Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2021) Volume 11, Issue 83
Azurin and its protein subunit P28 as an alternative approach to anticancer therapeutics.
Faheem uz zaman*, Rabia Arooj, Mubeen Fatima, Asma Basharat, Aleena Sumrin
Department of Center for Applied Molecular Biology (CAMB), University of the Punjab, Lahore, Pakistan
- Corresponding Author:
- Zaman FU
Department of Applied Molecular Biology (CAMB),
University of the Punjab,
Lahore,
Pakistan
E-mail: faheem.phd20@camb.edu.pk
Accepted date: September 13, 2021
Abstract
In 2018 the second leading basis for death is cancer with 9.6 million deaths. Some conventional therapies are chemotherapy, surgery and radiotherapy for cancer. Formation of resistance against multidrug lack of toxicity against tumor cells are limitations and adverse side effects of these therapies. A redox protein is Azurin having 128 amino acids with a size of 14 kDa and secreted by Pseudomonas aeruginosa as a periplasmic protein. A peptide from Azurin has been found having target specificity, delivery and cytotoxity like Azurin but having lesser side effects as compared to Azurin. This amphipathic peptide is formed by residues from 50-77 AA of Azurin having 209 KDa size showing very surprising results named as P28. The anticancer activity of P28 is related with its amino acids residues from 11-18 which binds to P53. The clinical trials against adult and pediatric patients have been carried out, results showed that P28 is safe with no side effect and can be used as an alternative anti-cancer therapeutic protein. In this review paper an updated detail of Azurin and P28 about mechanism of action and therapeutic strategies is described.
Keywords
Azurin, Periplasmic protein, Pseudomonas aeruginosa, Amphipathic peptide.
Introduction
An indicative member of cupredoxin family is Azurin which is secreted by Pseudomonas aeuruginosa. It is a periplasmic copper containing protein consist of 128 AA of 14 KDa size. It is made up of eight antiparallel strands structure which is connected by 4 loops and linked by disulphide bridge. A study shows that cupredoxin especially Azurin has been used for the development of therapeutic proteins, which have anti-cancer as well as antiviral and anti-bacterial effects. Azurin is water soluble and stable protein which is small in size and can be easily purified in bacterial host.
Azurin with electron transfer activity have anticancer role both in vitro and in vivo. Anticancer activity of Azurin has shown the interaction between Azurin and P53 leads towards the rise in intracellular transcription factors and stabilization of P53. An experiment on single molecule atomic spectroscopy has elaborated the formation of strong and firm complex between Azurin and P53. Although Azurin have prominent and significant anticancer activity. But it produces some side effects which may cause problem in its pharmaceutical efficiency. Therefore, a peptide from Azurin has been found having same target specificity, delivery and cytotoxity like Azurin but having lesser side effects as compared to Azurin. This amphipathic peptide is formed by residues from 50-77 AA of Azurin having 209 KDa size showing very surprising results named as P28. The antitumor activity of P28 is related with its amino acids residues from 11-18 which binds to P53 [1].
Invasive tumors are a big threat to human beings, and after cardiovascular disease it is major leading cause of death. The expensive treatment of cancer patients highly disturbed the life style of patients and their families. Therefore, it is highly needed to explore efficient and cost effective alternative anticancer therapeutics. Tumor removal surgeries along with chemotherapy and radiotherapy is currently the most used treatment for cancer patients. Although there are side effects of traditional chemotherapy and radiotherapy along with the success of surgical procedures. In recent years, rapid development has seen with the aim of finding protein based specific targeted drugs to achieve the double benefit of tumor treatment and minimized side effects.
P53 is tumor suppressor and a transcription factor which plays very important role in genome stability and prevention of cancer development. It is a homo tetramer in which each monomer is comprises of 393 amino acid residues which further divided in three functional regions. An N terminal domain NTD which is comprises of 1-93 AA engaged in transcriptional function of P53 and growth suppression, a core DNA binding domain DBD which involved in site specific binding and comprises of 102-292 AA residues and C terminal domain CTD which is responsible for P53 tetramerization. P28 and Azurin target many pathways like P53 and receptor tyrosine kinase pathway. They have promising potential to be used as new anticancer drug because of not inducing resistance [2].
Features of Azurin and its Derived Peptides
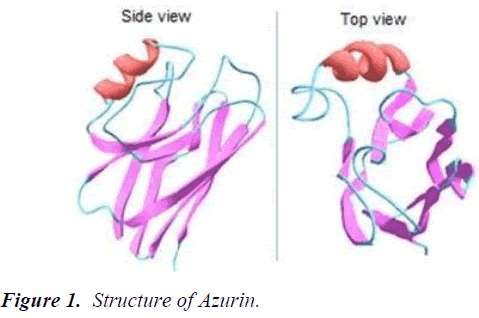
Azurin belongs to cupredoxin redox proteins family, which acts as an important anticancer drug due to its unique features. The redox protein azurin consists of 128 amino acid having a size of 14 kDa and secreted by Pseudomonas aeruginosa as a periplasmic protein. Azurin, copper-containing redox protein being an important bacteriocin has a structure similar to immunoglobulins represented by a structure similar to single antibody having 8 antiparallel strands forming a βeta-sandwich core of the immunoglobulin chain. Azurin contains protein transduction domain in an alpha helix as well as four regions are found in a C-terminal domain CD, GH, FG and EF. An immunoglobulin fold and β-sandwich core enables the peptide to show anticancer activity by escaping the immune response (Figure 1).
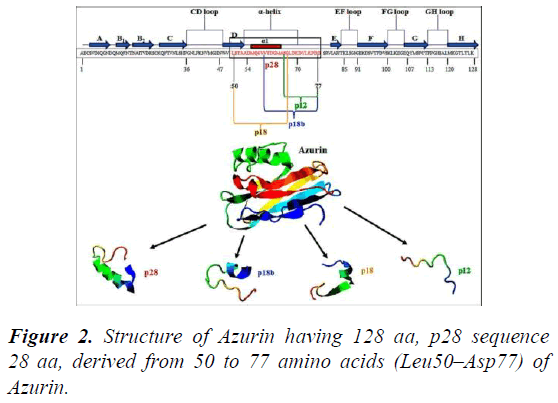
Azurin penetrates to the cancer cells through different caveosome independent or caveosome dependent and endocytic pathways via unique α-helical peptides resulting in the shrinkage of tumor cells. The multivalent therapeutic potential of azurin is associated with an α-helical peptide. One helical peptide consisting of 18 amino acids long stretch is known as p18, is the internal P28 fragment (L50–G67), while P28 is the other one with additional 10 amino acids (L50– D77), which enables entry of azurin into cancer cells without acting on normal cells (Figure 2).
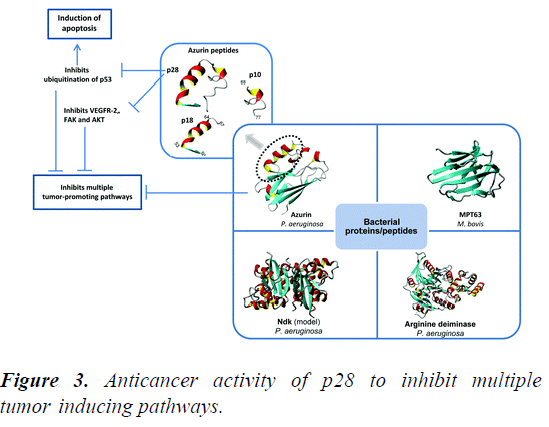
Azurin along with its derived peptide P28 shows apoptotic activity and helps in the process of angiogenesis by different mechanisms to treat cancers. P18 having protein transduction domain is internalized into the tumor cells, but its anticancer activity is very low. Therefore, it can be used as a diagnostic marker because it can preferentially enter and accumulate into cancer cells. While P28 has the ability to preferentially enter the tumor cells and exerts its anticancer response with the formation of a complex with DNA binding domain of P53, inhibiting its proteosomal degradation and ubiquitination, which leads to the higher levels of tumor suppressors, induction of apoptosis and cell cycle arrest in various type of cancer cells (Figure 3).
Mechanism of Anticancer Activity of Azurin
Synchronizationofredoxhomeostasis
There is a good number of Reactive Oxygen Species (ROS) like superoxide (O2-), hydroxyl radical (OH-) and hydrogen peroxide (H2O2) which have the ability to bring to terms redox signaling for various cellular functions. High levels of ROS cause cell death and this feature of ROS can be utilized in developing approaches for cancer treatment.
Azurin is a metallo-protein containing copper in it as a metal source. It is redox in nature and is involved in denitrification process of Pseudomonas aeruginosa, serving as an electron transfer shuttle. Being a redox protein, azurin generates exorbitant levels of ROS during macrophages treatment. The cytotoxic character of azurin cannot be attributed to the redox nature of this protein because many redox-negative mutants have been identified which still retain the ability to generate ROS brought about macrophage apoptosis. It has been disclosed by mechanistic analyses that azurin modulates redox homeostasis through a P53-mediated mechanism by forming complexes with P53 and increasing its protein level.
Maintenance of P53 protein level
Evidences have shown that the anticancer function of azurin is pivoted on the turning up of P53 protein. Azurin causes apoptosis promptly in cancer cells which express functional P53 than those with no P53 expression. The high level of P53 protein in cancer cells is indicative of azurin’s functioning through P53-pathway. There is a crucial E3 ubiquitin ligase named MDM2 that functions to regulate P53 degradation by attaching to its N-terminal transactivation domain (P53-TAD). Azurin also binds with P53-TAD but with a dissociation constant higher than that of MDM2-P53 TAD association. Moreover, azurin, P53 and MDM2 together can form a ternary complex. So, azurin cannot interfere with MDM2 binding with P53-TAD. Raman spectroscopy and molecular docking have established that azurin can increase structural stability of P53 protein by binding to flexible L1 and s7-s8 loops of P53 DNA- Binding Domain (DBD). So, the protein level and anticancer function of P53 may be linked to structural stability of P53- DBD [3].
Variations in cell membrane properties
The structural features of cell membrane are controlled by cell surface receptors. Cell-surface receptors manage this by adjusting cell shape, cell migration, attachment of cell to the surrounding extracellular matrix and membrane stiffness. So, ultimately these cell-surface receptors dictate the activity of anticancer drugs.
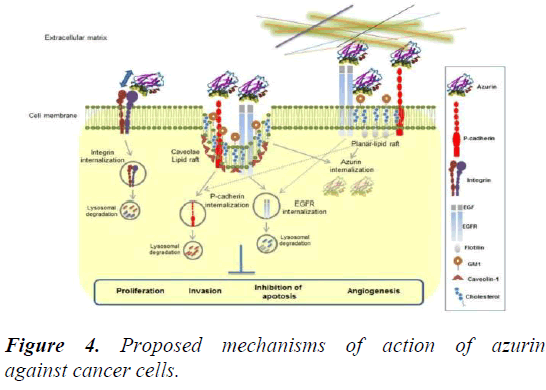
It has been studied that azurin lowers the expression of integrin β1 receptors, thus disturbing their distribution on the cell membrane of A549 lung cancer cells. Lately, it has also been investigated that azurin lessens the level of caveolin-I and arrangement of the cell membrane in HeLa cervical cancer cells and MCF-7 breast cancer cells. These practices offer cancer cells more exposure to anticancer drugs, for instance, epidermal growth factor receptor-specific inhibitors like erlotinib, gefitinib and chemotherapeutic drugs like doxorubicin, paclitaxel.
The case of cadherins is another example to cite in this regard. The regulation of cell-cell adhesion is a critical operation controlled by cadherins. Overexpression of P-cadherins is related to expanded cell invasion in several breast cancer cells. Studies have proclaimed that azurin diminishes the expression of P-cadherin and holds back cell invasion caused by P- cadherin [4]. The intracellular signaling responses of non- receptor tyrosine kinases may also be related with the regulation of different cell membrane features by azurin because usually the phosphorylation levels of Akt, FAK, Src and PI3K are weakened (Figure 4).
Intervention with Eph-Ephrin pathway
Tyrosine kinase receptors have largest family constituting Eph receptors. Cancer progression involves signaling pathways between Eph receptors and their respective ephrin ligands. This feature makes Eph receptors potential targets for therapeutic purposes. There is exceptional similarity between azurin and ephrins and this similarity enables azurin to bind with Eph receptors (EphB2) with five times higher affinity level (Kd=6 nM) than that of ephrinB2 (Kd=30 nM). As a result, azurin efficiently binds with EphB2 by competing with ephrinB2 and obstruct tyrosine phosphorylation pathway of EphB2. Experiments have indicated that azurin has a C-terminal segment (amino acids 88-113) which arbitrates this interactivity between azurin and EphB2. Additional investigations claim that azurin peptide treatment comes up with a route to decreased cell viability in LN-229 glioblastoma cells and retards growth in MCF-7 breast cancer cells.
Mechanism of Anticancer Activity of P28
Entry of P28 to carcinoma cells
A receptor mediated endocytosis process by which P28 a cancer homing peptide entered into the cancer cells. The preference of P28 to enter in tumor cells related to the normal cells is higher because of the high expression of caveolin receptors in tumor cells then the surface of normal cells [5]. However, the entry of P28 is independent of membrane bound glycosaminoglycans and but caveolin and cathrin independent pathways are the ways through which it can enter into the tumor cells. Thus, these characteristics represent that P28 possibly will be used to preferably change other peptides to tumor cells.
Interaction of P28 with cellular signaling pathway
Evidence shows that COOH terminal of 10-12 AA of P28 has important part in inhibition of growth of cell and increased its apoptotic activity. P28 interact with many signaling pathways related to cancer development which involved in post transcriptional increase in P53 and inhibition of angiogenesis and tumor growth. The inhibition can occur through hinderance of the activity of VEGFR-2 tyrosine kinase and by its phosphorylation. Inhibition in phosphorylation of AKT, FAK AND P13K, Hindering the bFGF (FGFR-1) effects on growth, by binding of 28 to DBD of P53 and use of COP1 to prevent ubiquitination. They all are results in inhibition of growth and apoptosis of tumor cells.
P53 protein stabilization
P28 is depending upon P53 status in cancer cells for its anticancer activity. MDM2 is negative regulator of tumor suppressor protein. To attach with P53, P28 does not strive with MDM2. But interaction of P28 occur with P53 DNA binding domain. By the use of biophysical characterization method, it was reported that dissociation constant for P28 and P53 DNA Binding Domain (DBD) complex is range from 7 µM to 0.7 nM. The interaction of P53-DBD with COP1 and E3 ubiquitin ligase cause the negatively regulation of the P53. P28 compete with COP1 to bind with P53-DBD and it reduces the COP1 binding in concentration dependent manner which is showed by GST pull down experiment. The competition of P28 with COP1 for binding is confirmed when it fixes to a pocket on P53 DNA Binding Domain (DBD). The P53 dissociation constant and COP1 complex is approximately 10 nM which is measured by surface plasma resonance and atomic force microscopy. Which is comparable to Kd value of P28 and P53 DNA binding domain complex. The COP1 mediated ubiquitination pathway is one possible mechanism for P53 stabilization by P28 in which inhibition of COP1 binding to P53 DNA binding domains is occurred by P28. High affinity for different P53 mutants achieved by rational designing of P28.
Angiogenesis inhibition
A cytokine VEGF vascular endothelial growth factor promote angiogenesis and over expressed in many tumors and blood cancers. VEGFR2 9 (vascular endothelial growth factor receptor 2) is present in several lymphocytes and carcinomas. It plays role in regulation of proliferation and endothelial migration by responding to vascular endothelial growth factor signal. So, VEGF/VEGFR2 is main therapeutic target in regulation of angiogenesis in tumors. The inhibition of P28 of BFGF (Basic Fibroblast Growth Factor) receptor inhibit VEGF induced migration, formation of capillary tube and NEO angiogenesis in multiple xenograft models. P28 colocalizes with VEGFR-2 by penetrating in human umbilical vein endothelial cells. It also results in irregular spreading of cell migration associated proteins by decrease in downstream phosphorylation of AKT and FAK and P28 reduce VEGFR-2 kinase activity in response to VEGFA. So, P28 inhibits angiogenesis in different way as compared to the other anti- angiogenic agent.
Therapeutic efficacy of azurin
Azurin shows significant anticancer and cytotoxic activity towards human breast cancer and melanoma cells. In a study azurin injection into human cancer cells xenografted nude mice resulted in increase of survival rate and regression of tumor in mice. P28 shows proapoptotic activity towards various tumor cell lines. Azurin exerts its anticancer activity by preferentially penetrating cancer cells leading to apoptotic and cytostatic effects without acting on normal cells [6]. Azurin domain representing anticancer potential is termed P28 and it spans 50-77 residues, adopting an alpha helical conformation. The therapeutic efficacy of azurin is not only limited to its anticancer activity, but also it protects host cell invasion by Plasmodium falciparum (malarial parasite), Toxoplasma gondii (toxoplasmosis-causing parasite) and AIDS causing HIV-1 virus. Azurin has shown significant potential in oral squamous carcinoma cells, also it enhanced the activity of other drugs conducted a study using Escherichia coli strain to target cancer which inhibited 4T1 breast tumors and mouse B16 melanoma by continuous azurin expression. The results showed significant regression in the growth of orthotopic 4T1 breast tumor and B16 melanoma, and also inhibited pulmonary metastasis in immunocompetent mice. Dr. Yamada conducted a study which showed that P28 elevated the effectiveness of antimitotic and cytotoxic DNA-damaging drugs in numerous cancer cells through Cdk2/p21/P53 pathways. A number of studies has confirmed that P28 has anticancer activity against breast cancer models. P28 decreased the size of tumor of MCF-7 xenografts in the mice model with 30 days exposure of 10 mg/kg dose of peptide. Dr. Ghasemi Dehkordi constructed a vector in order to express Mammaglobin-A and azurin to induce immune response towards breast cancer. Dr. Mehta also designed another carrier to express P53 and azurin simultaneously under the control of hypoxic promoter.
Phase I clinical trials of 28 are recently completed, which confirms its safety and anticancer activity in humans. One clinical trial was conducted on 15 metastatic solid tumor patients. After treatment, three patients exhibited partial response, one of them showed full response and 7 out of 15 patients showed stable disease for seven to sixty one weeks. Another trial was carried out in children having brain tumors. After treatment the results showed safety of P28, although it does not have high potential for malignancies of central nervous system.
Conclusion
An ideal therapeutic effect with limited antitumor drugs is a difficult task to achieve as tumor development is depends on various factors. Multiple signaling pathways are targeted by P28 and azurin against various cancers as demonstrated by many researchers previously. This therapeutic protein causes apoptosis by stabilizing P53 protein, by binding with different tyrosine kinases it inhibits downstream phosphorylation signaling pathways. Moreover, after binding with nanoparticles or liposomes, P28 has ability to achieve tumor targeted drug transfer. So that P28 can be used synergistically with other anticancer drugs or as an anticancer therapeutic alone. Structural information on binding of P28/Azurin with other partner bio molecules against tumors is limited. Therefore, it is instantly needed to find out structural details of P53/P28 DBD complex to improve the action of P28 and to understand the complete morphology of Azurine/P28.
References
- Bizzarri M, Brash DE, Briscoe J, et al. A call for a better understanding of causation in cell biology. Nat Rev Mol Cell Biol 2019; 20: 261–262.
- Qianli Zou, Manzar Abbas, Luyang Zhao, et al. Biological Photothermal Nanodots Based on Self-Assembly of Peptide–Porphyrin Conjugates for Antitumor Therapy. J Am Chem Soc. 2017; 139(5): 1921-1927.
- Yaghoubi A, Ramazani A. Anticancer DOX delivery system based on CNTs: Functionalization, targeting and novel technologies. J Control Rel. 2020; 327:198-224.
- Omarini C, Guaitoli G, Pipitone S, et al. Neoadjuvant treatments in triple-negative breast cancer patients: where we are now and where we are going. Cancer Manag Res. 2018; 10: 91-103.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497-506.
- Rabinowits G, Taylor CG, Day JM, et al. Exosomal MicroRNA: A Diagnostic Marker for Lung Cancer. Clin Lung Cancer. 2009; 10(1): 42-46.