Research Article - International Journal of Pure and Applied Zoology (2021) Volume 9, Issue 8
BIO-ECOLOGY OF AQUATIC AND SEMI-AQUATIC INSECTS OF ORDER HEMIPTERA IN THE WORLD
Hassan Vatandoost1,2*, Ali Jalilian1, Ghazal Tashakori1, Ramin Khaghani1
1Department of Medical Entomology & Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran,Iran
2Department of Chemical Pollutants and Pesticides, Institute for Environmental Research, Tehran University of Medical Sciences,Tehran, Iran
- Corresponding Author:
- Hassan Vatandoost
Department of Medical Entomology & Vector Control,
School of Public Health,
Tehran University of Medical Sciences,
Tehran,
Iran
E-mail: hvatandoost1@yahoo.com, vatando@tums.ac.ir
Received: 5th November, 2021; Accepted: 19th November, 2021; Published: 26st November, 2021
Abstract
Background: Hemiptera is an order of insects, commonly called true bugs, comprising over 80,000 species. Most hemipterans feed on plants. Other species have been used for biological control of insect pests. Hemipterans have been cultivated for the extraction of the dyestuff cochineal (also known as carmine) and for shellac. The bed bug is a persistent parasite of humans, and some kissing bugs can transmit Chagas disease. The bed bug, Cimex lectularius, is an external parasite of humans. It lives in bedding and is mainly active at night, feeding on human blood, Cicadas have been used as food. Hemipterans make use of a variety of modes of locomotion including swimming, skating on a water surface and jumping, as well as walking and flying like other insects.
Methods: In this research different databases including: “PubMed”, “Web of Knowledge”, “Scopus”, “Google Scholar”, “SID”, “random” and “thesis.research.ac.ir” were used.
Results: Results showed that there are significant varieties in the distribution of aquatic and semiaquatic Hemiptra worldwide. The main important aquatic and semiaquatic families were: Belostomatidae, Hydrophilidae, Dytiscidae, Corixidae, Nepidae, Notonectidae, Peleidae, Naucoridae, Gerridae, Vellidae, Hydrometridae, Gelasorocidae, Mesoveliidae, Heridae, and Corduliidae.
Discussion and conclusion: Aquatic and semi aquatic heteropterans form food in different trophic levels of fresh water ecosystems. Due to their poor dispersal capabilities, these bugs serve as zoogeographical indicators for diverse habitats. Many families of the bugs may be utilized in the biological control of mosquito larvae. Some are used as indicators of water quality.
Keywords
Aquatic, Semi-aquatic, Hemiptera, Worldwide.
Introduction
Aquatic insects are major components of both freshwater communities and adjacent terrestrial habitats. Larval insects serve as prey for larger aquatic insects, amphibians, and fishes, and the winged adult stages feed terrestrial predators such as birds, bats, and spiders [1]. Inland waters cover less than 1% of the Earth’s surface yet harbor 10% of all known animal species, of which 60% is composed of aquatic insects. This diversity today numbers close to 100,000 described species [2]. This is probably an underestimate, and with the taxonomic deficit skewed toward the insects, we estimate that aquatic insects may well number more than 200,000. A species and thereby make up 80% of aquatic animal diversity. Aquatic insects spend one or more stages of their life cycles in the water, with the majority living in water as eggs and larvae and moving to terrestrial habitats as adults [2].
Aquatic insects play an important role in aquatic ecosystem functioning [3]. They are an important component of invertebrate assemblages in an aquatic ecosystem where they are a controlling group in food webs. At the larval stage,they constitute the principal nutritive fauna of fish [4,5].
They play important ecological roles in both aquatic and terrestrial realms as primary consumers, detritivores, predators, and pollinators. The ecology of many groups is well studied, owing to their roles as bioindicators or disease vectors, but freshwaters have been largely overlooked as a hotbed of diversification, despite their disproportionate contribution to global biodiversity [2].
A review by Mayhew [6] Although belonging to 12 orders, aquatic insects may represent more than 50 separate invasions [2]. Ephemeroptera, Odonata, Plecoptera, Trichoptera, and Megaloptera are almost exclusively restricted to freshwater by an aquatic larval stage and makeup over 27,000 known species, of which over half belong to Trichoptera. The remaining diversity includes over 10% of the hemipteran suborder Heteroptera, approximately 30% of Diptera approximately 3% of Coleoptera, and very small proportions of Hymenoptera, Lepidoptera, Neuroptera, and Orthoptera.
The order Diptera is by far the largest group, containing nearly half of all aquatic insects. All major orders are cosmopolitan, with the notable exception of the Megaloptera, and have 50%-75% of known species in the tropics, except Plecoptera, of which 65% are Holarctic species [7].
Freshwaters are highly diverse and include ponds, lakes, springs, streams, rivers, wetlands, reservoirs, and ditches [8]. The transition to freshwaters demanded adaptation in mechanisms of thermo- and osmoregulation, respiration, feeding, and locomotion. Among the most notable characteristics of freshwaters are their daily and seasonal temperatures, which are more stable than air and soil temperatures. Freshwaters occupy a low position on the landscape where they accumulate nutrients and detritus [2].
Aquatic insects are excellent models for research on diversification. Their habitats exhibit marked spatial and temporal gradients instability and their amphibiotic lifestyles link strong habitat dependence with response to change via dispersal. This has likely led to varying contributions of extinction as well as allopatric and ecological speciation [9].
Standing waters appear to harbor more dispersive species than running waters, but there is little understanding of how this fundamental ecological difference has affected diversification [2].
In contrast to the lack of evolutionary studies, the ecology and habitat preferences of aquatic insects have been intensively studied, in part because of their widespread use as bioindicators. The combination of phylogenetics with the extensive ecological data provides a promising avenue for future research, making aquatic insects highly suitable models for the study of ecological diversification [2].
Aquatic and semi-aquatic heteropterans form food in different trophic levels of freshwater ecosystems. Due to their poor dispersal capabilities, these bugs serve as zoogeographical indicators for diverse habitats [10]. Further, many families of the bugs may be utilized in the biological control of mosquito larvae [10]. Some corixids are used as indicators of water quality. Studies also indicate that the quality of the aquatic environment is partially dependent on the abundance of bugs population [10].
The use of insect predators in mosquito control has been exploited in a limited fashion and there is much room for further investigation and implementation [9].
Insects that are recognized as having predatorial capacity concerning mosquito prey have been identified in the Orders Odonata, Coleoptera, Diptera (primarily aquatic predators), and Hemiptera (primarily surface predators). Although their capacity is affected by certain biological and physical factors, they could play a major role in mosquito control [9]. Hemipterans are widespread insects capable of colonizing nearly all types of aquatic habitats and are often one of the first successional stages in newly created water bodies, such as artificial ponds [11]. They are important food for many organisms including fish, amphibians, waterfowl, and other animals [12]. Certain families of the bugs may be utilized in the biological control of mosquito larvae [13]. Hemipterans are among the highly adapted colonizers of temporary ponds, and among hemipteran species, there will be strong selection to favor early migration to temporary ponds during springtime for reproduction, followed by the rapid development of the next generation [12].
Aquatic insects belonging to order Hemiptera are important as fish food, bioindicators, predators The role of Hemipteran predators in controlling mosquito larvae has been recognized since 1939 in New Zealand when stock troughs with Anisops assimilis were found to be free of mosquitoes whereas puddles in depressions surrounding the troughs contained mosquitoes [14]. Although the costs of colonization and mass production, coupled with the logistics of distribution, handling, and timing of release at the appropriate breeding site, impede the use of notonectids in mosquito control [15], results of a recent study for mass rearing and egg release of the predatory backswimmer Buenoa scimitar for the biological control of Cx. quinquefasciatus were impressive [16]. Production of backswimmer eggs was observed for 263 days and eggs that were released in artificial containers continued to produce new individuals until the adult stage. These backswimmers produced a significant reduction in mosquito larval density in 5 sampling dates out of 7 individuals until the adult stage. These backswimmers produced a significant reduction in mosquito larval density in 5 sampling dates out of 7.
Materials and Methods
In this research different databases including: “PubMed”, “Web of Knowledge”, “Scopus”, “Google Scholar”, “SID”, “random” and “thesis.research.ac.ir” of English and Persian articles published and graduated thesis from January 1990 to November 2020 were used. The study also revealed the rich diversity of aquatic and semi-aquatic heteropteran fauna occurring in the state. Under each species citation for the original description and other accompanying work necessary to understand the taxon or its occurrence in India is provided
An investigation on the aquatic insect community of 10 selected sites of Deepor Beel, the only Ramsar site of Assam situated on the southern side of River Brahmaputra was carried out from March to November 2013. During the study period, the aquatic insect community was represented by 31 species belonging to 18 families of 5 orders. Record of 17 species and 8 families of the order Hemiptera showed that it is the largest order in terms of aquatic insect diversity followed by order Coleoptera having 7 species and 5 families [3].
Results
The present study recorded 5 orders of aquatic insects (Hemiptera, Diptera, Coleoptera, Odonata, Ephemeroptera), 18 families (Corixidae, Nepidae, Mesoveliidae, Notonectidae, Gerridae, Pleidae, Veliidae, Belostomatidae of the order Hemiptera; Libellulidae, Coenagrionidae of order Odonata; Hydrophilidae, Dytiscidae, Chrysomelidae, Gyrinidae, Curculionidae of order Coleoptera; Baetidae of order Ephemeroptera; Chironomidae and Culicidae of order Diptera), 26 genera and 17 species of order Hemiptera, 7 species of order Coleoptera, 4 31 species. They were represented by species of order Odonata, 2 species of order Diptera, and 1 species of order Ephemeroptera.
The number of species recorded in the present investigation exceeded the findings of [17] from the same beel. It was found that the number of species of order Hemiptera was recorded highest [18] followed by order Coleoptera [19]. Hazarika and Goswami [20] recorded 14 species from two pond ecosystems in Gauhati University, Assam; while Das and Gupta [21] recorded 12 species of Hemiptera from rain pools and 10 species of Hemiptera from agricultural fields in Cachar district, Assam. Similarly, Das and Gupta [22] recorded 14 species of Hemiptera from a temple pond in Cachar district, Assam and Gupta and Narzary [23] recorded 5 species of Hemiptera from Phulbari anua, Assam. The number of recorded species in the present study signifies the rich diversity of aquatic insects in the only Ramsar site of Assam.
Many predaceous insects were found associated with both nuisance and mosquito vectors in temporary habitats such as manmade ponds, snowmelt pools, rain pools, floodwater pools, and other different pools [24]. Found that mosquitoes colonized the man-made ponds within one day of formation followed by predaceous Coleoptera, Hemiptera, and then Odonata. A significant inverse relationship was noted between mosquitoes and predators densities in 3 out of 4 trials [9]. A large number of different predator fauna have been associated with Anopheles larvae in different aquatic bodies [25] found that the most abundant and diverse predators associated with Anopheles albimanus larvae in various hydrological types in southern Mexico, were aquatic Coleoptera (20 genera) followed by Hemiptera and Odonata (each with 16231 genera).
Belostomatidae, Nepidae, and Notonectidae are the most important families of predaceous Hemipteran bugs. The backswimmers (Family: Notonectidae) are the most common bugs preying upon mosquito larvae, an important factor in reducing the immature mosquito population and considered promising in mosquito control [9]. Surface predators all belonged to order Hemiptera and they were less abundant than aquatic predators [26] identified seven families (Hydrophilidae, Dytiscidae, Corixidae, Nepidae, Notonectidae, Belostomatidae, and Corduliidae) of larval mosquito predators from the larval habitats(drainage ditches, cow hoofprints, and disused goldmines) of the malaria vector An. gambiae s.l. in natural habitats in Western Kenya Highlands. Predator density in disused goldmines was significantly higher than that of other habitat types. The samples were taken from rice fields at 6 different phases of maturity (fallow, ploughed, nursery, newly transplanted, after tillering, mature). Dytiscidae, Anisoptera, and Zygoptera were the primary aquatic predators in fallow or mature fields while Hydrophilidae and Notonectidae had no clear succession patterns. Nepidae was collected only from mature fields. Among surface predators, Vellidae was most abundant in fallow fields(in one study site) and in planted fields (in the other study site) and other predators were rare [27]. These results indicated that the abundance of aquatic predators decreased at the onset of ploughing and then recovered slowly as rice plants grew. In the case of surface predators, the pattern is similar but less conspicuous.
The water bugs Diplonychus rusticus (Fabricius) (Heteroptera: Belostomatidae) and Anisops bouvieri (Kirkaldy) (Heteroptera: Notonectidae) co-occur in wetlands sharing mosquito larvae as prey. As a consequence, an asymmetrical Intra Guild Predation (IGP) involving D. rusticus as IG predator and A. bouvieri as IG prey can be possible, the outcome of which may vary with the relative density of interacting species. Based on this proposition density-dependent effects on the IG prey and shared prey mortality was assessed in the laboratory using varying numbers of IG predator and shared prey (IV instar Culex quinquefasciatus larva). In contrast to the single predator system, mosquito larvae were proportionately less vulnerable to predation in IGP, at a low density of shared prey. An increase in density of mosquitoes decreased the mortality of IG prey (A. bouvieri), but the mean mortality of the IG prey increased with the density of IG predators, in the IGP system. An increase in density of mosquito and D. rusticus enhanced the risk of predation of a mosquito while reducing the mortality of A. bouvieri. Interaction between D. rusticus and A. bouvieri as a part of the IGP system provides a possible reason for coexistence of mosquito immature along with predators in wetlands. Biological regulation of mosquitoes may be affected if appropriate predator numbers are not available in the habitats [28].
Carnivores (39%), and periphyton feeders (10%) were predominant (Figure 1). A diversified carnivorous group, composed mainly by genera of Odonata, Hemiptera, and Coleoptera fed on aquatic insects. The detritivorous group was as diversified as the carnivores, although composed mainly by Diptera genera [29].
Twenty-six of the 28 artificial retention ponds were found to support aquatic hemipterans. In total, 9989 specimens were identified as species, representing 26 species from five families (Notonectidae, Corixidae, Belostomatidae, Nepidae, Pleidae) [30].
Corixidae was the most species-rich family; 17 corixid species were identified, one of which, Corisella inscripta Uhler, had not been previously documented in Wisconsin [31]. Although some species were very common and abundant, others were relatively rare, occurring in low numbers and a few sites [30].
Notonecta undulata (Notonectidae) was by far the most common and abundant species, occurring in 23 ponds, and accounting for 39% of the total species abundance Sex ratios of notonectid species and Hesperocorix acorixid species were generally not different from 1:1, while the sex ratios of Sigara and Trichocorixa corixid species were generally female- biased [30]. The percentage of adults generally increased from June to September [30].
Aquatic hemipteran richness ranged from one to 16 species in the occupied ponds; seven was both the mean and the mode species richness value. Corixid richness ranged from one to 12 species, with two species in the mode and 4.6 species in the mean. The 26 ponds also showed variation in morphology, biotic variables, and percent composition of each watershed land cover category [30]. Sixteen of the 28 ponds supported fish, with a total of five species (all predatory) collected. The fathead minnow Pimephales promelas, a pioneer species highly tolerant of a wide range of environmental conditions was most common, occurring in 11 ponds [30].
Over two sample periods occurrence indicates the number of ponds that the species was found in, while abundance indicates the total number of individuals that were sampled from those ponds. The sex ratio (%Fem) indicates the percentage of adults that were female; the age ratio (% Ad) indicates the percentage of the species that were adults, separated by sample date. Blank cells are shown for sex or age ratios not acquired. Age ratios were not acquired for corixid species (blank cells) due to the inability to identify the instars of this taxon [30,31]. The age ratio of Corixidae (16 species combined) was 52% adults in June and 79% in September. Due to their low numbers and similarity in habitat and resource use, Ranatra species were combined in our analyses; R. fusca was the most common of the three species. Hemipteran species with occurrence values of less than three were not included in the NMS ordination or cluster analysis.
Strong seasonality was expressed by the notonectids Buenoa margaritacea, Torre-Bueno and Notonecta September [30]. Insulata Kirby, both of which expressed consistently positive (B. margaritacea) or consistently negative changes (N. insulate) in presence/absence status in the ponds between June and Buenoa margaritacea was found in six ponds in June (total abundance=29) and in six additional ponds in September (total abundance=474) [30].
In contrast, N. insulata was found in 13 ponds in June (total abundance=407), and no longer found in six of those ponds in September, when its total abundance dropped to 79. The highest instability values were found for the corixid species Sigara alternata Say (10 changes) and Hesperocorixa vulgaris Hungerford (nine changes), the changes of which were only weakly seasonal (Figure 1) [30]. Belostoma flumineum Say and Hesperocorixa laevigata Uhler was also relatively unstable, non-seasonal species (Figure 1). Species that were fairly consistent in their occurrence across seasons (detected by the combination of low instability and low seasonality values) included both very rare (e.g., Sigara solensis Hungerford) and very common species (e.g., N. undulate) [30].
Occurrence instability and seasonality of 25 aquatic hemipteran species sampled from 28 ponds in June and September. Species whose seasonality values were highly positive tended to be absent in June but present in September particularly when the seasonality value was equal to the instability value (e.g., B. margaritacea). Species with highly negative seasonality values tended to decline in September, again, particularly when the seasonality value (absolute value) was equal to the instability value (e.g., N. insulate). Species with seasonality values close to zero were highly consistent in their presence/absence status in ponds, if their instability value was also low. Low seasonality values coupled with high instability values indicates that the species had several countering (non-directional) occurrence changes (e.g., B. flumineum).
A total of 37 species of semiaquatic bugs were recorded from the reserves during this survey, with 36 species found in the Central Catchment Nature Reserve and 17 species in the Bukit Timah Nature Reserve. Of the 24 forest species, 13 are considered rare in Singapore and they are mostly distributed in the Central Catchment Nature Reserve. Eight species; Tenagogonus octopunctatus, Ventidius modulatus, Microvelia albolin.eolata, Neoalardus typicus, Hydrometra carin. ata, H. lon. gicapitis, H. okin. awana and H. papuan were recorded from Singapore for the first time [32]. Several other recently published records were also based on the materials collected in the Nature Reserves notably, Cryptobates rufus, Rhagovelia sin. gaporen. sis and R. rudischuhi [33,34].
The poor diversity of Gerromorphan bugs in the Bukit Timah Nature Reserve was due to the small and relatively short streams with poorly grown aquatic vegetation and the total absence of swamp [32]. The middle and lower reaches of the streams were either at the edge of the forest or in the open country habitats [32]. Inside the forest, parts of the streams dried up easily during the dry season as these were exposed due to a large number of fallen big trees in recent years. They probably also suffered from the drying effects of the numerous walking trails constructed in the reserve [35]. The isolated location and the small stream at a higher elevation in Jungle Fall Valley probably accounted for the lowest number of forest species of (6 out of 24) found in this primary forest [32]. The central catchment nature reserve has many swampy forest streams under well-shaded forest and these provide different microhabitats that are not available in the Bukit Timah Nature Reserve [32].
Ten forested species found in the forest of the Central Catchment Nature Reserve Amemboa brevifasciata, Cryptobates rufus, Rheumatogonusintermedius, Tenagogonus (L.) octopunctatus, T quin quemaculatus, Ventidius harrisoni V. modulatus Microvelia plumbea, Rhagovelia singaporensis and Strongylovelia sp. were not found in Bukit Timah Nature Reserve [32]. With exception of R. intermedius (moderate to fast flowing water species), the other nine species were either found on swampy puddles or in slow flowing streams [32]. Four species of water measurers, Hydrometra carinata, H. longicapitis, H. okinawana and H. papuan. were collected from a weedy pool, in a semi-open country habitat, near the Nee Soon swamp forest These were new records for Singapore and found only in this location [32].
H. papuana is very rare in Peninsular Malaysia and was only recorded from lowland swamp forests [36].
The Nee Soon Forest has the highest species diversity recorded in this study. Twenty [83%] of the 24 forest species and 9 (69%) of the 13 rare species were found in this location. This swamp forest was also the type locality for two recently described species, Rhagovelia singaporensis [34,36]. The latter is rare (Figure 1) and distributed only in a few swampy streams, under well shaded forest, either near headwaters or in areas with iron hydroxide deposits [37].
Metrocoris tenuicornis, Rhagovelia sumatrensis and R. rudischuhi were very common and were widely distributed in all forest streams, along with the less common Tenagogonus insularis at the swampy or quiet edges of the streams in all forested areas. Ptilomera tigrina was also common in most flowing forest streams except for the stream at the Jungle Fall Valley [32]. Three gerrids, Cylindrostethus malayensis Polhemus, 1994 (C. costa/is Cheng & Fernando, 1969), Esakia fernandoi Cheng and Ventidius hungerfordi Cheng, previously collected from Sungei seletar in 1965 [38] were not found in this study [32]. Sungei seletar was the biggest stream in the Central Catchment Nature Reserve before it was converted into a reservoir in the early 1970s. The interruption of the water system probably accounted for the possible extinction of these three species that inhabited larger flowing water bodies. The survival of the present-day swamp forest species, especially those rare and localized ones will, therefore, be threatened by the change, loss or pollution of the swamp forest [32].
Entomovlia doversi previously recorded from the Mac Ritchie forest Murphy, et al. was also not found in this study [32-37]. It could have been carried over through the pipeline from the river in Johore (Malaysia) to the Upper Peirce Reservoir. Only a single specimen was collected after a heavy downpour that could have caused the water from the reservoir to flow into the forest stream. This species is common in pristine forest streams in Peninsular Malaysia. The single record of Ventidius modulatus was also from the same area [32]. A study in the wetlands of Donana National Park (SW Spain) about the aquatic bug Trichocorixa verticalis verticalis (Hemiptera: Corixidae) was performed. Overall, sampled 134 different sites in the Donana wetlands situated within a polygon of 54,000 Ha. Some of these points were sampled during several years. Sampled sites included artificial ponds (11 fish ponds, 14 shallow, new temporary ponds and 30 deep waterholes (zacallones) and natural water bodies (four streams, 12 points in temporary marshes and 63 natural ponds).We detected the presence of T. v. verticalis on 66 occasions more than half of these (53%) being in artificial ponds. In contrast, artificial water bodies were only 41% of the total points sampled [39].
Veta la Palma fish ponds and new temporary ponds were the only two areas where reproductive populations of T. v. verticalis were recorded [39]. In both areas T. v. verticalis was the dominant species, apparently outcompeting native ones. In Vetala Palma, sampled during 2001 and 2002, 179 samples were gathered with a total of 738 adult corixids, 96% of which were T. v. verticalis, the remaining adults being Sigara stagnalis and S. scripta. Abundance peaked in spring and summer, with the lowest densities in autumn and winter.
There was a highly significant difference in densities between seasons [40].
The presence of juveniles suggests that reproduction continues throughout the year in this site. In 2006, we detected a second reproductive population in the new temporary ponds in caracoles, where 307 adult corixids were retrieved from 14 ponds of which 92% were T. v. verticalis. The other three species that occurred in this area were Sigara lateralis (4%), S. stagnalis (2%), and S. scripta (2%). Both here and in Vetala Palma, we also identified freshly molted adult and juvenile corixids that were surely T. v. verticalis. In the three new temporary ponds with the highest T. verticalis density (30 in our sample), conductivity was particularly high, ranging from 17.3 to 54.6 m S/cm [39].
In the other places where T. v. verticalis occurred, no matter the year, only adults were detected, surely these individuals were vagrant adults (we captured juvenile corixids but we identified them as other species). On these occasions T. v. verticalis always occurred in small numbers, and making a small proportion of the total corixids sampled. In 2007, we captured and retrived 1881 adult corixids throughout the year but only 37 of those were T. v. verticalis [39].
This study show the relative proportion of T. v. verticalis out of the other corixid species present but only in the ponds where the exotic species occurred T. v. verticalis was detected coexisting with another seven species of corixids in the area. Only one other corixid species was present in more water bodies than T. v. verticalis in 2006, and only three other species in 2007. Paracorixa concinna was the only species that was never observed coexisting with T. v. verticalis in the same pond. T. v. verticalis was more likely to be found in ponds than the rarer native corixids (Sigara scripta, S. stagnalis, and S. selecta). Remarkably, T. v. verticalis has not been recorded in the main body of the temporary marsh in the samples studied as yet [39].
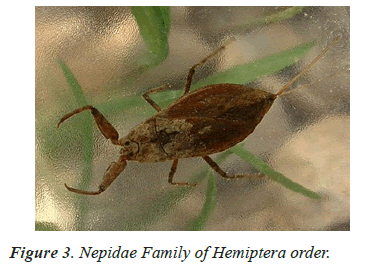
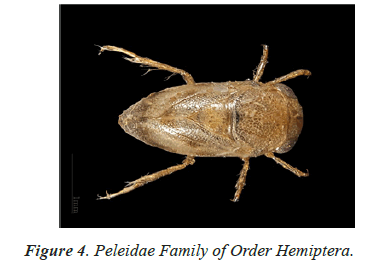
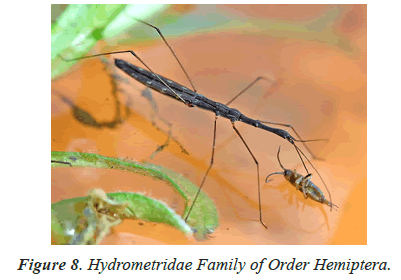
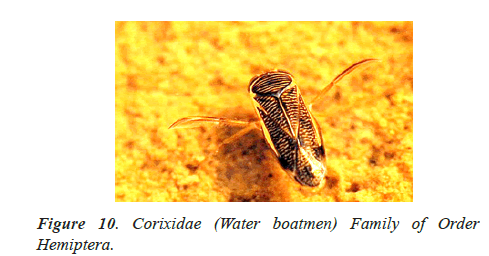
In the study of life cycle of the giant water bug of northwestern Patagonian wetlands analyzed the effect of hydro period and water temperature on the life cycle of the giant water bug Belostoma bifoveolatum in two wetlands of northwestern Patagonia, Argentina. In each wetland, we estimated adult and nymph abundance and monitored water depth and temperature throughout the study period [40,41]. We determined the age structure of the giant water bug population in each wetland, and estimated the cumulative Degree-Days [DD] needed for eggs to hatch and for nymphs to complete their development. Individuals of B. bifoveolatum colonized temporary wetlands at the beginning of spring when daylight lasts 12 h. The breeding period varied with hydro period length and showed both univoltine and bivoltine strategies, with a relatively constant breeding season. Egg- bearing males appeared in October, carrying between 35 and 144 eggs per individual [41]. Hatching success was high (~80% of eggs) and cumulative temperature for the hatching event was between 250 and 300 DD (which represents 3–4 weeks in nature), while complete development occurred between 800 and 1220 DD (~7–8 weeks). Individuals were more abundant in shallow and sunny patches of the wetlands, where the temperature was comparatively high than in deeper or shaded sites. These results showed that hydro period duration and temperature could be good regulators of voltinism and development in B. bifoveolatum, driving the population structure of this giant water bug at the southern end of its distribution range [41]. Figures 1-13 shows different families of Hemiptera as and semi and aquatic insects.
Discussion and Conclusion
Aquatic insects are major components of both freshwater communities and adjacent terrestrial habitats. Larval insects serve as prey for larger aquatic insects, amphibians and fishes, and the winged adult stages feed terrestrial predators such as birds, bats and spiders.
Aquatic and semi aquatic heteropterans form food in different trophic levels of fresh water ecosystems. Due to their poor dispersal capabilities, these bugs serve as zoogeographical indicators for diverse habitats. Further, many families of the bugs may be utilized in the biological control of mosquito larvae. Some corixids are used as indicators of water quality. Studies also indicate that the quality of the aquatic environment is partially dependent on the abundance of the bug population.
Aquatic insects belonging to order Hemiptera are important as fish food, bio indicators and predators. The role of hemipteran predators in controlling mosquito larvae has been recognized since 1939 in New Zealand, when stock troughs with Anisops assimilis were found to be free of mosquitoes whereas puddles in depressions surrounding the troughs contained mosquitoes.
Acknowledgment
This research is supported by Ministry of Health and Medical Education under code number of NIMAD 995633.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
Ministry of Health and Medical Education under code number of NIMAD 995633.
Author Contributions
All the authors were involved.
References
- Power, M., Rainey, W. (2000). Food webs and resource sheds: towards spatially delimiting trophic interactions. Ecological Consequences of Habitat Heterogeneity., 1: 291-314.
- Dijkstra, K-DB., Monaghan, MT., Pauls, SU. (2014). Freshwater biodiversity and aquatic insect diversification. Annual Review of Entomology., 4(59):143-163
- Choudhury, D., Gupta, S. (2015). Aquatic insect community of Deeporbeel (Ramsar site), Assam, India. Journal of Entomology and Zoology Studies., 3(1):182-192.
- Minshall, GW. (2003). Responses of stream benthic macroinvertebrates to fire. Forest Ecology and Management., 178(1-2):155-161.
- Tachet, P., Richoux, H., Bournaud, P., Usseglio-Polatera, M. (2003). Invertébrés d'eau douce: Systématique, biologie, écologie edition. CNRS Paris P., 588.
- Mayhew, PJ. (2007). Why are there so many insect species? Perspectives from fossils and phylogenies. Biological Reviews., 82(3):425-454.
- Balian, E., Lévêque, C., Segers, H., Martens, K. (2008). Freshwater Animal Diversity Assessment. Developments in Hydrobiology: Springer
- Wetzel, R. (2001). Inland waters: understanding is essential for the future. Limnology lake and river ecosystems 3rd ed. Academic Press San Diego CA., 8:25-41.
- Shaalan, EA-S., Canyon, DV. (2009). Aquatic insect predators and mosquito control. Tropical Biomedicine., 26:223-261.
- Thirumalai, G. (2004). A checklist of aquatic and semi-aquatic Hemiptera (Insecta) of Karnataka. Records of the Zoological Survey of India., 102(1-2):57-63.
- Papáček, M. (2013). Small aquatic and ripicolous bugs (Heteroptera: Nepomorpha) as predators and prey. The question of economic importance EJE., 98(1):1-12.
- Tara, J., Kour, R. (2014). Biology and morphometric studies of aquatic bug, Sphaerodema molestrum (Hemiptera: Belostomatidae) from Jammu (J&K, India). Journal of Entomology and Zoology Studies., 2(3):82-85.
- Saha, N., Aditya, G., Bal, A., Saha, GK. (2007). A comparative study of predation of three aquatic heteropteran. Bugs on Culex quinquefasciatus larvae., 1:73-80.
- Kumar, R., Hwang, J-S. (2006). Larvicidal efficiency of aquatic predators: a perspective for mosquito biocontrol. Zoological Studies-TAIPEI., 45(4):447-450.
- Legner, E. (1995). Biological control of Diptera of medical and veterinary importance. Journal of Vector Ecology., 20(1):59-120.
- Rodriguez-Castro, V., Quiroz-Martinez , H., Solis-Rojas, C., Tejada, L. (2006). Mass rearing and egg release of Buenoa scimitra bare as biocontrol of larval Culex quinquefasciatus. Journal of the American Mosquito Control Association., 22(1):123-125
- Chetri, G., Sheikh, M., Kalita, J., Dutta, A. (1997). Population abundance of aquatic insects in Deepar Beel Assam. Insect Environment., 3(1):14-15.
- Abhijna, U., Ratheesh, R., Kumar, AB. (2013). Distribution and diversity of aquatic insects of Vellayani lake in Kerala. Journal of Environmental Biology., 34(3):605-611.
- Sivaramakrishnan, K., Venkataraman, K., Sridhar, S., Marimuthu, M. (1995). Spatial patterns of benthic macroinvertebrate distributions along river Kaveri and its tributaries (India). International Journal of Ecology and Environmental Science., 21:141-161.
- Hazarika, R., Goswami, M. (2010). Aquatic Hemiptera of Gauhati University, Guwahati, Assam, India. Journal of Threatened Taxa., 2(3):778-782.
- Das, K., Gupta, S. (2010). Aquatic Hemiptera community of agricultural fields and rain pools in Cachar District, Assam, North East India. Assam University Journal of Science and Technology., 5(1):123-128.
- Das, K., Gupta, S. (2012). Seasonal variation of Hemiptera community of a temple pond of Cachar District, Assam, northeastern India. Journal of Threatened Taxa., 5:8.
- Gupta, S., Narzary, R. (2013). Aquatic insect community of lake, Phulbari anua in Cachar, Assam. Journal of Environmental Biology., 34(3):591-600.
- McDonald, G., Buchanan, G. (1981). The mosquito and predatory insect fauna inhabiting fresh‐water ponds, with particular reference to Culex annulirostris Skuse (Diptera: Culicidae). Australian Journal of Ecology., 6(1):21-27.
- Danis-Lozano, R., Rodriguez, MH., Arredondo-Jimenez, J., Hernandez-Avila, M., Mallorca, C. (1997). Aquatic insects associated with Anopheles albimanus (Diptera: Culicidae) breeding sites in southern Mexico. Environmental Entomology., 26(4):828-838.
- Munga, S., Minakawa, N., Zhou, G., Githeko, AK., Yan, G. (2007). Survivorship of immature stages of Anopheles gambiae sl (Diptera: Culicidae) in natural habitats in western Kenya highlands. Journal of Medical Entomology., 44(5):758-764.
- Mogi, M., Miyagi, I. (1990). Colonization of rice fields by mosquitoes (Diptera: Culicidae) and larvivorous predators in asynchronous rice cultivation areas in the Philippines. Journal of Medical Entomology., 27(4):530-536.
- Brahma, S., Aditya, G., Sharma, D., Saha, N., Kundu, M., Saha, G. (2014). Influence of density on intraguild predation of aquatic Hemiptera (Heteroptera): implications in biological control of mosquito. Journal of Entomological and Acarological Research., 46(1):6-12.
- Motta, R., Uieda, VS. (2004). Diet and trophic groups of an aquatic insect community in a tropical stream. Braz. J. Biol., 64:809-817.
- Foltz, SJ., Dodson, SI. (2009). Aquatic Hemiptera community structure in stormwater retention ponds: a watershed land cover approach. Hydrobiologia., 621(1):49-62.
- Hilsenhoff, WL. (1995). Aquatic Hydrophilidae and Hydraenidae of Wisconsin (Coleoptera). I. Introduction, Key to Genera of Adults, and Distribution, Habitat, Life Cycle, and Identification of Species of Helophorus helophorus Fabricius, fabricius, Hydrochus hydrochus Leach, and Leach, and Berosus Leach (Hydrophilidae), and Hydraenidae. Aquatic., 28:278-287.
- Yang, C., Lua, H., Yeo, K. (1997). Semi-aquatic bug (Heteroptera: Gerromorpha) fauna in the nature reserves of Singapore. Garden’s Bulletin., 49:313-319.
- Polhemus, DA., Polhemus, JT. (2002). The Trepobatinae (Gerridae) of New Guinea and surrounding regions, with a review of the world fauna Part 6 Phylogeny, biogeography, world checklist, bibliography and final taxonomic addenda. Insect Systematics & Evolution., 33(3):253-290.
- Yang, C., Polhemus, D. (1994). Notes on Rhagovelia mayr (Hemiptera: Veliidae) from Singapore, with description of a new species. Raffles Bulletin of Zoology., 42(4):987-993.
- Corlett, RT., Bukit, T. (1988). The history and significance of a small rain-forest reserve. Environmental Conservation., 15(1):37-44.
- Polhemus, JT., Polhemus, DA. (1995). Revision of the genus Hydrometra Latreille in Indochina and the western Malay Archipelago (Heteroptera: Hydrometridae): Bishop Museum Press.
- Murphy, D. (1990). Walkers on water–An account of the pleuston of Singapore. Essays in Zoology, Papers Commemorating the 40th Anniversary of the Department of Zoology, National University of Singapore Singapore. Walkers on water., 8:153-68.
- Cheng, L., Fernando, CA. (1969). Taxonoimic study of the Malayan Gerridae (Hemiptera: Heteroptera) with notes on their biology and distribution. Oriental Insects., 3(2):97-160.
- Rodríguez-Pérez, H., Florencio, M., Gómez-Rodríguez, C., Green, AJ., Díaz-Paniagua, C., Serrano, L. (2009). Monitoring the invasion of the aquatic bug Trichocorixa verticalis verticalis (Hemiptera: Corixidae) in the wetlands of Doñana National Park (SW Spain). Pond Conservation in Europe Springer., 3:65-73.
- Rodríguez Pérez, H. (2006). Efectos de las aves acuáticas sobre los macrófitos y los invertebrados en las marismas de Doñana.Ph.D thesis.
- Jara, FG., Perotti, MG. (2018). The life cycle of the giant water bug of northwestern patagonian wetlands: The effect of hydro period and temperature regime. Invertebrate Biology., 137(2):105-115.