018;154(10):1152-8.
- Gonzalez-Gronow M, Kaczowka S, Gawdi G, et al. Dipeptidyl peptidase IV (DPP IV/CD26) is a cell-surface plasminogen receptor. Front Biosci. 2008;13:1610?8.
- Hofmann SC, Voith U, Sch