- Biomedical Research (2013) Volume 24, Issue 4
Application of mechanical circulatory support in treatment of infants with severe congenital heart disease and cardiac arrest.
Xiaoyang Hong1,2, Gengxu Zhou3, Yuhang Liu3, Hui Wang3, Yingyue Liu2, Zhichun Feng2*1PICU, Affiliated Bayi Children's Hospital, Bayi Clinical Medical Institute, Southern Medical University, Beijing 100007, China
2PICU, Affiliated Bayi Children's Hospital of General Hospital of Beijing PLA Military Region, Beijing 100007, China
3Pediatric Cardiology & Cardiac Surgery, Affiliated Bayi Children's Hospital of General Hospital of Beijing PLA Military Region, Beijing 100007, China
- *Corresponding Author:
- Zhichun Feng
PICU, Affiliated Bayi Children's Hospital of General
Hospital of Beijing
PLA Military Region
Beijing 100007, China
Email: feng520131450@aliyun.com
Accepted August 13 2013
citation: Hong X, Zhou G, Liu Y, Wang H, Liu Y, Feng Z . Application of mechanical circulatory support in treatment of infants with severe congenital heart disease and cardiac arrest. Biomedical Research 2013; 24 (4): 515-520.
Abstract
Mechanical circulatory support (MCS) for saving infants with severe congenital heart disease who are receiving traditional cardio-pulmonary resuscitation has never been conducted before. The selection of timing and indications of MCS is associated with clinical ethics, and most of the doctors are more cautious in the application of extracorporeal membrane oxygenation (ECMO) for infants with severe disease undergoing cardio-pulmonary resuscitation. ECMO was used for the treatment in 3 infants of complex congenital heart disease and cardiac arrest from October 2012 to January 2013 in our hospital. Among these 3 patients, ECMO was successfully evacuated in 2 cases. For infants with severe congenital heart disease who suffered from cardiac arrest, timely and quickly establishing a circulatory support to correct further injury due to hypoxia and hypoperfusion, is an effective treatment method.
Keywords
Mechanical circulatory support (MCS), Cardiopulmonary bypass (CPB), Extracorporeal membrane oxygenation (ECMO), Cardiac arrest, Congenital heart disease, Cardiopulmonary resuscitation
Introduction
Mechanical circulatory support (MCS) mainly includes cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO) which was developed from CPB and is a life support technology that can replace partially or completely cardio-pulmonary function within a long period, maintain oxygen supply to various organs of the body and perform a prolonged cardiopulmonary support for patients with serious cardiorespiratory failure. In 1971, Hill first adopted extracorporeal circulatory equipment to successfully treat a 24-year-old patient with multiple organ damage combined with acute respiratory distress syndrome (ARDS) [1]. Through long-term unremitting efforts of clinical medical personnel, EMCO has got considerable progress and achieved better clinical effect with the development in equipment and management technique. While in China, EMCO was applied and carried out late, and only one study on ECMO for treatment of severe disease in 12 infants from 2007 to 2011 was reported by Lin et al [2]. Mechanical circulatory support for saving the infants with severe congenital heart disease who are receiving traditional cardio-pulmonary resuscitation has never been conducted before. The selection of timing and indications of MCS is associated with clinical ethics, and most of the doctors are more cautious in the application of ECMO for infants with severe disease undergoing cardio-pulmonary resuscitation. ECMO was used for the treatment in 3 infants of complex congenital heart disease and cardiac arrest from October 2012 to January 2013 in our hospital, and the details were introduced and analyzed as follows.
Case report
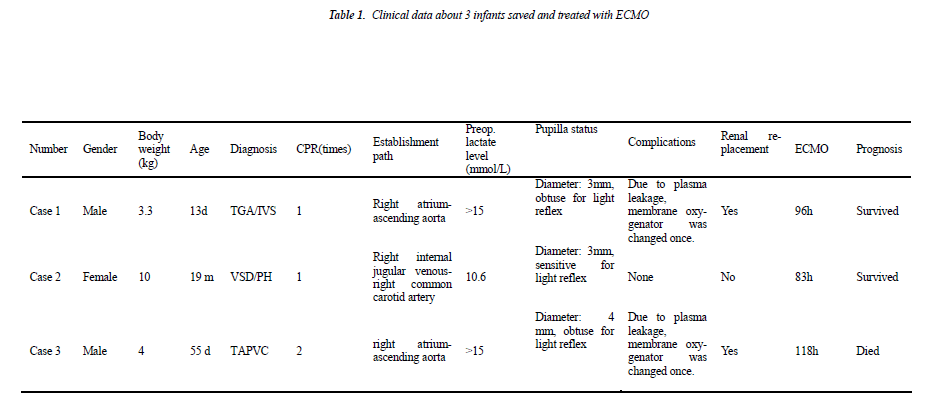
Case 1 (male, body weight of 3.3 kg, 13 days after birth) was diagnosed as “transposition of great arteries/intact ventricular septum/patent ductus arteriosus (TGA/IVS/- PDA)”. Continuous intravenous prostaglandin E1 was given immediately after admission due to severe hypoxemia, but the symptom did not improve significantly and cardiac arrest happened. Cardio-pulmonary resuscitation was started immediately and emergent cardio-pulmonary bypass (CPB) was established, and then he underwent “arteries switch operation (ASO)”. Low cardiac output syndrome and pneumorrhagia was exhibited postoperatively when weaning from CPB, so CPB was switched to ECMO support, and he was transferred to the intensive care unit under ECMO support with delayed sternal clousure.
Case 2 (female, body weight of 10 kg, 21 months) was diagnosed as “residual shunt after repair of interventricular septal defect, severe pulmonary hypertension (pressure gradient between left and right ventricle is 10 mmHg) and left ventricular dysfunction (EF: 40%)”. She underwent “residual ventricular septal defect repair” and experienced post-operative pulmonary hypertensive crisis and severe left ventricular dysfunction (EF dropped to 15-20%). The patient suffered from cardiac arrest on the operative day and then cardio-pulmonary resuscitation and emergency ECMO installation at the bedside was carried out at the same time.
Case 3 (male, body weight of 4 kg, 45 days after birth) was diagnosed as “total anomalous pulmonary venous connection (supracardiac), severe pulmonary hypertension”. On the operative day he had severe hypoxemia and hypotension and experienced cardiac arrest twice. Cardiopulmonary resuscitation was started immediately after cardiac arrest and the baby was transferred into emergency operating room for establishing CPB and “TAPVC correction surgery”. CPB was switched to ECMO support for being hard to wean from machine, and then he was transferred to the intensive care unit with delayed sternal closure.
For cases 1 and 3, ECMO was established in the operating room. At the end of surgery, after exclusion of residual uncorrected cardiac malformations and three attempts to wean the babies from CPB, ECMO installation was immediately carried out for patients after obtaining the informed consent of the parents.
The original superior vena cava cannula was kept in the right atrium and the ascending aorta cannula was reserved. The venous and aortic cannula was connected to ECMO system and CPB was stopped and switched to ECMO support. Heparin was half antagonized, maintaining ACT between 150-200 seconds. The two babies were transferred to intensive care unit under ECMO assist and a delayed sternal closure. Because case 2 exhibited a severe low cardiac output syndrome and cardiac arrest on the evening of the operative day, cardiopulmonary resuscitation as well as bedside emergency ECMO installation was given. A transverse skin incision 1cm above the right clavicle was made on the neck to expose the right common carotid artery and the right internal jugular vein. After a dose of 0.5mg/kg heparin was given and ACT maintained above 150 seconds, right common carotid artery and right internal jugular vein were cannulated with 14F cannula respectively. The cannula tip was confirmed at right position by bedside ECHO examination.
During ECMO support, blood flow and volume was adjusted according to blood pressure, central venous pressure and other hemodynamic parameters. Meanwhile, inotropics were reduced gradually and even stopped, ECMO flow was maintained from 50 to 100ml·kg-1·min-1 with gas-blood ratio of 1:1 and a oxygen concentration of 0.5-0.6. Mixed venous oxygen saturation was monitored continuously and kept at >75%, and HCT (hematokrit) was maintained at about 35%; the arteriovenous pressure of ECMO circulation was monitored, and the arterial and venous pressure can be kept at <150mmHg and >- 100mmHg, respectively within the above flow range. Mechanical ventilation was maintained, and synchronized intermittent mandatory ventilation (SIMV) mode was adopted, and a high positive end expiratory pressure (PEEP) (6~8cmH2O), low frequency (20~25 per min), low oxygen concentration (0.3~0.5) and longer inspiratory time mode (0.6~0.7 seconds) was kept to maintain alveolar patency. Gas-blood ratio was monitored once every 6 hours, and the homeostasis was maintained in accordance with gas and blood condition. When apparent water-sodium retention, reduced urine volume and renal insufficiency, and even acute renal failure (BUN>- 28.56mmol·L-1, Cr>530.4umol·L-1 and serum potassium> 6.5mmol·L-1) occurred, CRRT treatment was given in the method of parallel connection with ECMO circuit with blood flow of 5~10ml·kg-1·min-1, displacement liquid therapeutic dose of 15ml·kg-1·min-1 and ultrafiltration rate of 2ml·kg-1·min-1 during the treatment which can be adjusted based on the hemodynamic parameters in infants in the actual treatment process.
The recovery of the lung and heart function of infants was evaluated everyday, based on bedside chest X-ray and echocardiography. When improvement of the heart and lung function was observed, blood flow of EMCO would be gradually reduced, and the dose of vasoactive agents would be gradually increased, and ACT time would be prolonged appropriately. When the blood flow was reduced to 10% of the full flow for about 1 hour, and a good arteriovenous gas-blood was reviewed. ECMO can be considered to be removed. After removing the arteriovenous cannula, sternal closure or repair of arteria carotis communis and jugular vein was performed.
In these 3 infants, 2 cases survived, and 1 case (case 3) died. Case 1 and 2 exhibited an immediate and rapid decrease in lactate level after the treatment with ECOM. After they were assisted with ECMO for 93 hours and 83 hours, respectively, EMCO was evacuated. The survived infants did not exhibit any short-term complications. Case 3 was assisted for a total of 118 hours and exhibited continuous hyperlactacidemia; meanwhile, he had irreformable acidosis and then exhibited multiple organ failure (liver, kidney and heart), so the family gave up the treatment, and he died after evacuation of ECMO. During ECMO supporting, 3 infants all exhibited plasma leakage of oxygenator in extracorporeal membrane oxygenation, in which, membrane oxygenator was changed once in case 1 and 3; when plasma leakage occurred in case 2, ECMO was evacuated by considering that the heart function was basically recovered in this infant, and membrane oxygenator was not changed. Case 1 and 3 both exhibited reduced urine volume, increased creatinine and urea nitrogen and other clinical manifestations of renal failure at day 3 after beginning ECMO for treatment and then were given continuous CRRT.
Discussion
CPB technology has been well used in surgery for infants with congenital heart disease for life support currently; ECMO technology, as an effective life support mean in patients with cardiorespiratory failure, has been well conducted and applied in clinic in western developed countries, but its development is late in China. Based on the data provided by extracorporeal life support organization (ELSO), the survival rate of adults and infants with ECMO circulation support is 32% and 45%, respectively [3,4]. By 2010, in about 17 countries, 110 or more advanced life support technical medical centers with the capability for conducting ECMO were established, and 35, 000 patients received ECMO treatment. At present, the experts have a cautious attitude and more controversies in ECMO treatment for the patients who undergoing cardiopulmonary resuscitation, because this involves in the ethical issues (e.g. how to evaluate the organ function of the patients at the time). Meert el al [5] reported the study data from many centers consisting of 15 hospitals, and in 353 patients who experienced cardiac arrest in the hospital and received ECMO support, 49% survived and left the hospital. In China, carrying ECMO technology is late, and is seriously lagging especially in the medical application of the infants with severe diseases. It is more rarely reported how to adopt CPB, ECMO and other mechanical circulatory supports in saving the infants with severe congenital heart disease who underwent cardiac arrest.
In recent years, the clinical data shows that the application of ECMO in saving and treating the children with respiratory failure is gradually reducing, while its proportion in circulatory support of the children with heart failure is increasing steadily, especially in the heart failure after heart surgery. According to the current reports, the survival rate of the infants with heart failure with ECMO for circulatory support is about 45% 3. For the infants with severe diseases needing ECMO treatment for circulatory support, it is a key for successfully treating such infants, successfully establishment and right timely initiation of ECMO. In the data about this group of cases, for case 1 and 3, we changed the atrial cannulation and ascending aorta cannulation of CPB to ECMO circuit for support at the end of heart surgery; for case 2, the emergency cannulation was given at the bedside through right common carotid artery and right internal jugular venous cannulation to establish ECMO. Provided that the cannula models and size are selected appropriately, ECMO can be established quickly in the two methods above, and a satisfactory blood flow can be reached within the acceptable circuit pressure range. In order to grasp optimal opportunity of EMCO initiation, it is required to rapidly, fully and accurately evaluate the function of all systems in infants. Different from the application of ECMO support for respiratory failure, the current application of ECMO in the infants with severe circulatory failure and especially those undergoing cardio-pulmonary resuscitation has no generally accepted clear indications and contraindications, and it is always required to evaluate continuously during ECMO support process whether recoverability exists in heart and lung function of the infants. It is very difficult to determine whether recoverability exists in the heart and lung function of the infants before deciding to adopt mechanical circulatory support; while for the evaluation of neurologic function, we can perform a preliminary judgement only by observing pupillary reflex and autonomous respiration. The survival of case 1 and case 2 may be related to the effective cardio-pulmonary resuscitation and timely establishment of circulatory support (Extracorporeal circulatory support for case 1, and cardio-pulmonary resuscitation and starting circulatory support time < 1 hour); while for case 3 due to the factors of guardian, the interval from the start of cardio-pulmonary resuscitation to the establishment of circulatory support was longer (about 3 hours), and the rapid establishment of the effective circulatory support failed. For the infants who underwent cardiac surgery, the serum lactate levels can predict the prognosis of the infants with critical disease and are closely related to the mortality of the infants with sever diseases [6-9].
In 3 infants, the lactate level before the start of mechanical circulatory support is more than 10mmol/L (in which, >15 mmol/L in case 1 and 3); after obtaining effective circulatory support, the lactate levels of the survived case 1 and 2 both reduced to < 5mmol/L; and during ECMO support, the lactate level in case 3 is always >9mmol/L without significant decrease even if SVO2 >75% is always kept, which may be associated with the preoperative delay in the effective circulatory support, histanoxia and serious damage, and oxygen utilization disorder occurred in the body in spite of the adequate oxygen supply after ECMO support. Compared to the lactate level before the mechanical circulatory support, the dynamic change in the level after the establishment of the effective circulatory support may be a more sensitive indicator indicating the prognosis, but this still needs more case studies for support.
It is reported that bleeding and renal insufficiency is still the most common complication during ECMO treatment [10-12]. In case 1 and 3, postoperative delayed sternal closure was given, CPB was stopped and changed to ECMO support, and protamine (the ratio of protamine and heparin of 1:1) was given at the same time for neutralization; catheterization through the arteries or veins of the neck was performed in case 2. ACT was adjusted as 150- 200s for all infants, and blood routine examination was performed daily. Platelet level was kept at >50×109/L. During ECMO support, no obvious bleeding was observed in 3 infants. In ECMO support process, 3 infants all exhibited a certain degree of renal insufficiency, and even case 1 and 3 had obvious oliguria and were given CRRT by the parallel connection of Asahi Kasei iQ21 in ECMO pipeline (blood flow velocity of 5-10ml·kg-1·min-1, and ultrafiltration flux rate of 2mg·kg-1·min-1). In 2 infants, the circulatory stability was maintained, and a satisfying renal replacement therapy effect was obtained. Compared to the traditional connection with ultrafilter in ECMO circuit alone for renal replacement therapy, the parallel connection with CRRT in ECMO pipleline can precisely control the blood flow velocity and ultrafiltration flux rate with renal replacement therapy to maintain the infant capacity and circulatory stability, and is particularly significant in the younger patients with low body weight. According to the preliminary search, the parallel application of ECMO and CRRT in ECMO treatment for infants has not been reported. In the mechanical complications, 3 patients in this group show a plasma leakage of membrane oxygenator and decline of oxygenation at about 80 hours after ECMO treatment, which is mainly associated with the quality of ECMO supplies. At present, for ECMO in treating infants in our country, alternative supplies are fewer and only Medtronic pediatric kit can be selected. While the hollow-fiber membrane oxygenator of Medtronic pediatric kit has a limited service life (plasma leakage will occur at about 70 hours), so the prolonged ECMO support (> 5 days) can be achieved only by changing membrane oxygenator. In order to improve the management level of the prolonged ECMO support in infants, the appropriate silicone membrane oxygenator for infants is still needed.
The medical experts who are specialized in critical diseases at home and abroad hold a cautious attitude in rapidly establishing mechanical circulatory support to save patients with critical diseases undergoing cardio-pulmonary resuscitation, which is associated with greater difficulty in establishing circulatory support while giving cardio- pulmonary resuscitation at the same time and evaluating whether recoverability exists in heart, lung and nervous system function under emergency condition [13,14]. From the experience form our hospital, for infants undergoing cardiac arrest, timely and effective cardiopulmonary resuscitation and rapid establishment of circulatory support (including CPB and ECMO) under a full and accurate assessment of the function of heart, lung and nervous system to gain time for further treatment should be able to improve the success rate in treating such infants.
References
- Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxy- genation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972; 286: 629-634.
- Lin R, Zhang CM, Tan LH, Shi LP, Xiong QX, Zhang EW, et al. Emergency use of extracorporeal membraneoxygenation in pediatric critically ill patients. Zhong-hua Er Ke Za Zhi 2012; 50: 649-652.
- Ayad O, Dietrich A, Mihalov L. Extracorporeal mem- brane oxygenation. Emerg Med Clin North Am. 2008; 26: 953-959.
- Karagiannidis C, Philipp A, Buchwald D. Extracorpo- real membrane oxygenation, Dtsch Med Wochenschr 2013; 138: 188-191.
- Meert KL, MethenyN. Placement of postpyloric tubes using electromagnetic guidance. Pediatr Crit Care Med. 2009; 10: 271-273.
- Hannan RL, Ybarra MA, White JA, Ojito JW, Rossi AF,Burke RP. Patterns of lactate values after congenital heart surgery and timing of cardiopulmonary support. Ann Thorac Surg 2005; 80: 1473-1474.
- Hatherill M, Sajjanhar T, Tibby SM, Champion MP, Anderson D, Marsh MJ, et al. Serum lactate as a pre- dictor of mortality after paediatric cardiac surgery. ArchDis Child 1997; 77: 235-238.
- Hatherill M, Waggie Z, Purves L, Reynolds L, Argent Mortality and the nature of metabolic acidosis in children with shock. Intensive Care Med 2003; 29:286-291.
- Dietl CA, Wernly JA, Pett SB, Yassin SF, Sterling JP, Dragan R, et al. Extracorporeal membrane oxygenationsupport improves survival of patients with severe Han-tavirus cardiopulmonary syndromes. J Thorac Cardio- vasc Surg 2008; 135: 579-584.
- Oliver WC. Anticoagulation and coagulation manage-ment for ECMO. Semin Cardiothorac Vasc Anesth 2009; 13: 154-175.
- Khaja WA, Bilen O, Lukner RB, Edwards R, Teruya J.Evaluation of heparin assay for coagulation manage- ment in newborns undergoing ECMO. Am J Clin Pa-thol 2010; 134: 950-954.
- Combes A, Leprince P, Luyt CE, Bonnet N, TrouilletJL, Léger P, et al. Outcomes and long-term quality-of- life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008; 36: 1404-1411.
- Liu Y, Cheng YT, Chang JC, Chao SF, Chang BS. Ex- tracorporeal membrane oxygenation to support pro- longed conventional cardiopulmonary resuscitation in adults with cardiac arrest from acute myocardial infarc- tion at a very low-volume centre. Interact Cardiovasc Thorac Surg 2011; 12: 389-393.
- Kagawa E, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S, et al. Assessment of outcomes and differ- ences between in- and out-of hospital cardiac arrest pa- tients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation. 2010; 81:968-973.