Research Article - Gynecology and Reproductive Endocrinology (2017) Volume 1, Issue 1
Bioengineered human endometrium in vitro- A novel model for implantation research
Patki Satish M*, Patki Shweta S, Patil Rajendra
Patki Hospital and Research foundation, 693E, Shahupuri, 3rd lane, Kolhapur 416001, Maharashtra, India
- *Corresponding Author:
- Patki Satish M
M. D[ObGy], Head of Institution
Patki Hospital and Research foundation, 693 E
Shahupuri, 3rd lane, Kolhapur 416001 Maharashtra, India
Tel: +91-9823388858
E-mail: satish.patki@rediffmail.com
Accepted date: November 27, 2017
Citation: Satish MP, Shweta SP, Rajendra P. Bioengineered human endometrium in vitro- A novel model for implantation research. Gynecol Reproduct Endocrinol -UK. 2017;1(1):31-34
DOI: 10.35841/2591-7994.1.1.31-34
Visit for more related articles at Gynecology and Reproductive EndocrinologyAbstract
Objective: To develop the tissue culture technique for 3 dimensional creation of bioengineered human endometrium in a culture dish for morphological and molecular studies of its components. Settings and design: This is a laboratory based observational study during the period of 2 years from May 2014 to May 2016. Patients/ Intervention: 100 samples of the endometrium were obtained by the procedure of single biopsy in a proliferative phase of the cycle. The cases of uterine and endometrial pathologies were excluded. The cells were trypsinised and cultured on matrigel. The culture was supplemented with sequential estradiol and progesterone. Main outcome measures: Development of glanduloepithelial, stromal and vascular components of the endometrium in the culture dish. Induction of pinopodes after progestogenic stimulation on estradiol primed endometrium. Results: The stromal cells were observed like fibroblasts at the floor of culture dish while the glanduloepithelial components were observed at the surface. The epithelial cells were differentiated in to pinopodes between day 3 to 5 exposure of progesterone which are the hallmarks of the process of implantation. Conclusion: The human endometrium can be bioengineered in the culture dish, which can be a novel model for implantation research. It can avoid the need of repeated invasive endometrial biopsies and replace studies based on animal models.
Keywords
Bioengineered Endometrium, Pinopodes, Implantation, Matrigel, 3D culture
Introduction
In spite of the astounding advances in the field of Assisted Reproductive Technology (ART), the implantation rate of various procedures has still remained 25% to 30% [1]. Failure of implantation is the result of either noncompeting embryo or nonreceptive endometrium or both [2]. The research in the field of creating good quality embryos is progressing rapidly. The introduction of new culture media, embryo monitoring systems like embryoscopes and metabolomics and methods to assess the genetics of the embryos like Preimplantation Genetic Screening (PGS) and Preimplantation Genetic Diagnosis (PGD), all help in creating and identifying the best embryos before they are transferred to the uterus.
However, the methods for assessment of the receptivity of the endometrium at a cellular level are not fully developed. The only reason is inability to get the endometrial tissue on multiple occasions, for morphological assessment and molecular studies as it requires the invasive procedures of endometrial biopsies.
The procedure of the endometrial biopsy cannot be performed during the conception cycle. Hence, most of the data on endometrial receptivity is based on noninvasive procedures like vaginal sonographic and colour doppler assessment of the endometrium. However, their positive predictive value is limited [3,4]. Doing repeated endometrial biopsies in the preembryo transfer cycle and identification of genes expression by Endometrial Receptivity Array (ERA) test are expensive and have limitations.
With this background, there is a need to develop a bioengineered human endometrium in a petri dish which will be useful as a tool for ‘Implantation Research’. Once such bioengineered endometrium in vitro is available, researchers can do morphological and molecular studies on the cells in culture dish. Similarly, the in vitro studies of the effects of various hormones and agents on each component of the endometrium can be done.
There will be no longer need of the traumatic endometrial biopsies. Additionally, the bioengineered endometrium can be used for co-culturing the embryo which will be sensitized before actually it is transferred to the endometrial cavity. The present study is an attempt to culture the human endometrium in a 3D (3 Dimensional) pattern.
Materials and Methods
The study was approved by the Institutional Review Board (IRB) and informed consent was obtained from all the patients. The study duration was of two years from May 2014 to May 2016. The patients who had decided to undergo the treatment of ‘In vitro Fertilization (IVF)’ in subsequent cycles were randomly selected.
The patients having proven uterine and endometrial pathologies like congenital uterine anomalies, fibroids, Asherman syndrome and genital tuberculosis were excluded. The patients with reduced ovarian reserve like those with premature ovarian failure (POF) and peri and post-menopausal status were also excluded. The patients having chronic pelvic inflammatory disease, endometriosis, adenomyosis and having chronic pelvic congestion were also excluded. The patients having endocrine disorders like thyroid dysfunction and hyperprolactinemia were excluded.
After the basic hematological, biochemical and endocrine work up, they were advised to report between day 10 to day 12 of the unstimulated cycle (during proliferative phase) without any other medication. The transvaginal sonography was performed to note the endometrial thickness. The sample size for the present study was of 100 patients with the mean age of 32.5 years. The endometrial biopsy was taken only once when the endometrial thickness was between 8 to 10 mm by vagino sonography under short sedation by taking all aseptic precautions.
In only five cases the embryos were co-cultured with the bioengineered endometrium, to study the rate of their in vitro growth. However, none of these embryos were transferred. It was planned as a different project.
Tissue culture protocol for the development of 3D bioengineered endometrium in culture dish
The entire procedure was performed in a class 10000, tissue culture laboratory, well equipped with air and temperature managed by air handling unit (AHU). The cell handling was performed in a laminar air flow with class 100 atmospheres. All the disposable ware was made up of tissue culture grade polypropylene and culture media of Sigma-Aldrich were used.
Human endometrium is a layered tissue and to develop it in a 3D culture, matrigel (BD) was used which acts as an extracellular matrix platform to culture all the layered components.300 microlitres of red matrigel were added to each well of four well dish and the dish was then kept in the carbon dioxide incubator for 30 minutes for the solidification of matrices.
The endometrial bits were initially washed several times with PBS (Phosphate Buffered Saline) to remove the red blood cells. These bits were taken in a 35 mm petri dish and were minced with number 20 surgical blade and forceps. The endometrial glands were isolated by mechanical dissection under stereo zoom microscope. The fine paste of the tissue so formed was transferred to 10 ml conical tube. 0.5 ml of enzyme Trypsin was added to the cell paste for 3 minutes and vigorous mixing of the cells with enzyme was performed at room temperature. The tube was then kept for 2-3 minutes at 37°C temperature for optimum action of trypsin. To prevent the over action of trypsin and to avoid over digestion of the stromal cells and glands, 1 ml of filtered human serum (patient’s own) was added, to neutralize the action of trypsin. The tube was then centrifuged at 2000 RPM for 20 minutes to get rid of trypsin. The supernatant was removed and 5 ml of Dulbecco’s Modified Eagle Medium (DMEM) and Ham F12 was added to the call pellet. This cell pellet was finally seeded over the matrigel floor in a 4 well dish.
The cell culture was done in the carbon dioxide incubator at 37°C at 5% CO2 atmosphere. The medium was replenished every 48 hours. 3-5 days after the primary 3D culture, the culture medium was supplemented with 17 β-estradiol dissolved in DMEM. After 4 to 5 days of estrogenisation, VEGF (Vascular Endothelial Growth Factor) was added to culture to induce angiogenesis. After 7 days of culture, the aqueous progesterone was added to the culture medium. The cellular changes were studied under phase contrast as well as scanning electron microscope (SEM), sequentially every day.
Results
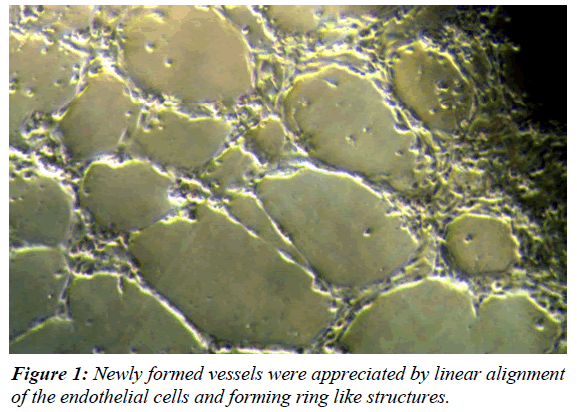
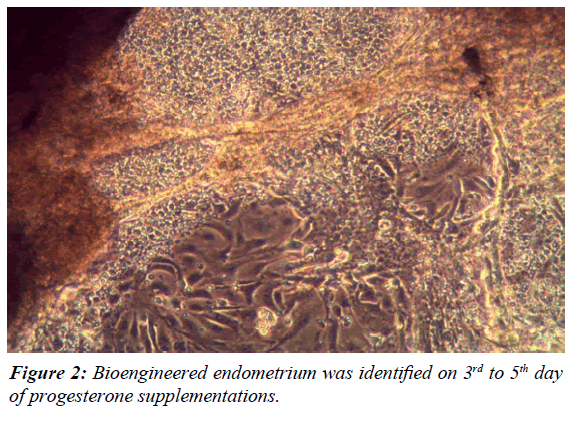
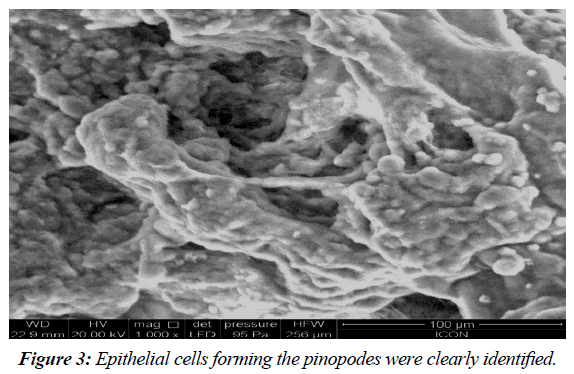
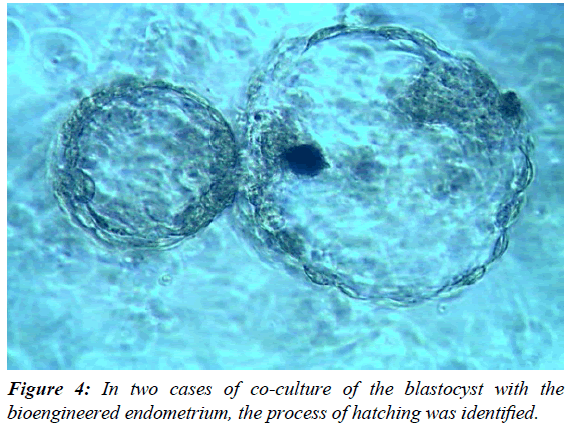
At 0 hours of cell culture, the stromal cells predominated in the culture plate which looked like circular isolated cell. The epithelial cells and glands looked like brighter circular cells which cultured in clumps. After estradiol stimulation the epithelial as well as stromal cells started rapid proliferation. The stromal cells got attached to the floor of the dish and elongated like fibroblasts. The epithelial cells started proliferating in the superficial plane and formed a classical cobble stone appearance with glandular differentiation. The addition of VEGF to the culture induced the process of angiogenesis. The newly formed vessels were appreciated by linear alignment of the endothelial cells and forming ring like structures (Figure 1). Finally, addition of the progesterone induced glandular proliferation and a sheet of 3D-bioengineered endometrium was identified on 3rd to 5th day of progesterone supplementations (Figure 2). The culture dish at this stage was subjected for scanning electron microscopy. The epithelial cells forming the pinopodes were clearly identified. Interestingly, the pinopodes were not only covering the entire surface of the endometrium but were also entering into the lumens of the glands (Figure 3)The results were reproducible and were consistently obtained in all the cases. In two cases of co-culture of the blastocyst with the bioengineered endometrium, the process of hatching was identified (Figure 4).
Discussion
The endometrium plays an important role in the process of implantation, in fact more than the embryo. However, assessment of the endometrial components especially during the crucial period of the window of implantation in a sequential day wise manner is almost not possible. The window of implantation is a period in which the endometrium becomes extremely receptive for embryo implantation and lasts only for a short period of 3-4 days [5]. During this period, all the three components of the endometrium namely-epithelium and glands, stroma and vessels support the process of implantation of blastocyst through cytokines, growth factors and adhesion molecules [6-8].
The present study has shown the successful isolation, propagation as well as differentiation of the components of the endometrium. In a conventional tissue culture, it is difficult to culture all the 3 components of the endometrium.
In such cultures, only stromal cells dominate and the culture dish is fully confluent with their fibroblast like appearance. However, in the present 3D culture system, the proportion of the glanduloepithelial component, vessels and stroma remain like their in vivo existence. Interestingly, the in vitro cultured endometrium responds very well to the exogenous sequential endocrine stimuli of estradiol followed by progesterone. This microenvironment mimics in vivo endocrine events occurring during the window of implantation. The most notable change in the epithelium between 3rd to 5th day of progesterone exposure is the appearance of pinopodes. The pinopodes are indeed hallmark of the process of implantation [9]. They are believed to be involved in the process of embryo opposition and its subsequent attachment [10,11].
Scientists have made attempts to culture the glanduloepithelial as well as stromal cells in the canine [12] and rabbits [13] endometrial model. However, the animal studies cannot mimic the human conditions. In mice, NK cells are not present in the endometrium and during pregnancy there is minimal trophoblastic invasion [14]. Present study has the additional advantage of getting the human endometrial tissue with all its functional components, in the petri dish. This will definitely avoid repeated invasive procedures of endometrial biopsies.
Apart from such morphological studies such bioengineered endometrium can also be used for molecular studies. Some of the genes in the endometrium are upregulated while some are downregulated during the window of implantation making a gene signature [8]. Such molecular studies can be done cells obtained from the bioengineered endometrium.
In the present study, in two cases where the blastocysts were co-cultured with the bioengineered endometrium, the process of hatching was observed. It is hypothesized that such sensitized embryos will have better chances of implantation after transferring them actually to the endometrial cavity. It needs further research and evaluation.
The culture model of the human endometrium and cell sheet engineering has been tried in earlier studies [15,16]. However, the present study has gone further in depth and can be clearly used as a model for implantation research.
References
- Boomsma CM, Macklon MS. What can the clinician do to improve implantation? Reprod Biomed Online 2006;13:845-855.
- Nardo LG, LiTC, Edwards RG, et al. Introduction: Human embryo implantation failure and recurrent miscarriages: Basic science and clinical practice. Reprod Bio Med Online 2006;13:11-12.
- Kazar JL. Three dimensional ultrasound assessment of endometrial receptivity: A review. Reprod Bio Endocrinol 2006;4:56.
- Ng EH, Chan CC, Tang OS, et al. Factors affecting endometrial and sub endometrial blood flow measured by three-dimensional power doppler ultrasound during IVF treatment. Hum Reprod 2006;2:1062-1069.
- Navot D, Scott RT, Droesch K, et al. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril 1991;55:114-118.
- Kammerer U, Von WM, Markert UR, et al. Immunology of human endometrium. Immunobiology 2004;209:569-574.
- Giudice LC. Implantation and endometrial function. Famser BC (edn), Reproductive Medicine: Molecular, Cellular and Genetic Fundamentals. New York NY. Parthenon Publishing 2002:439-465.
- Dimitriadis E, White CA, Jones R, et al. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update 2005;11:613-630.
- Qiong Z, Jie H, Yonggang W, et al. Clinical validation of pinopode as a marker of endometrial receptivity: A randomized controlled trial. Fertil Steril 2017;10B:513-517.
- Anderson TL, Olson GE, Hoffman LH, et al. Stage-specific alterations in the apical membrane glycoproteins of endometrial epithelial cells related to implantation in rabbits. Bid Reprod 2000;15:67-73.
- Bentin LU. Relevance of endometrial pinopodes for human Blastocyst implantation. Hum Reprod 2000;15:67-73.
- Bartel C, Tichy A, Schoenkypl S, et al. Effects of steroid hormones on differentiated glandular epithelial and stromal cells in a three dimensional cell culture model of the canine endometrium. BMC Vet Res 2013;24:79-86.
- Wang HB, Lo SH, Lin QX, et al. Reconstruction of endometrium in vitro via rabbit uterine endometrial cells expanded by sex steroid. Fertil Steril 2010;93:2385-2395.
- Carson DD, Bagchi I, Dey SK, et al. Embryo Implantation. Dev Biol 2000;223:217-237.
- Blauer M, Heinonen PK, Martikainen PM, et al. A novel organotypic culture model for normal human endometrium: Regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum Reprod 2005;20:864-871.
- Takagi S, Shimizu T, Kuramoto G, et al. Reconstruction of functional endometrium-like tissue in vitro and in vivo using cell sheet engineering. Biochem Biophys Res Commun 2014;446:335-340.