Research Article - Biomedical Research (2017) Volume 28, Issue 1
Association of genetic polymorphisms of MERTK with multiple sclerosis among Jordanians
Rand Zaza1, Leena Ibayyan1, Mohammed El-Khateeb1, Yacoub G Bahou2, Elham Khreisat3, Wafa Al- Khateeb3 and Mamoun Ahram4*1Department of Pathology, Microbiology, and Forensic Medicine, The University of Jordan, Amman, Jordan
2Department of Intenral Medicine, The University of Jordan, Amman, Jordan
3Health Insurance Directorate, Ministry of Health, Amman, Jordan
4Department of Physiology and Biochemistry, Faculty of Medicine, the University of Jordan, Amman, Jordan
- *Corresponding Author:
- Mamoun Ahram
Department of Physiology and Biochemistry, Faculty of Medicine
The University of Jordan, Jordan
Accepted date: June 10, 2016
Abstract
Multiple sclerosis (MS) is an autoimmune disease characterized by demyelination of nerve axons in the central nervous system (CNS) through inflammatory and degenerative processes. Both genetic and environmental factors play a role in disease development with unique distribution according to latitude and association of a number of genetic factors. Among the genetic factors associated with MS is the MER tyrosine kinase (MERTK) gene, which has a plethora of immune-related functions including regulation of phagocytosis. MERTK also functions in the process of myelination. Although a number of SNPs have been associated with MS, studies that analyze these SNPs are lacking. In this study, three SNPs (rs17174870, rs867311 and rs1516629) with strong linkage disequilibrium have been analyzed in connection to MS. The SNP variants in 200 MS patients and 200 healthy subjects were investigated using a simple method of DNA amplification followed by restriction fragment length polymorphism. Within the MERTK gene, only rs687311 had a significant association with the disease where the homozygous (GG) variant of the SNP was more prevalent among MS subjects in our population than the other genetic variants (p-value=0.004). These results suggest an association of MERTK with MS with, particularly, rs687311 as a potential genetic marker of the disease.
Keywords
Multiple sclerosis, MERTK, Polymorphism, Rs17174870, Rs867311, Rs1516629.
Introduction
Multiple Sclerosis (MS) is a neurodegenerative, demyelinating disease of the central nervous system (CNS) affecting over 2 million people worldwide [1,2]. This disease is characterized by myelin loss, chronic inflammation, axonal and oligodendrocyte pathology, and progressive neurological dysfunction [3]. MS has been classified into 4 major clinical types based on the disease course; relapsing-remitting MS (RR-MS), secondary-progressive MS (SP-MS), primary progressive MS (PP-MS), and progressive-relapsing MS (PRMS). About 85% of MS patients are diagnosed with RR-MS, which is marked by exacerbations of symptoms followed by periods of remission where symptoms improve or disappear. RR-MS disease may progress in some patients into SP-MS where the disease course continues to worsen with or without periods of remission. PP-MS affects approximately 10% of MS patients with gradual worsening of symptoms from the onset of the disease with no relapses or remissions, but occasional plateaus. PR-MS is a rare form affecting fewer than 5% of patients. It is progressive from the start with intermittent flareups of worsening symptoms along the way and no periods of remission. The Kurtzke expanded disability status scale (EDSS) has been utilized to express the degree of disability in MS patients, reflecting disease progression and severity through quantitative neurologic examination [4].
The incidence of MS varies in different parts of the world with cases clustering in northern and southern hemispheres and high prevalence among populations of European ancestry. This distribution of the disease suggests that environmental factors can be risk factors or influential on the disease course [5]. The prevalence of the disease in Jordan, specifically, is reported to be 20-60 cases per 100,000 individuals [6,7]. According to the classification of Kurtzke, the prevalence of MS in Jordan is considered to be intermediate to high [8]. The onset of MS begins in late adolescence reaching a peak among individuals in their late 20s and early 30s with females being twice at risk of developing MS [9-13]. All gender ratio reports from Arabic populations, including Jordanians, indicate that females are more often affected than males [7,14-16].
As for the genetic contribution, the most highly associated genetic factor is the human leukocyte antigen locus (HLA) [17]. The advent of genome wide association studies (GWAS) has facilitated the identification of genes outside the HLA genetic regions [18-21]. Many of these genes have regulatory functions in the immune cells strengthening the notion that the development of MS is related to dysregulation of the immune system. Single nucleotide polymorphisms (SNPs) within the intronic region of the MER tyrosine kinase (MERTK) gene have also been identified by GWAS (5; 33). Me et al. confirmed the association of some of these SNPs with MS. MERTK is a member of the TAM (Tyro-3, Axl, and Mer) family of receptor tyrosine kinases. The TAM family plays essential roles in a number of major cellular processes: cell survival and proliferation, immunomodulation and phagocytosis [22]. Genetic or experimental alteration of TAM receptor function can contribute to a number of disease states, including coagulopathy, autoimmune diseases, retinitis pigmentosa, and cancer [23].
MERTK is a single-pass transmembrane receptor that is highly expressed in monocytes/macrophages and epithelial cells including the retinal pigment epithelium [23,24]. This protein and the other TAM family receptors have been shown to be important regulators of both oligodendrocyte survival and microglial activation in the CNS and of significant role during demyelination and remyelination in both the animal model of MS and the human disease [22,25,26]. Mice with mutations in MERTK as well as MERTK-deficient mice were shown to have impaired clearance of apoptotic cells, defective phagocytosis, and to develop autoimmune diseases like rheumatoid arthritis and lupus [23,24,27]. Additionally, mutations of MERTK have been linked to retinal dystrophy caused by a defect in the removal of apoptotic cells by macrophages [24]. It has been suggested that a defect in the clearance of apoptotic cells can lead to autoimmunity [28]. Collectively, MERTK contributes to phagocytosis of dead cells studies via regulating the dynamics of the actin cytoskeleton of macrophages [29]. It was also shown that MERTK-knock-out mice suffer from splenomegaly and loss of inhibitory receptor signaling in dendritic cells, which are chief players in innate immune responses and also in the activation of T lymphocytes [30].
MERTK has two major vitamin K dependent ligands: growth arrest-specific gene 6 (Gas6) and protein S [24,25,31]. Gas6 is important in the process of myelination via oligodendrocytes [26]. In addition, deficiency of Gas6 or blocking its receptor binding can lead to microglial activation, loss of oligodendrocytes, and inability of remyelination following drug-induced demyelination [26]. Interestingly, soluble Mer and Axl receptors, which have been shown to be present in human multiple sclerosis lesions, correlate to decreased level of Gas6 in these lesions and prevent Gas6 binding to the membrane receptor and, hence, block signaling [32]. Several SNPs within the MERTK gene have suggestive association with MS, the data generated for these SNPs through GWAS and replication studies suggest that MERTK is a novel risk gene for MS [25]. Due to the relevant role of MERTK in regulating the immune cells and its association with MS, we investigated 3 of the previously identified SNPs. Two of them, rs17174870, rs867311, are present in the first intron, and the third SNP, rs1516629, is positioned in the second intron. Molecular analyses of the SNPs were carried out using a simple methodology of DNA amplification followed by restriction fragment length polymorphism (PCR-RFLP).
Materials and Methods
Study population and study design
A total of 200 Jordanian MS patients were recruited during the period December 2011 to December 2012. Patients attended the Directorate of Health Insurance, Jordan University Hospital (JUH), and the Jordanian Multiple Sclerosis Society. A definite MS diagnosis was confirmed based on neurological and clinical examination by a committee of neurologists. This study was approved by the local IRB and ethics committee of the Ministry of Health and JUH. Written informed consent was obtained from all study participants in accordance with the Declaration of Helsinki. In addition, samples from 200 healthy participants were obtained from the National Center of Diabetes, Endocrinology, and Genetics. The volunteers were previously consented as part of the “The National Vitamin D Study” [33] and used as control samples in this study. None of the control subjects had a diagnosis with MS. Control subjects with Diabetes mellitus or Rheumatoid arthritis were excluded from the study.
Data collection
Demographic data were collected directly from the patients and clinical data were obtained from the neurologists or the control subject’s file.
General procedures of sample collection and analysis
Three to five milliliters of blood were drawn and collected in EDTA-containing vaccutainer tubes using standard phlebotomy procedures. Genomic DNA was extracted from patients’ blood samples using the Wizard Genomic DNA Purification Kit (Promega, USA) according to manufacturer’s guidelines. The total volume of polymerase chain reaction (PCR) mixture prepared for each of the PCR reactions was 25 μl consisting of: forward primer, reverse primer, 2X KAPATaq ready PCR mix (Kapa Biosystems, USA), DNA sample, and nuclease free water. All primers were purchased from Integrated DNA Technologies (IDT, USA). The primers were designed based on MERTK sequence obtained from the NCBI website (http:// www.ncbi.nlm.nih.gov), checked for being suitable using the “Primer 3” software (http://primer3.wi.mit.edu). Restriction enzymes were chosen via the restriction mapper software (http://www.restrictionmapper.org) and purchased from Thermo Scientific (Lithuania, EU). Gel electrophoresis and documentation was carried on 2 stages: a 2% gel electrophoresis to ensure a successful PCR reaction and a 3% gel electrophoresis to analyze and confirm the restriction cleavage pattern.
Analysis of rs17174870
A 345-bp DNA fragment containing rs17174870 was amplified using the forward primer (5’- AGATTGTGCCACTGCACTCCAG-3’) and the reverse primer (5’-ACATCATTCTCCCTGATCCTGA-3’) according to the following PCR program: an initial step at 95°C for 5 minutes followed by 35 cycles of 95°C for 30 sec, 56°C for 30 sec, and 72°C for 45 sec and a final step of 72°C for 5 min. PCR amplification of 52 control samples was not successful despite repeated trials. This could be due to the quality of the DNA samples. The restriction enzyme TruI (MseI) was used to analyze for the presence of T (major allele) or C (minor allele) at the SNP site. The PCR product was incubated with the enzyme at 65°C for 1-16 hrs.
Analysis of rs867311
A 369-bp DNA fragment containing this SNP was amplified using the forward primer (F: 5’ATCTGGCAACCCAATGCCAACA-3’) and the reverse primer (R: 5’-ACTCACTTGACCTGATGGATCA-3’). The same PCR program was used as above except for a 58°Cannealing temperature. The presence of the major allele (G) or the minor allele (T) was tested by the restriction enzyme MnlI that was incubated with the DNA fragment for 1-16 hrs at 37°C.
Analysis of rs1516629
Amplification of a 384-bp fragment containing rs1516629 was done using the primers (F: 5’- CCCAGAGGGTCCTTCAGTCT-3’) and (R: 5’- CGTGTTAGCCAGGTTGGTCT-3’) as described above with an annealing temperature of 60°C. The genetic profile of the SNP (major allele, T; minor allele, C) was tested with the restriction enzyme, NciI, which was incubated with the PCR fragment at 37°C for 1-16 hrs.
Statistical analysis
Statistical analysis was performed using Statistical Package of Social Science Program version 17 (SPSS v.17). Continuous data are presented as mean ± SD (standard deviation) and qualitative data are presented as frequencies. Descriptive statistical analysis was used for demographic. Frequencies and proportions were found by Chi-square test. The association between genetic polymorphisms and MS risk was analyzed by calculating the crude odds ratio (OR) and 95% confidence interval (95% CI) using binary logistic regression. A pvalue< 0.05 was considered as statistically significant.
Results
Demographic characteristics of the study population
Two hundred MS patients and 200 healthy controls were involved in the study. The mean age of our MS group was 33 years (Table 1). Of these, 136 (68%) were females and 64 (32%) were males. The mean age of the control group was 37 years and their age range was 16-64 years with 162 (81%) females and 38 (19%) males. Four Types of MS were encountered among patients: benign MS, RR-MS, SP-MS, and PR-MS. Of our 200 patients, 173 (86.5%) were diagnosed with RR-MS. The mean age at disease onset was 28 years with a range of 4-52 years. EDSS scores for patients were in the range of 0-8 with a mean of 3. A Pearson Chi-Square test for association between gender and type of MS diagnosis revealed no significant association in our study population.
| Gender (n=200) | |
| Female | 136 (68%) |
| Male | 64 (32%) |
| Age (range) | 33.40 ± 9 (17-58) |
| Type of diagnosis | |
| Benign | 3 (1.5%) |
| RR-MS | 173 (86.5%) |
| SP-MS | 23 (11.5%) |
| RR-MS | 1 (0.5%) |
| EDSS | 2.77 ± 1.77 (0.0-8.0) |
Table 1. Summary of patient information.
Prevalence of rs17174870 and disease association
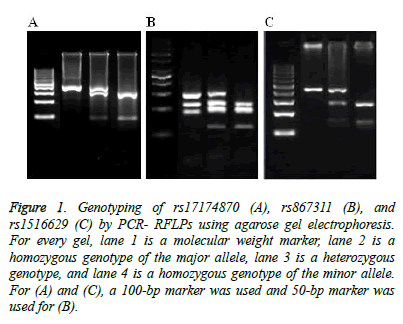
In order to investigate for frequencies of allelotyping and genotyping of rs17174870, the restriction enzyme, TruI (MseI), was used. The presence of the major allele (C) blocks cleavage of the 345-bp DNA fragment by this enzyme. On the other hand, a (T) allele results in cleavage of the fragment generating 261- and 82-bp fragments (Figure 1A). The distribution of both alleles was similar between the control and MS groups (Table 2).
Figure 1: Genotyping of rs17174870 (A), rs867311 (B), and rs1516629 (C) by PCR- RFLPs using agarose gel electrophoresis. For every gel, lane 1 is a molecular weight marker, lane 2 is a homozygous genotype of the major allele, lane 3 is a heterozygous genotype, and lane 4 is a homozygous genotype of the minor allele. For (A) and (C), a 100-bp marker was used and 50-bp marker was used for (B).
| SNP | Cohort | % Major allele | % Minor allele | p-value | Genotype Major/Major | Genotype Major/Major | Genotype Major/Major | p-value |
|---|---|---|---|---|---|---|---|---|
| rs17174870a | MS | 80.75 | 19.25 | 0.48 | 66 | 29.5 | 4.5 | 0.304 |
| Controls | 76.7 | 23.3 | 58.1 | 37.2 | 4.7 | |||
| rs867311b | MS patient | 83 | 17 | 0.47 | 70.5 | 25 | 4.5 | 0.004 |
| Controls | 79 | 21 | 59.5 | 39 | 1.5 | |||
| rs1516629c | MS patient | 80.25 | 19.75 | 0.85 | 65.5 | 29.5 | 5 | 0.101 |
| Controls | 81.25 | 18.75 | 64 | 34.5 | 1.5 | |||
| aThe major allele for rs17174870 is C and the minor allele is T bThe major allele for rs867311 is G and the minor allele is T cThe major allele for rs1516629is T and the minor allele is C |
||||||||
Table 2. Allele and genotype frequencies of mertk in ms patients and controls.
A homozygous wild (CC) genotype was found among 132 MS patients (66%) and 86 control individuals (58.1%), whereas 59 MS patients (29.5%) and 55 control subjects (37.2%) had a heterozygous (CT) genotype, and 9 MS patients (4.5%) and 7 control subjects (4.7%) had a homozygous variant (TT) genotype. No significant association between rs17174870 and MS incidence was found (Tables 2 and 3). In both tested groups, the genotype frequencies did not deviate from the Hardy-Weinberg equilibrium.
| SNP | Genotype | OR (95% CI)* | p-value |
|---|---|---|---|
| rs17174870 | CC | 1 reference | 0.305 |
| CT | 0.699 (0.443-1.104) | 0.124 | |
| TT | 0.838 (0.301- 2.333) | 0.735 | |
| rs867311 | GG | 1 reference | 0.005 |
| GT | 0.541 (0.352-0.832) | 0.005 | |
| TT | 2.532 (0.670-9.566) | 0.171 | |
| rs1516629 | TT | 1 reference | 0.125 |
| TC | 0.835 (0.547-1.277) | 0.407 | |
| CC | 3.257 (0.876-12.107) | 0.078 | |
| *OR: Odds Ratio, CI: Confidence Interval | |||
Table 3. Prediction of genotyping the SNPS with multiple sclerosis.
Prevalence of rs867311 and disease association
Upon cleavage of rs867311 containing the major allele G by MnlI, four fragments of sizes 147, 110, 85, 21, and 6 bp would be generated (Figure 1B). The presence of the minor allele (T) allows further cutting of the 147-bp fragment into two smaller ones, 93 and 54 bp.
Although there was no significant difference in the allele frequencies of rs867311 between the two groups, differences of genotyping were statistically significant (Tables 2 and 3). Genotyping in 200 MS patients and 200 control individuals revealed a homozygous wild genotype (GG) among 141 (70.5%) of MS patients and 119 (59.5%) of the control subjects (Table 2). On the other hand, 50 (25%) MS patients and 78 (39%) controls had a heterozygous variant genotype (GT), and 9 (4.5%) MS patients and 3 (1.5%) control subjects had a homozygous variant genotype (TT). The difference of genotyping between patients and controls was of statistical significance (p=0.004). Whereas the homozygous wild genotype (GG) of rs867311 polymorphism was significantly more frequent in patients than in controls (p-value=0.005, OR=1, reference), the heterozygous variant genotype (GT) of rs867311 polymorphism was significantly more frequent in control subjects than in patients (p-value=0.005; OR=0.541; 95% CI=0.352-0.832) (Table 3). In both tested groups, the genotype frequencies were not significantly different from the Hardy-Weinberg equilibrium.
Prevalence of rs1516629 and disease association
Utilization of the restriction enzyme, NciI, can differentiate the presence of the major allele (C) and the minor allele (T) in rs1516629. Whereas the presence of a (T) allele blocks cleavage of the 378-bp DNA fragment, the enzyme can cleavage the DNA fragment with a (C) allele generating in 247- and 137-bp fragments (Figure 1C). The distribution of both alleles was similar between the control and MS groups (Table 2). Among 200 MS patients, 131 (65.5%) were homozygous for the wild genotype (TT) of rs1516629 polymorphism, 59 (29.5%) were heterozygous for the variant genotype (TC), and 10 (5%) were homozygous for the variant genotype (CC). Among 200 control subjects, 128 (64%) were homozygous for the wild genotype (TT), 69 (34.5%) were heterozygous for the variant genotype (TC) and 29 (14.5%) were homozygous for the variant genotype (TT) of rs1516629. Neither allelotype frequencies not genotype frequencies of rs1516629 polymorphism significantly differed between patients and control subjects. In addition, the genotype frequencies were not significantly different from the Hardy- Weinberg equilibrium in both tested groups.
Discussion
Multiple Sclerosis is a complex autoimmune disease caused by multiple environmental and genetic factors. Numerous genes have been implicated in the etiology of multiple sclerosis [34,35]. GWAS studies of MS have offered compelling evidence to strengthen the contribution of genetic factors that regulate the immune system. Two studies have found strong association of SNPs within the MERTK gene to MS [19,21]. Further analysis of a large number of these intronic SNPs has replicated the GWAS findings into a shorter list [25]. Recently, a GWAS study has linked a number of genetic variants with functional roles within the MERTK gene with MS [36]. Due to absence of additional studies on the association of genetic polymorphism of this gene with MS, we investigated the association of three of these SNPs with MS using a simple PCR-RFLP methodology.
The GWAS studies of Ma et al., Sawcer et al., and more recently Binder et al. revealed the association of rs17174870 with MS. Ma et al. also identified additional 7 SNPs of high association including rs867311 and rs1516629. The SNPs were found to be in strong linkage disequilibrium. Contrary to the aforementioned study, we have only found significant differences between rs867311 with MS in our subject population. More specifically, a higher proportion of the (GG) genotype are found among MS patients than in the control group, which has a higher frequency of the heterozygote (GT) genotype. Prediction of the effect of the (GG) and (GT) genotypes on disease outcome is evident. The exact significance of the association of the non-coding genotype on the incidence of MS has yet to be elucidated. Although this SNP may not have functional significance on the function of the protein, it may lead to identification of nearby and relevant molecular markers within the coding regions [37]. The absence of any significant association of the other two genetic markers could be due to the small population in our study. In addition, genetic heterogeneity among populations could be a reason for not finding an association. For example, a SNP within the interleukin-7 receptor alpha gene has frequently been reported to be associated with MS among populations of European decent, but it was another SNP located within the promoter region that has been found to be associated with MS among Jordanian patients [38].
The three SNPs had an identical frequency of 22.3% among the Australian population in the study of Ma et al. On the other hand, minor variation has been observed in our study where the frequencies of the minor allele for rs17174870, rs867311 and rs1516629 are slightly lower (19.25, 17, and 19.25%, respectively). Not only genetic studies analyzing these polymorphisms in MS are lacking, no such studies exist in other populations in general. Hence, it is early to offer any further comparative analyses. In summary, we have designed detection protocols for the SNPs in MERTK that are reproducible, inexpensive. Based on our study, only rs867311 in MERTK gene shows suggestive association with MS risk. However, further investigation of this SNP and others using a larger sample size SNP is needed. The effect of sample size seen in previous reports emphasizes the importance of conducting large-scale, genetic analyses in order to better investigate the association of these variants of modest effect on MS susceptibility. In addition, performance of functional analyses of the protein may elucidate how it can contribute to MS.
Acknowledgment
We would like to offer our gratitude to the MS patients and the staff at the Health Insurance Doctorate, Ministry of Health, and the Multiple Sclerosis Society for their cooperation. Special thanks to the team of the genetics laboratory at The National Center for Diabetes, Endocrinology & Genetics. This study was funded by the Deanship of Academic Research.
References
- Hoffjan S, Akkad DA. The genetics of multiple sclerosis, an update 2010. Mol Cell Probes 2010; 4: 237-243.
- Dutta R, Trapp BD. Gene Expression Profiling in Multiple Sclerosis Brain. Neurobiol Dis 2012; 45: 108-114.
- Oksenberg JR, Baranzini SE. Multiple sclerosis genetics-is the glass half full, or half empty? Nat Rev Neurol 2010; 6: 429-437.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis, an expanded disability status scale (EDSS). Neurology 1983; 33: 1444-1452.
- Kantarci O, Wingerchuck D. Epidemiology and natural history of multiple sclerosis, new insights. Curr Opin Neurol 2006; 19: 248-254.
- World Health Organization. Atlas multiple sclerosis resources in the world 2008. Geneva, WHO Press, 2008.
- El-Salem K, Al-Shimmery E, Horany K, Al-Refai A, Al-Hayk K, Khader Y. Multiple sclerosis in Jordan, A clinical and epidemiological study. J Neurol 2006; 253: 1210-1216.
- Kurtzke JF. A reassessment of the distribution of multiple sclerosis. Acta Neurol Scand 1975; 51:110-157.
- Dyment DA, Sadovnick AD, Ebers GC, Sadnovich AD. Genetics of multiple sclerosis. Hum Mol Genet 1997; 10: 1693-1698.
- Al-Omaishi J, Bashir R, Gendelman HE. The cellular immunology of multiple sclerosis. J Leukoc Biol 1999; 65: 444-452.
- Evsyukova I, Somarelli JA, Gregory SG, Garcia-Blanco M. Alternative splicing in multiple sclerosis and other autoimmune diseases. RNA Biol 2010; 7: 462-473.
- Gourraud P, Mcelroy JP, Caillier SJ, Britt A, Santaniello A, Hauser SL, Oksenberg JR. Aggregation of MS genetic risk variants in multiple and single case families. Ann Neurol 2011; 69: 65-74.
- Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat rev Neurol 2012; 8: 602-612.
- Al-Din AS, El-Khateeb M, Kurdi A, Mubaidin A, Wriekat A, al-Shehab A. Multiple sclerosis in Arabs in Jordan. J Neurol Sci 1995; 131: 144-149.
- Ahram M, El-Omar A, Baho Y, Lubad MA. Association between human herpesvirus 6 and occurrence of multiple sclerosis among Jordanian patients. Acta Neurol Scand 2009; 120: 430-435.
- Benamer TS, Ahmed ES, Al-Din AS, Grosset DG. Frequency and clinical patterns of multiple sclerosis in Arab countries, A systematic review. J Neurol Sci 2009; 278: 1-4.
- Jersild C, Svejgaard A, Fog T. HLA antigens and multiple sclerosis. Lancet 1972; 1: 1240-1241.
- The International Multiple Sclerosis Genetic Consortium. Risk Alleles for Multiple Sclerosis Identified by a Genome wide Study. N Engl J Med 2007; 357: 851-862.
- Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene). Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 2009; 41: 824-828.
- Patsopoulos NA. Genomewide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann Neurol 2011; 70: 897-912.
- Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011; 476: 283-293.
- Binder MD, Kilpatrick TJ. TAM receptor signalling and demyelination. Neurosignals 2009; 17: 277-287.
- Linger RM, Keating AK, Earp HS, Douglas KG. TAM Receptor Tyrosine Kinases, Biologic Functions, Signaling, and Potential Therapeutic Targeting in Human Cancer. Adv Cancer Res 2008; 100: 35-83.
- Strick DJ, Vollrath D. Focus on molecules, MERTK. Exp Eye Res 2010; 91: 786-787.
- Ma GZ, Stankovich J, Kilpatrick TJ, Binder MD, Field J. Polymorphisms in the receptor tyrosine kinase MERTK gene are associated with multiple sclerosis susceptibility. PloS One 2011.
- Binder MD, Xiao J, Kemper D, Ma GZ, Murray SS, Kilpatrick TJ. Gas6 increases myelination by oligodendrocytes and its deficiency delays recovery following cuprizone-induced demyelination. PloS one 6 2011.
- Rosen A, Casciola-Rosen L. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Nat Med 2001; 7: 664-665.
- Gaipl US, Voll RE, Sheriff A, Franz S, Kalden JR, Herrmann M. Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmun Rev 2005; 4:189-194.
- Tibrewal N, Wu Y, D'mello V, Akakura R, George TC, Varnum B, Birge RB. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J Biol Chem 2008; 283: 3618-3627.
- O’Neill LA. TAMpering with toll-like receptor signaling. Cell 2007; 131: 1039-1041.
- Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 2007; 109: 1026-1033.
- Weinger JG, Omari KM, Marsden K, Raine CS, Shafit-Zagardo B. Up-Regulation of Soluble Axl and Mer Receptor Tyrosine Kinases Negatively Correlates with Gas6 in Established Multiple Sclerosis Lesions. Am J Pathol 2009; 175: 283-293.
- Batieha A, Khader Y, Jaddou H, Hyassat D, Batieha Z, Khateeb M. Vitamin D status in Jordan, dress style and gender discrepancies. Ann Nutr Metab 2010; 58: 895-911.
- Goldenberg MM. Multiple sclerosis review. Pharmacy Theraputics 2012; 37: 175-184.
- Inglese M. Multiple Sclerosis, New Insights and Trends, Am J Neuroradiol 2006; 27: 954-957.
- Binder MD, Fox AD, Merlo D, JohnsonLJ, Giuffrida L, Calvert SE, Akkermann R. Common and Low Frequency Variants in MERTK Are Independently Associated with Multiple Sclerosis Susceptibility with Discordant Association Dependent upon HLA-DRB1*15:01 Status. PLoS Genet 2016; 12: e1005853.
- Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biol 2010; 8: e1000294.
- Ibayyan L, Zaza R, Dahbour S, El-Omar A, Samhouri B, El-Khateeb M, Ahram M. The promoter SNP, but not the alternative splicing SNP, is linked to multiple sclerosis among Jordanian patients. J Mol Neurosci 2014; 52: 467-472.