Research Article - Biomedical Research (2017) Volume 28, Issue 16
Association between food allergy and duodenal mast cells in patients with functional dyspepsia
Xiuzhen Ren1#, Zhen Li2#, Xinzhu Li3, Xin Li4, Haipeng Yuan5* and Xiaohong Wang5*
1Departments of Respiratory, Tai’an Central Hospital, Tai’an, Shandong, PR China
2Departments of and Clinical Psychology, Tai’an Central Hospital, Tai’an, Shandong, PR China
3Department of Ultrasonography, the First People’s Hospital of Tai’an, Tai’an, Shandong, PR China
4Department of Clinical Laboratory, Maternal and Child Care Service Centre of Tai’an, Tai’an, Shandong, PR China
5Department of Gastroenterology, Tai’an Central Hospital, Tai’an, Shandong, PR China
#These authors have equally contributed to this study
- *Corresponding Authors:
- Haipeng Yuan
Department of Gastroenterology
Tai’an Central Hospital
PR China - Xiaohong Wang
Department of Gastroenterology
Tai’an Central Hospital
PR China
Accepted date: July 29, 2017
Abstract
Functional Dyspepsia (FD) is one of the most common functional gastrointestinal disorders, which remains as a great burden to the healthcare system. Although the etiology and the pathogenesis of FD are not yet completely clear, the duodenum has been suggested as the key position of FD. In order to examine the association between food allergy and duodenal mast cells in patients with FD, the current study performed a retrospective review on 48 patients. Mucosal tissue specimens were obtained from the duodenal bulb and descending duodenum. IgG antibody in the serum was then detected using enzymelinked immunosorbent assay. Toluidine blue staining was used to identify mast cell counts and degranulation rates. According to the results of Pearson rectilinear correlation analysis, mast cells were significantly increased in patients with FD compared with the number in healthy volunteers (P<0.01). Degranulation ratios (%) of mast cells in patients were also significantly increased (P<0.01). Positive rates of IgG to certain types of food in patients with postprandial distress syndrome and epigastric pain syndrome were significantly increased, and the kinds of food allergen-specific antibodies and scores of IgG in the serum were significantly increased compared with the controls (P<0.01). Allergen-specific IgG scores and types demonstrated positive correlations with cell counts (r=0.247, P=0.038; r=0.243, P=0.041) and degranulation rates (r=0.307, P=0.011; r=0.326, P=0.007) of mast cells. Thus, these findings suggested that food allergy in patients with FD may increase cell counts and degranulation ratios of duodenal mast cells.
Keywords
Functional dyspepsia, Food allergy, IgG, Duodenum, Mast cell
Introduction
Functional Dyspepsia (FD) is one of the most common functional gastrointestinal disorders, which remains a great burden to the healthcare system and has a considerable negative socioeconomic impact [1-4]. It is diagnosed when upper gastrointestinal endoscopy reveals no organic lesions that can explain the dyspeptic symptoms. According to the Rome III criteria, FD is divided into two subgroups, namely Postprandial Distress Syndrome (PDS) and Epigastric Pain Syndrome (EPS), in order to simplify the intricate heterogeneity of this symptom complex and to help select an appropriate treatment strategy [5]. PDS is characterized by the presence of meal-induced dyspeptic symptoms, including early satiation and postprandial fullness, while EPS is defined as meal-unrelated symptoms with the presence of burning sensation or epigastric pain [6].
Although the etiology and pathogenesis of FD are not completely clear yet, the duodenum has been suggested as a key position of FD [7]. According to earlier studies, patients with FD also suffered from food allergies at the same time [8,9]. Food allergy involves the immune system. Mast cells in the duodenum are important local immune effector cells, and have been found to be involved in the pathogenesis of FD [10-12]. It has also been demonstrated that in the gastric corpus and antrum of adults with FD, mucosal mast cell density was evidently increased, which is generally isolated to the stomach of adults with FD [13]. Yuan et al. observed that the counts and degranulation rate of mast cells in the bulb of the duodenum are significantly increased in FD patients [14].
Mast cells express multiple IgG Fc receptors, which function as important participants in protective immune responses to pathogens, cross-linking elicit mast cell activation in allergy and also serving a vital role in autoimmunity [15]. Although it remains unclear whether the presence of food-specific IgGs in patients with adverse food reactions can be utilized for diagnosis, serum food-specific IgG tests have been used in patients with chronic symptoms due to their convenience [16,17].
According to previous studies, the present study proposed the hypothesis that food allergy is closely associated with mast cells in the duodenum. The current study aimed to detect the variations of mast cells in the duodenum and food-specific IgGs, as well as to analyze the association between food allergy and mast cells in the duodenum by using food-specific IgG tests. The study also further investigated the impact of food allergy in the pathogenesis of FD.
Patients and Methods
Ethical statement
All animal procedures in the present study were approved by the Ethics Committee of Tai’an Central Hospital (Tai’an, China).
Study population
A systematic review was performed for 48 patients diagnosed with FD between May 2011 and June 2011 in the Tai’an Central Hospital, including 13 males and 35 females, with ages of 22-71 y (mean age, 44.35 ± 11.64 y). All the patients with FD were diagnosed according to the Rome III criteria [18]. The control group was constituted of 21 healthy volunteers, including 9 males and 12 females, with ages of 19-66 y (mean age, 45.53 ± 10.58 y). There was no significant difference in the age and gender composition between patients and controls. A complete medical history and physical examination were performed to exclude surgery and infection, gastroesophageal reflux, esophagitis, malignancy and anaphylactic diseases in all participants.
Dyspeptic symptom scores
According to the Rome III criteria, dyspeptic symptoms of the upper gastrointestinal tract were divided into epigastric distension, epigastric pain, early satiety and epigastric ignition. The scores of severity were divided into four grades as follows: 0, asymptomatic; 1, mild symptoms; 2, moderate symptoms, but with no influence on work and life of patient; 3, severe symptoms, affecting the work and life of patient.
Toluidine blue staining
A gastroscope was used to collect two mucosal tissue specimens of the Duodenal Bulb (D1) and the Descending Duodenum (D2) from patients and controls. Mucosal tissues were then fixed in 10% formaldehyde solution, embedded in paraffin and cut into 3 μm sections. Next, sections were stained in 0.5% toluidine blue for 20-30 min and differentiated in 0.5% glacial acetic acid until the nucleus and cytoplasm particles were distinct. Subsequent to rinsing for a few seconds, the sections were dried and enveloped with neutral balsam. Mast cells in the sections were then counted under a microscope. The degranulation rate (%) of mast cells was calculated as the degranulation cell count over the mast cell count.
Detection of food allergy-specific IgG antibody
An IgG detection kit was purchased from Biomerica, Inc. (Irvine, CA, USA). Briefly, 3 ml venous blood collected from each subject was used to perform detection of food allergy-specific IgG antibodies for 14 types of food, according to the kit instructions. The food types investigated included beef, chicken, codfish, corn, crab, egg, mushroom, milk, pork, rice, shrimp, soybean, tomato and wheat. Serum IgG scores and specific antibodies detected for food allergens were recorded.
Statistical analysis
The results were analyzed with SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation. Means comparison of multi-samples were analyzed with analysis of variance. Means in two samples were compared with t-test. The correlations of IgG with mast cell counts and degranulation rate were analyzed by Pearson correlation analysis. For all tests, a P-value of <0.05 was considered to indicate a statistically significant difference.
Results
Symptom evaluating
Among the 48 FD patients, 23 patients were diagnosed with PDS, including 5 males and 18 females, with ages between 32 and 64 y (mean age, 44.56 ± 7.30 y). The remaining 25 patients were diagnosed with EPS, including 8 males and 17 females, aged between 22 and 71 y (mean age, 47 ± 15.72 y). The different dyspeptic symptoms of the upper gastrointestinal tract and the severity scores of these symptoms in the patients are presented in Table 1.
| Group | n | Epigastric distension | Early satiety | Epigastric pain | Epigastric burning |
|---|---|---|---|---|---|
| Description, n (%) | |||||
| FD | 48 | 38 (79) | 17 (35) | 33 (69) | 19 (40) |
| PDS | 23 | 23 (100) | 10 (43) | 8 (35) | 4 (17) |
| EPS | 25 | 15 (60) | 7 (28) | 25 (100) | 15 (60) |
| Scores, na | |||||
| FD | 48 | 1.85 ± 0.67 | 2.24 ± 0.66 | 1.73 ± 0.67 | 1.95 ± 0.52 |
| PDS | 23 | 2.13 ± 0.61 | 2.50 ± 0.53 | 1.38 ± 0.52 | 2.25 ± 0.50 |
| EPS | 25 | 1.40 ± 0.57 | 1.86 ± 0.69 | 1.84 ± 0.69 | 1.87 ± 0.52 |
| aMean ± Standard Deviation; FD: Functional Dyspepsia; PDS: Postprandial Distress Syndrome; EPS: Epigastric Pain Syndrome. | |||||
Table 1: FD symptoms and severity scores of these symptoms in the included patients.
Histological examination
Staining with haematoxylin-eosin demonstrated that the D1 and D2 tissues were normal in terms of histological structure. Furthermore, symptoms of acute duodenitis, intestinal parasite infections and tumor signs were not identified in any of the patients and controls.
Analysis of correlations between mast cell counts and symptom severity scores
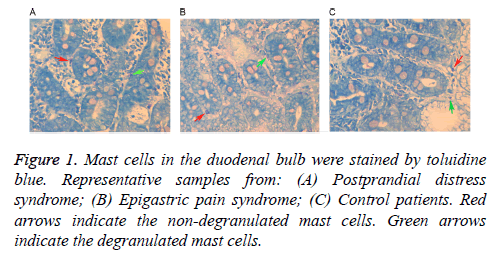
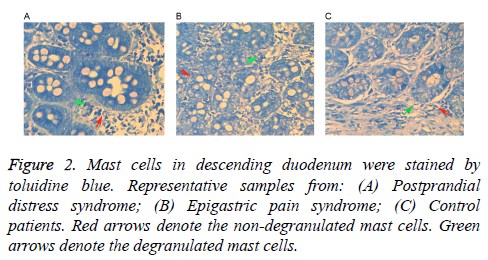
Following staining with toluidine blue, nuclei were stained with a blue color and cytoplasm particles appeared red. Normal cells presented uniform cytoplasm and a clear membrane. Cells with degranulation were irregular with a broken membrane, releasing particles around the membrane. As shown in Table 2, mast cell counts and degranulation ratios in the D1 and D2 samples obtained from the FD patients (including PDS and EPS) were significantly increased when compared with the control samples. Mast cells in a representative D1 tissue samples are shown in Figure 1, while cells in a D2 tissue sample are shown in Figure 2. As shown in Table 3, the associations of cell counts and degranulation ratios with the symptom severity scores were analyzed, and the results demonstrated no statistically significant correlation (P>0.05).
| Group | n | D1 MC | P-value | D2 MC | P-value |
|---|---|---|---|---|---|
| MC counts | |||||
| Controls | 21 | 104.29 ± 20.81 | 108.62 ± 7.64 | ||
| FD | 48 | 120.94 ± 13.31 | 0.002 | 123.28 ± 13.40 | <0.001 |
| PDS | 23 | 121.00 ± 13.75 | 0.001 | 124.32 ± 16.53 | <0.001 |
| EPS | 25 | 120.88 ± 13.13 | 0.001 | 122.24 ± 9.54 | <0.001 |
| Degranulation ratios (%) | |||||
| Controls | 21 | 25.38 ± 2.32 | 30.66 ± 2.89 | ||
| FD | 48 | 60.23 ± 5.10 | <0.001 | 66.97 ± 5.30 | <0.001 |
| PDS | 23 | 59.84 ± 4.50 | <0.001 | 66.63 ± 5.37 | <0.001 |
| EPS | 25 | 60.58 ± 5.66 | <0.001 | 67.28 ± 5.32 | <0.001 |
| Data are presented as the mean ± standard deviation. MC: Mast Cell; D1: Duodenal bulb; D2: Descending Duodenum; FD: Functional Dyspepsia; PDS: Postprandial Distress Syndrome; EPS: Epigastric Pain Syndrome. | |||||
Table 2: MC counts and degranulation ratios (%) in the D1 and D2 samples of the FD patients (including PDS and EPS patients).
| Sample | Epigastric distension | Early satiety | Epigastric pain | Epigastric burning |
|---|---|---|---|---|
| D1 MC count | ||||
| r-value | 0.096 | 0.266 | 0.048 | 0.167 |
| P-value | 0.583 | 0.32 | 0.803 | 0.508 |
| D2 MC count | ||||
| r-value | 0.096 | 0.266 | 0.034 | 0.086 |
| P-value | 0.583 | 0.32 | 0.859 | 0.734 |
| D1 degranulation ratio | ||||
| r-value | 0.109 | 0.126 | 0.037 | 0.273 |
| P-value | 0.551 | 0.653 | 0.853 | 0.273 |
| D2 degranulation ratio | ||||
| r-value | 0.139 | 0.416 | 0.185 | 0.259 |
| P-value | 0.439 | 0.109 | 0.337 | 0.299 |
| MC: Mast Cell; D1: Duodenal Bulb; D2: Descending Duodenum. | ||||
Table 3: Correlation of mast cell counts and degranulation ratios with the symptom severity scores (P>0.05).
Detection of food-specific antibodies in the serum
The positive rates of specific IgG antibodies were calculated, and are presented in Tables 4 and 5. In the 23 PDS patients, the positive rates of IgG antibody specific to beef, chicken, crab, shrimp and wheat were significantly increased in comparison with the controls (P<0.05). In the 25 EPS patients, positive rates of IgG antibody specific to beef, chicken and soybean were significantly increased (P<0.05, Table 4). Furthermore, positive scores and types of food-specific IgG antibodies in patients were significantly increased when compared with the controls (P<0.005, Table 5).
| Allergen | PDS | EPS | FD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | + | ++ | +++ | Positive rate | P-value | - | + | ++ | +++ | Positive rate | P-value | - | + | ++ | +++ | Positive rate | |
| Beef | 8 | 6 | 4 | 5 | 0.65 | <0.001 | 11 | 8 | 6 | 0 | 0.56 | 0.003 | 19 | 0 | 2 | 0 | 0.1 |
| Chicken | 6 | 8 | 5 | 4 | 0.74 | <0.001 | 11 | 7 | 6 | 1 | 0.56 | <0.001 | 21 | 0 | 0 | 0 | 0 |
| Codfish | 18 | 2 | 1 | 2 | 0.22 | 0.803 | 22 | 2 | 0 | 1 | 0.12 | 1 | 18 | 3 | 0 | 0 | 0.14 |
| Corn | 19 | 0 | 3 | 1 | 0.17 | 0.749 | 16 | 5 | 2 | 2 | 0.36 | 0.08 | 19 | 0 | 0 | 2 | 0.1 |
| Crab | 3 | 9 | 6 | 5 | 0.87 | 0 | 16 | 3 | 2 | 4 | 0.36 | 0.346 | 17 | 2 | 1 | 1 | 0.19 |
| Egg | 14 | 2 | 3 | 4 | 0.39 | 0.259 | 17 | 4 | 1 | 3 | 0.32 | 0.51 | 17 | 1 | 3 | 0 | 0.19 |
| Mushroom | 22 | 0 | 0 | 1 | 0.04 | 1 | 24 | 0 | 0 | 1 | 0.04 | 1 | 21 | 0 | 0 | 0 | 0 |
| Milk | 19 | 2 | 2 | 0 | 0.17 | 1 | 22 | 2 | 1 | 0 | 0.12 | 0.802 | 17 | 3 | 1 | 0 | 0.19 |
| Pork | 18 | 3 | 2 | 0 | 0.22 | 0.23 | 21 | 3 | 0 | 1 | 0.16 | 0.457 | 20 | 1 | 0 | 0 | 0.05 |
| Rice | 21 | 1 | 1 | 0 | 0.09 | 1 | 21 | 2 | 0 | 2 | 0.16 | 0.457 | 20 | 1 | 0 | 0 | 0.05 |
| Shrimp | 13 | 6 | 4 | 0 | 0.43 | 0.009 | 19 | 1 | 4 | 1 | 0.24 | 0.162 | 20 | 0 | 1 | 0 | 0.05 |
| Soybean | 20 | 1 | 1 | 1 | 0.13 | 0.668 | 7 | 6 | 8 | 4 | 0.72 | <0.001 | 20 | 1 | 0 | 0 | 0.05 |
| Tomato | 17 | 1 | 3 | 2 | 0.26 | 0.844 | 19 | 4 | 1 | 1 | 0.24 | 0.963 | 17 | 2 | 2 | 0 | 0.19 |
| Wheat | 15 | 3 | 3 | 2 | 0.35 | 0.036 | 23 | 0 | 0 | 2 | 0.08 | 1 | 20 | 1 | 0 | 0 | 0.05 |
| PDS: Postprandial Distress Syndrome; EPS: Epigastric Pain Syndrome; FD: Functional Dyspepsia. | |||||||||||||||||
Table 4: Positive rate of food-specific IgG antibodies in patients.
| Group | n | IgG score | P-value | IgG species | P-value |
|---|---|---|---|---|---|
| Control | 21 | 1.90 ± 2.36 | 1.29 ± 1.52 | ||
| PDS | 23 | 6.96 ± 5.09 | <0.001 | 4.19 ± 2.83 | <0.001 |
| EPD | 25 | 6.43 ± 4.46 | <0.001 | 3.60 ± 2.42 | 0.001 |
| FD | 48 | 6.69 ± 4.73 | <0.001 | 3.89 ± 2.62 | 0.004 |
| PDS: Postprandial Distress Syndrome; EPS: Epigastric Pain Syndrome; FD: Functional Dyspepsia. | |||||
Table 5: Types and scores of food-specific IgG antibodies.
Analysis of correlations of IgG
In FD patients, symptom scores were analyzed with no correlations observed with positive kinds and positive scores of IgG (P>0.05), as shown in Table 6.
| Parameter | Epigastric distension | Early satiety | Epigastric pain | Epigastric burning |
|---|---|---|---|---|
| Positive kinds of IgG | ||||
| r-value | 0.164 | 0.161 | 0.011 | 0.296 |
| P-value | 0.319 | 0.536 | 0.95 | 0.219 |
| Positive scores of IgG | ||||
| r-value | 0.145 | 0.326 | 0.089 | 0.281 |
| P-value | 0.378 | 0.202 | 0.624 | 0.244 |
Table 6: Correlations of symptom scores with positive kinds and positive scores of IgG.
By contrast, a positive association was identified between the mast cell counts and presence of IgG antibody (P<0.05), as shown in Table 7.
| Parameter | D1 MC | D1 degranulation rate | D2 MC | D2 degranulation rate |
|---|---|---|---|---|
| Positive kinds of IgG | ||||
| r-value | 0.247 | 0.307 | 0.185 | 0.221 |
| P-value | 0.038 | 0.011 | 0.123 | 0.068 |
| Positive scores of IgG | ||||
| r-value | 0.243 | 0.326 | 0.19 | 0.17 |
| P-value | 0.041 | 0.007 | 0.113 | 0.162 |
| MC: Mast Cell; D1: Duodenal Bulb; D2: Descending Duodenum. | ||||
Table 7: Correlations of mast cell counts with the positive kinds and positive scores of IgG.
Discussion
In the present study, it was observed that the positive rates of food-specific IgG antibodies for beef, chicken, crab, shrimp and wheat were significantly increased in PDS patients compared with the controls. In addition, the positive rates of food-specific IgG antibodies for beef, chicken and soybean were significantly increased in EPS patients. Furthermore, kinds and scores of food-specific IgG antibodies in both PDS and EPS groups were significantly increased. These findings suggested that food-specific IgG antibodies were involved in the pathogenesis of FD.
Food may not be entirely digested due to the lack of a specific digestive enzyme. The remainder proteins enter the blood or lymph fluid in the form of large molecules, which are recognized as antigens by the body’s immune system, thus inducing food-specific IgG antibodies [19]. Park et al. demonstrated that food-specific IgG antibodies, particularly IgG4, increased significantly in patients with irritable bowel syndrome, indicating that IgG4 was associated with visceral hypersensitivity in certain subtypes of IBS patients [9]. However, the present study observed that there were no correlations between allergen-specific IgG antibody and symptom scores, which were consistent with the findings of other earlier studies. This phenomenon may be associated with the following factors: (i) Food-associated symptoms may be due to food intolerance rather than food allergy, and thus there is no change in IgG; (ii) Food-associated symptoms may be caused by other factors, such as saccharides, cellulose and caffeine in food [20].
The current study demonstrated that mast cell counts and activation ratios in the duodenum significantly increased in FD patients compared with those in the controls, while the mucosal immune was also activated in FD patients. According to previous studies, mast cells in the antral gastric mucosa were increased in FD patients, and were associated with gastric emptying and gastric electrical activity in these patients [21,22]. In addition, mast cells were increased in post-infectious FD patients. Thus, due to its close distance to the nerve fibre, mast cells along with medium may participate in the pathogenesis of post-infectious FD [23]. The present study indicated that mast cells in the duodenum may also be involved in the pathogenesis of FD in addition to the mast cells in the stomach.
The kinds and positive rates of food-specific IgG antibodies were positively correlated with the activation of mast cells in the duodenum in the present study, indicating that the effect of food allergy-specific IgG antibodies on gastrointestinal functions was possibly on the basis of mast cell activation in the duodenum. Food allergen leads to the infiltration of mast cells in the intestinal tract [24,25]. Mast cells are activated by IgG subsequent to binding to IgG receptors on the surface of cells. Another factor involved in mast cell activation is the antigen-antibody complex, which refers to the combination of IgG and the relevant antigens [26]. Furthermore, IgG was suggested to be a protective antibody of IgE hypersensitivity, since food allergen-specific antibodies of IgG and IgE increased simultaneously in patients with functional gastric or intestinal disease. Therefore, it was further speculated that in FD patients, IgE activates mast cells and generates anaphylaxis, followed by the production of IgG, and the anaphylaxis was limited in the gastrointestinal mucosa by certain unclear factors, resulting in food allergy-associated gastrointestinal symptoms, but without other symptoms observed [27].
In conclusion, mast cell counts and degranulation ratios were significantly increased in patients with FD compared with healthy volunteers. Positive rates of IgG against certain types of food in patients with PDS and EPS were significantly increased, while the allergen-specific IgG scores and kinds demonstrated positive correlations with mast cell counts and degranulation rates. Therefore, food allergy in patients with FD may increase the cell counts and degranulation ratios of duodenal mast cells.
References
- Tack J, Talley NJ. Functional dyspepsia-symptoms, definitions and validity of the Rome III criteria. Nature Rev Gastroenterol Hepatol 2013; 10: 134-141.
- Ghoshal UC, Singh R, Chang FY, Hou X, Wong BC, Kachintorn U. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil 2011; 17: 235-244.
- Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil 2008; 20: 121-129.
- Nellesen D, Yee K, Chawla A, Lewis BE, Carson RT. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm (JMCP) 2013; 19: 755-764.
- Geeraerts B, Tack J. Functional dyspepsia: past, present, and future. J Gastroenterol 2008; 43: 251-255.
- Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterol 2006; 130: 1466-1479.
- Walker MM, Warwick A, Ung C, Talley NJ. The role of eosinophils and mast cells in intestinal functional disease. Curr Gastroenterol Rep 2011; 13: 323-330.
- Soares RL, Figueiredo HN, Maneschy CP, Rocha VR, Santos JM. Correlation between symptoms of the irritable bowel syndrome and the response to the food extract skin prick test. Brazil J Med Biol Res 2004; 37: 659-662.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? A systematic review. J Eur Gastrointestinal Motil Soc 2006; 18: 595-607.
- Schappi MG, Borrelli O, Knafelz D, Williams S, Smith VV, Milla PJ, Lindley KJ. Mast cell-nerve interactions in children with functional dyspepsia. J Paediatric Gastroenterol Nutrit 2008; 47: 472-480.
- Yamamoto T, Kodama T, Lee J, Utsunomiya N, Hayashi S, Sakamoto H, Kuramoto H, Kadowaki M. Anti-allergic role of cholinergic neuronal pathway via alpha7 nicotinic ACh receptors on mucosal mast cells in a murine food allergy model. PLoS One 2014; 9: e85888.
- Song S, Song Y, Zhang H, Li G, Li X, Wang X, Liu Z. Increased counts and degranulation of duodenal mast cells and eosinophils in functional dyspepsia- a clinical study. Med Glas (Zenica) 2014; 11: 276-282.
- Friesen CA, Schurman JV, Colombo JM, Abdel-Rahman SM. Eosinophils and mast cells as therapeutic targets in pediatric functional dyspepsia. World J Gastrointestinal Pharmacol Therap 2013; 4: 86-96.
- Yuan HP, Li Z, Zhang Y, Li XP, Li FK, Li YQ. Anxiety and depression are associated with increased counts and degranulation of duodenal mast cells in functional dyspepsia. Int J Clin Exp Med 2015; 8: 8010-8014.
- Brown MA, Hatfield JK. Mast Cells are important modifiers of autoimmune disease: with so much evidence, why is there still controversy? Frontiers Immunol 2012; 3: 147.
- Hochwallner H, Schulmeister U, Swoboda I, Twaroch TE, Vogelsang H, Kazemi-Shirazi L, Kundi M, Balic N, Quirce S. Patients suffering from non-IgE-mediated cow's milk protein intolerance cannot be diagnosed based on IgG subclass or IgA responses to milk allergens. Allergy 2011; 66: 1201-1207.
- Pelsser LM, Frankena K, Toorman J, Savelkoul HF, Dubois AE, Pereira RR, Haagen TA, Rommelse NN, Buitelaar JK. Effects of a restricted elimination diet on the behaviour of children with attention-deficit hyperactivity disorder (INCA study): a randomised controlled trial. Lancet 2011; 377: 494-503.
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterol 2006; 130: 1377-1390.
- Ou-Yang WX, You JY, Duan BP, Chen CB. Application of food allergens specific IgG antibody detection in chronic diarrhoea in children. Zhongguo dang dai er ke za zhi (Chin J Contemp Pediatr) 2008; 10: 21-24.
- Xiuli Zuo YL, Wenjie Li, Yuting Guo, Juan Zeng. Role of food antigen-specific IgG and IgE in the Pathogenesis of functional gastrointestinal disorders. Gastroenterol 2008; 13: 95-99.
- Hall W, Buckley M, Crotty P, O'Morain CA. Gastric mucosal mast cells are increased in Helicobacter pylori-negative functional dyspepsia. Clin Gastroenterol Hepatol 2003; 1: 363-369.
- Friesen CA, Lin Z, Singh M, Singh V, Schurman JV, Burchell N, Cocjin JT, McCallum RW. Antral inflammatory cells, gastric emptying, and electrogastrography in paediatric functional dyspepsia. Digest Dis Sci 2008; 53: 2634-2640.
- Li X, Chen H, Lu H, Li W, Chen X, Peng Y, Ge Z. The study on the role of inflammatory cells and mediators in post-infectious functional dyspepsia. Scand J Gastroenterol 2010; 45: 573-581.
- Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in post-infective gut dysfunction. Gut 1999; 44: 400-406.
- Smout A, Azpiroz F, Coremans G, Dapoigny M, Collins S, Muller-Lissner S, Pace F, Stockbrugger R, Vatn M. Potential pitfalls in the differential diagnosis of irritable bowel syndrome. Digest 2000; 61: 247-256.
- Malbec O, Daeron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev 2007; 217: 206-221.
- Kalliomaki MA. Food allergy and irritable bowel syndrome. Curr Opinion Gastroenterol 2005; 21: 708-711.