- Biomedical Research (2016) Volume 27, Issue 3
Association of CagA-positive Helicobacter pylori infection with severity of obstructive sleep apnea syndrome.
Oguzhan Yildirim*, Ilhan Bali, Feti Tulubas, Rafet Mete, Birol Topcu, Yuksel Seckin, Yasir Furkan Cagin, Yilmaz Bilgic, Recep Alp
Faculty of Medicine, Malatya Inonu University, Malatya, Turkey
Accepted date: March 31, 2016
Abstract
Aim: To investigate the association between Helicobacter pylori cytotoxin-associated gene-A (CagA) status and the severity of OSA syndrome in infected patients.
Methods: Ninty-six patients with obstructive sleep apnea syndrome (OSAS) and 30 age- and sexmatched control subjects with no history of OSA or gastrointestinal complaints were included in the study. Patients’ apnea-hypopnea index (AHI) was determined by polysomnography (PSG), and serum H. Pylori IgG and cytotoxin-associated gene-A IgG was assayed by enzyme-linked immunosorbent assay (ELISA). Based on their AHI score, subjects were assigned to one of three groups: a control group (AHI<5), a mild-moderate OSAS group (AHI ≥ 5 and <30), and a severe OSAS group (AHI ≥ 30).
Results: The prevalence of H. pylori IgG seropositivity was significantly higher in the severe OSAS group compared to the mild-moderate OSAS group [29 (90.6%) patients versus 41 (64%) patients, (p=0.007)]. In addition, CagA seropositivity was present in 10 control patients (58.8%), 23 mildmoderate OSAS patients (56%), and 25 severe OSAS patients (86.2%). There was a significantly higher prevalence of CagA seropositivity in the severe OSAS group compared to mild-moderate OSAS group (p=0.027). There was no significant difference in CagA seropositivity between the mild-moderate OSAS group and the control group (p=0.059).
Conclusion: Our findings suggest that H. pylori strains expressing CagA may be considered a risk factor in the severity of OSAS.
Keywords
Helicobacter pylori, Cytotoxin-associated antigen, Obstructive sleep apnea syndrome.
Introduction
H. pylori is a slow growing, spiral shaped, microaerophilic, gram negative bacterium that colonizes the human stomach and establishes long-term infection of the gastric and duodenal mucosa [1]. Long-term infection with H. pylori causes various gastro-duodenal diseases, such as chronic gastritis, peptic ulcer, mucosa-associated lymphoid tissue lymphoma (MALT), and gastric cancer [2].
H. pylori synthesizes numerous virulence factors, such as CagA, VacA, IceA, which are related to its pathogenicity. Cytotoxin-associated antigen (CagA), a “pathogenicity island” encoded by genes of H. pylori, is one of the main H. pylori virulence factors and results in clinically important outcomes [3,4]. CagA-positive H. pylori strains are associated with higher frequencies of duodenal ulcer, precancerous lesions, and gastric cancer compared to CagA-negative strains [5,6].
Further, H. pylori strains producing CagA cause more intense tissue inflammation and induce cytokine production [7,8]. In recent years, H. pylori has been reported with increasing prevalence in patients with some extragastric inflammatory diseases, such as autoimmune, cardiovascular, and skin diseases [9]. Helicobacter pylori is also associated with many respiratory disorders, including chronic obstructive pulmonary disease (COPD), bronchiectasis, asthma, lung cancer, and chronic nonspecific pharyngitis [10-14].
Obstructive sleep apnea syndrome is a very common disorder caused by complete or partial upper airway obstruction during sleep and decreasing oxyhemoglobin saturation. Obstructive sleep apnea syndrome is characterized by the cardinal symptoms of disrupted sleep, consequent excessive daytime sleepiness, and snoring [15]. Increased seroprevalence of H. pylori in patients with OSAS has been reported in the past decades [16].
Ye et al. found an association between increased severity of OSAS and higher seroprevalence of H. pylori [17], but CagA positive H. pylori-infected patients with OSAS have not been evaluated. In this study, we evaluated the relationship between CagA positivity and the severity of disease in OSAS patients infected with H. pylori.
Material and Methods
Patients
One hundred twenty-six sequential patients (84 M, 42 F) diagnosed with OSAS by PSG and 30 control subjects without any clinical OSAS or gastrointestinal symptoms were taken for polysomnographic evaluation in a neurology clinic between July 2014 and March 2015. The mean age of patients was 51.9 ± 12 years (range18–81 years). Patients who had previously received H. pylori eradication treatment, antibiotics, and antiinflammatory or acid suppresive treatment in last 2 months, and those with a history of systemic inflammatory and autoimmune disorders, gastroesophageal reflux disease, were excluded. Body mass index (BMI) was measured for each patient. Index values were calculated by dividing weight in kilograms by the square of height in meters. The study was planned according to the ethics guidelines of the Helsinki Declaration, informed consent was obtained from all participants and was approved by the Institutional Research Ethics Board of our hospital.
Polysomnography examination
An all-night polysomnography was performed using a computerized system (EmblaN7000; Somnologica, Broomfield, Colorado) that recordings were analyzed manually, based on standard criteria in our center [18]. The neurological variables were the electroencephalogram, electrooculogram and electromyogram. Flow tracing was measured using a nasal cannula and thermistor, while thoraco-abdominal movement was measured with thoracic and abdominal bands. Oxygen saturation was assessed with a pulse oximeter. Cardiac parametries such as heart rate, were analyzed automatically. Apnea was defined as cessation of airflow for ≥ 10 seconds, and hypopnea was defined as oxygen desaturation of ≥ 3% or a reduction in thoracic excursion of ≥ 50%. The number of apnea and hypopnea events per hour of sleep were measured as the apnea hypopnea index (AHI). There are three levels of OSAS: mild (AHI ≥ 5 and <15), moderate (AHI ≥ 15 and <30), and severe (AHI ≥ 30) [8]. In this study, the lowest oxygen saturations were obtained for each subject during the PSG. Those with an AHI score of ≥ 5 were included in the study. Patients were divided into three groups according to their AHI score. Those with an AHI ≥ 5 and <30 were assigned to the mild-moderate OSAS group; those with an AHI ≥ 30 were assigned to the severe OSAS group, and subjects with an AHI <5 served as controls.
Blood samples
Peripheral blood was collected from each patient after PSG. Sera obtained by centrifugation were stored at −20°C and analyzed simultaneously by technicians who were blind to group allocation. IgG antibodies against H.pylori were analyzed by ELISA (DIA.PRO Diagnostic, Bioprobes S.r.l, Milan, Italy). All subjects with positive H. pylori antibodies underwent a serologic assay for specific IgG antibodies against CagA protein, which was analyzed by enzyme immunoassays (DIA.PRO Diagnostic, Bioprobes S.r.l, Milan, Italy). CagA antibody titers ( ≥ 8 U/mL) were classified as positive, per manufacturer instructions.
Statistical analysis
Data were analyzed using Predictive Analytics Soft Ware (PASW) statistics version 18 for Windows (SPSS Inc., Chicago, IL, USA). Data normality was analyzed using the Kolmogorov-Smirnov test. Variance analysis (Anova) was used for the comparison of three or more independent groups. Independent group comparisons were performed with Mann Whitney U test for variables with non-normal distribution, with independent sample t test for variables with normal distribution. Categorical variables were analysed with the χ2 test. A p-value <0.05 was considered to be statistically significant.
Results
The study involved 126 patients, 96 of whom were included in the OSAS groups, with the remaining 30 in the control group. In the OSAS groups, 64 patients (66%) were in the mildmoderate group, and 32 patients (33%) were in the severe group. There were no significant differences between all different groups in terms of age or sex (p>0.05).
There was no significant difference between the mild-moderate and control group in BMI (p>0.05), while it was observed to be significantly higher in the severe OSAS group compared to control and mild-moderate groups (p=0.01). See Table 1 for comparisons. The average lowest oxygen saturation (SaO2) was significantly low in the mild-moderate and severe groups compared to controls (p<0.05). When the two OSAS groups were compared, the average SaO2 in the severe group was significantly lower than in the mild-moderate group (see Table 1).
| Controls (n=30) |
Mild-moderate (n=64) |
Severe (n=32) |
P-value | |
| Male n (%) | 20 (66.6) | 42 (65.6) | 22 (68.7) | 0.987* |
| Age(years) | 53,30 ± 11,35 | 51,92 ± 11,59 | 50,59 ± 13,79 | 0.681* |
| BMI(kg/m2) | 29.52 ± 3.98 | 31.01 ± 3.88a | 33.79 ± 5.54 | 0.004** |
| Lowest SaO2 | 89.73 ± 2.93 | 86.03 ± 4.68a | 78.68 ± 9.04 | 0.000** |
| H.pyloriIgG (+) n (%) | 17(56.6) | 41(64)b | 29(90.6) | 0.007** |
BMI, Body mass index; SaO2, arterial oxygen saturation; H.pylori, helicobacter pylori.
Data presented as the mean and SD (±), comparison made using ANOVA.
Data presented as the absolute frequency and relative frequency (%), comparison performed using Chi-square test.
**P <0.05 Severe vs. Mild-moderate
*P >0.05 Between all groups
aP <0.05 Mild-moderate vs. controls
bP >0.05 Mild-moderate vs. controls
Table 1. Clinical and biochemical variables in the controls and different severity in OSAS patients.
H. pylori incidences were 56.6% (17/30) in the controls, 64% (41/64) in the mild-moderate group, and 90.6% (29/32) in the severe group. There was significant difference between the severe and mild-moderate groups (p=0.007), while there was no significant difference between the mild-moderate and the controls (p>0.05 Table 1).
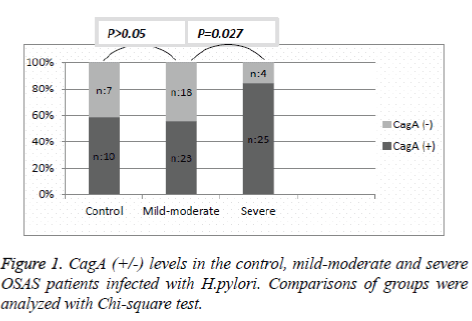
Helicobacter pylori IgG (+) OSAS patients were examined as CagA (+) and CagA (-). Ten patients in the control group (58.8%), 23 patients in the mild-moderate group (56%), and 25 patients in the severe group (86.2%) were found to be CagA (+). The difference in CagA (+) incidence between the mildmoderate and control group was not significant (p=0.059). However, comparing the OSAS groups, CagA (+) was significantly higher in the severe group compared to the mildmoderate group (p=0.027) (Figure 1).
Discussion
We found a significant relationship between H. pylori infection and OSAS, and also between severe OSAS and CagA-positive H. pylori infection. As far as we know, no previous study has demonstrated a relationship between CagA positive H. pylori infection and OSAS. In the literature, the data demonstrating a relationship between H. pylori infection and OSAS is limited [16,17]. The results of our study confirm those of two previous studies in this regard. First, a preliminary study by Unal et al. found H. pylori seropositivity in 17 of 19 OSAS patients (89.5%). This result indicated a strong relationship between H. pylori and OSAS [16]. The second study, conducted on 155 patients, found a statistically significant difference in H. pylori seropositivity between OSAS patients and a control group (75.5%, p=0.000) [17]. Additionally, the study found a significant relationship between disease severity and an increase in H. pylori seroprevalance in moderate and severe OSAS groups compared to the control group.
Several factors have been associated with an increased risk of OSAS, including age, male gender, obesity and alcohol abuse [19,20]. Our study results confirms previous findings that obesity is a major risk factor for OSAS. However, there was no significant difference regarding age and sex. Other risk factors such as alcohol use and smoking are not included in our study. Sufficient information for alcohol consumption and smoking could not be obtained because patients did not provide consistent information. And also recent studies showed that alcohol and smoking are not related risk factors with the increasing prevalence of H.pylori infection [21,22]. A previous study has found CagA seropositivity 69.5% in dyspeptic patients and a case-control study in a Turkish population showed all subjects were positive for CagA gene in patient groups including gastric cancer and duodenal ulcer while it was 62.5% in controls with no gastrointestinal symptoms [5,6]. Also our study results corroborates previous findings.
The main finding of our study was a positive relationship between CagA-positive H. pylori infection and OSAS severity. We found that CagA positivity was significantly increased in H. pylori-infected patients in the severe OSAS group compared to those in the control group. The potential pathogenic mechanism underlying this relationship is strong systemic inflamation caused by the CagA(+) H. pylori infection. Various studies have shown the relationship between H. pylori infection and systemic inflammation. As a result of chronic H. pylori infection, cytokines such as CRP, TNF-α, IL-8, IL-1, and IL-6, which signal systemic inflamation, are induced [23-26]. Additionally, CagA gene-positive strains are more virulent than CagA gene-negative strains and lead to significant increases in polimorfo nuclear leukocytes (PNL) and mononuclear cells, thus triggering inflammation in distant organs through the bloodstream [27]. Determining the role of systemic inflammation in OSAS etiology is important. A metaanalysis of 51 studies found the prevalence of systemic inflammatory markers was higher in OSAS patients than in control group patients [28]. Local and systemic inflammatory processes may cause stenosis and collapse in the upper airway in relation to disease severity and lead to oropharyngeal muscle disfunction [29,30]. IL-6 and TNF-α increases have been shown to have a role in OSAS pathogenesis and have been linked to the cardinal symptoms of OSAS, such as sleepiness and fatigue [31]. These two cytokines also have been found to increase in vitro in H. pylori-related gastritis subjects. Increased H. pylori-related inflammatory mediators thus may play a pivotal role in OSAS development.
Another potential pathogenic mechanism is that H. pylori colonizes in the upper respiratory tract [32]. In an extensive cohort study, the incidence of gastroesophageal reflux disease was reported to have increased notably in OSAS patients [33]. Gastroesophageal reflux into the laryngopharynx may cause inflammation-related upper airway disorders by directly exposing the pharynx and larynx to H. pylori [34]. This relationship suggests H. pylori infection may lead to airway stenosis, which also plays a role in OSAS etiology.
This study has some limitations. Although the specificity of the H. pylori antibody test is lower than methods such as endoscopical biopsy, the urea-breath test, and the stool antigen test, its specificity is still very respectable at nearly 95% [35]. Additionally, the H. pylori antibody test is convenient because it is noninvasive, inexpensive, easily administered, is not affected by PPI or antibiotic usage, and can easily be used with OSAS patients. The failure to quantify alcohol consumption and smoking may have been a limitation to the study. However, these limitations are not a mitigating factor and do not affect the result of our study because we found a high prevalence of CagA seropositivity in the study population.
In conclusion, we identified high CagA seroprevelance in patients infected with H. pylori who were diagnosed with severe OSAS. CagA-gene positive H. pylori strains may be a risk factor in increasing the severity of OSAS.
Conflict of Interest
None declared
References
- Blaser MJ. Ecology of Helicobacter pylori in the human stomach. J Clin Invest 1997; 100: 759-762.
- Perez-Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004; 1:1-6.
- van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W.Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori Gastroenterology 1998 115:58-66.
- Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. ProcNatlAcadSci USA 1993; 90:5791-5795.
- Weel JF, van der Hulst RW, Gerrits Y, Roorda P, Feller M, Dankert J, Tytgat GN, van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolatingcytotoxin, and Helicobacter pylori-related diseases. J Infect Dis 1996;173:1171.
- Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH.Meta-analysis of the relationship between cagAseropositivity and gastric cancer. Gastroenterology. 2003;125:1636.
- Figura N. Helicobacter pylori exotoxins and gastroduodenal diseases associated with cytotoxic strain infection. Aliment PharmacolTher. 1996;1:79.
- Spechler SJ, Fischbach L, Feldman M. Clinical aspects of genetic variability in Helicobacter pylori. JAMA 2000;283:1264.
- Suzuki H, Franceschi F, Nishizawa T, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter 2011;16: 65-69.
- Gencer M, Ceylan E, YildizZeyrek F, Aksoy N. Helicobacter pylori seroprevalence in patients with chronic obstructive pulmonary disease and its relation to pulmonary function tests. Respiration 2007;74:170-175.
- Tsang KW, Lam SK, Lam WK, Karlberg J, Wong BC, Hu WH, Yew WW, Ip MS. High seroprevalence of Helicobacter pylori in active bronchiectasis. Am J RespirCrit Care Med 1998; 158:1047-1051.
- Jun ZJ, Lei Y, Shimizu Y, Dobashi K, Mori M. Helicobacter pylori seroprevalence in patients with mild asthma. Tohoku J Exp Med 2005; 207:287-291.
- Najafizadeh K, FalahTafti S, Shiehmorteza M, Saloor M, Jamali M. H pylori seroprevalence in patients with lung cancer. World J Gastroenterol 2007; 13:2349-2351.
- Aladag I, Bulut Y, Guven M, Eyibilen A, Yelken K. Seroprevalence of Helicobacter pylori infection in patients with chronic nonspecific pharyngitis: preliminary study. J LaryngolOtol 2008; 122:61-64.
- Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo ClinProc 2011;86:549-554.
- Unal M, Oztürk L, Oztürk C, Kabal A. The seroprevalence of Helicobacter pylori infection in patients with obstructive sleep apnea: preliminary study. ClinOtolaryngol Allied Sci 2003; 28:100-102.
- Ye XW, Xiao J, Qiu T, Tang YJ, Feng YL, Wang K, Ou XM. Helicobacter pylori seroprevalence in patients with obstructive sleep apnea syndrome among a Chinese population. Saudi Med J 2009; 30:693-697.
- Iber C, Ancoli-Israel S, Chesson A, Quan S. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328: 1230-1235.
- Dagan Y, Doljansky JT, Green A, Weiner A. Body Mass Index (BMI) as a first-line screening criterion for detection of excessive daytime sleepiness among professional drivers. Traffic InjPrev 2006;7: 44-48.
- Dzhumabaev MN, Dzhumanova RG, Sabirov IS. The Interdependence between smoking, alcohol, tooth pathology and prevalence of Helicobacter pylori among ethnic groups in Kyrgyzstan. EkspKlinGastroenterol. 2015;16-20.
- Brenner H, Rothenbacher D, Bode G, Adler G. Relation of smoking and alcohol and coffee consumption to active Helicobacter pylori infection: cross sectional study. BMJ. 1997; 315:1489-1492.
- Oshima T, Ozono R, Yano Y, Oishi Y, Teragawa H, Higashi Y, Yoshizumi M, Kambe M. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am CollCardiol 2005; 45:1219-1222.
- Perri F, Clemente R, Festa V, De Ambrosio CC, Quitadamo M, Fusillo M, Grossi E, Andriulli A. Serum tumour necrosis factor-alpha is increased in patients with Helicobacter pylori infection and CagA antibodies. Ital J GastroenterolHepatol 1999; 31:290-294.
- Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut1991; 32:1473-1477.
- Roussos A, Philippou N, Mantzaris GJ, Gourgoulianis KI. Respiratory diseases and Helicobacter pylori infection: is there a link?Respiration 2006;73:708-714.
- Graham DY, Osato MS, Olson CA, Zhang J, Figura N. Effect of H.pylori infection and CagA status on leukocyte counts and liver function tests: extra-gastric manifestations of H. pylori infection. Helicobacter 1998; 3:174-178.
- Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Naseem J, Loomba R. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9:1003-1012.
- Inancli HM, Enoz M. Obstructive sleep apnea syndrome and upper airway inflammation. Recent Pat Inflamm Allergy Drug Discov 2010; 4:54-57.
- Hatipoğlu U, Rubinstein I. Inflammation and obstructive sleep apnea syndrome pathogenesis: a working hypothesis. Respiration 2003; 70:665-671.
- Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J ClinEndocrinolMetab2000; 85:1151-1158.
- Kariya S, Okano M, Nishizaki K. An association between Helicobacter pylori and upper respiratory tract disease: fact or fiction? World J Gastroenterol 2014; 20:1470-1484.
- Basoglu OK, Vardar R, Tasbakan MS, Ucar ZZ, Ayik S, Kose T, Bor S. Obstructive sleep apnea syndrome and gastroesophageal reflux disease: the importance of obesity and gender. Sleep Breath 2015; 19:585-592.
- Rubin JS, Benjamin E, Prior A, Lavy J, Ratcliffe P. The prevalence of Helicobacter pylori infection in benign laryngeal disorders. J Voice 2002; 16:87-91.
- Us D, Hascelik G. Seroprevalence of Helicobacter pylori infection in an Asymptomatic Turkish population. J Infect 1998; 37:148-150.