Research Article - Biomedical Research (2017) Volume 28, Issue 1
Anti-inflammatory effect of combination therapy with rosiglitazone and alltrans retinoic acid on high glucose-induced MCP-1 response in rat mesangial cells
Dae Hee Kim1#, Geon-Cheol Lee2#, Cy-Hyun Kim1, Se Won Oh3, Kum Hyun Han3, Sang Youb Han1,3*1Clinical Research Center, Inje University Ilsan-Paik Hospital, Goyang, Korea
2Department of Urology, Inje University Ilsan-Paik Hospital, Goyang, Korea
3Department of Internal Medicine, Inje University Ilsan-Paik Hospital, Goyang, Korea
#These authors contributed equally to the work
- *Corresponding Author:
- Sang Youb Han
Division of Nephrology Department of Internal
Medicine Inje University Ilsan-Paik Hospital Korea
Accepted on June 29, 2016
Abstract
Peroxisome Proliferator-Activated Receptor-γ (PPAR-γ) agonists and retinoid acid have antiinflammatory and anti-proliferative effects; however, their synergistic effects are not well known. We investigated the combined anti-inflammatory effect of rosiglitazone, which is a PPAR-γ agonist, and retinoid acid in rat mesangial cells (RMCs) stimulated by a high glucose (HG) concentration (30 mmol of D-glucose). The doses of rosiglitazone and all-trans retinoic acid (ATRA) which inhibited MCP-1 mRNA expression by 20-40% were selected for the study. At 48 h following incubation of RMCs in HG, MCP-1 mRNA expression was significantly increased. Rosiglitazone and ATRA lowered MCP-1 mRNA expression in a dose-dependent manner. Among the effective doses, 1.0 and 5.0 μmol/L of rosiglitazone, and 0.1 and 1.0 μmol/L of ATRA were selected for further studies. HG-induced MCP-1 mRNA expression was inhibited by combined treatment with rosiglitazone and ATRA. A combination of 1.0 μmol/L rosiglitazone and 0.1 μmol/L ATRA tended to decrease MCP-1 mRNA expression compared to the individual treatments. A combination of 5.0 μmol/L rosiglitazone and 1.0 μmol/L ATRA significantly inhibited MCP-1 mRNA expression. MCP-1 protein levels were significantly increased after 48 h of incubating the RMCs in HG. The 5.0 μmol/L dose of rosiglitazone significantly lowered MCP-1 protein synthesis while 1 μmol/L ATRA decreased MCP-1 expression stimulated by HG. Combined treatment with rosiglitazone and ATRA caused a larger decrease in MCP-1 protein synthesis compared to either treatment alone. In conclusion, the data obtained show the possibility of a synergistic effect on MCP-1 mRNA expression by rosiglitazone and ATRA.
Keywords
Rosiglitazone, Retinoic acid, MCP-1, Mesangial cell, Inflammation.
Introduction
Inflammation plays a key role in the pathogenesis of diabetic nephropathy. Infiltration of chemokines, cytokines, and inflammatory cells into the kidney has been reported in both human and animal diabetic models [1,2]. Recently, several clinical trials have been conducted with the aim of controlling inflammation in patients with diabetic nephropathy. Peroxisome proliferator-activated receptor-γ (PPAR-γ) and retinoid X receptor (RXR) are nuclear receptors involved in various cellular processes. PPAR-γ regulates lipid and glucose metabolism and is implicated in pathological conditions including obesity, diabetes, and atherosclerosis [3]. RXR regulates reproduction, cellular differentiation, and hematopoiesis [4]. RXR is critical in the development of the kidneys. A deficiency of retinoic acid results in a reduction in nephron mass [5]. PPAR-γ and RXR agonists are beneficial in various renal diseases. PPAR-γ is involved in the protection of the renal tissues from inflammatory and fibrotic responses [6,7]. Retinoic acid has also shown anti-inflammatory and antiproliferative effects in multiple animal models of glomerular disease [8-10]. PPAR-γ and RXR form heterodimers within the nucleus and activate transcriptional factors [11]. Although their anti-inflammatory and anti-proliferative effects have been known well, their synergistic effect in kidney diseases is not clear. We therefore investigated the anti-inflammatory effect of a combined treatment with PPAR-γ and RXR agonists in rat mesangial cells (RMCs) stimulated by a high glucose (HG) concentration.
Materials and Methods
RMC culture and experimental design
RMCs were obtained by culturing glomeruli isolated from the kidneys of male Sprague-Dawley rats by conventional sieving methods as previously described [12]. Briefly, the mesangial cells were cultured in Dulbecco’s modified Eagle’s medium containing 20% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 44 mmol/L NaHCO3, and 14 mmol/L N-2-hydroxy-ethylpiperazine-N’-2-ethane sulfonic acid, at 37°C in a humidified atmosphere of 95% air and 5% CO2. Subconfluent cells were cultured in 0.1% fetal bovine serum for 24 h before the experiments. The cells were then incubated with 5.6 (control) or 30 mmol/L D-glucose in the presence of rosiglitazone (GlaxoSmithKline, Middlesex, UK) and/or alltrans retinoic acid (ATRA; Sigma-Aldrich, St. Louis, MO, USA). Cells were cultured in triplicates and harvested at 6 and 48 h, for extraction of total RNA and protein. Bioassays for monocyte chemoattractant peptide-1 (MCP-1) protein were performed on supernatants collected from the samples. All experiments were performed using cells between the 6th and 8th passages.
Quantitative real-time polymerase chain reaction
Total RNA was extracted using TRIzol and reverse transcribed using a cDNA synthesis kit (Fermentas, Burlington, Canada) as previously described [13]. Gene expressions were measured by quantitative real-time polymerase chain reaction (qRT-PCR). The RT-PCR was performed by following a standard three-step cycling condition using SYBR Green Master mix. Primers were designed from the respective gene sequences using Primer3 software. The nucleotide sequences of each primer were as follows: MCP-1, sense 5’ ATGCAGTTAATGCCCCACTC 3’ and anti-sense 5’ TTCCTTATTGGGGTCAGCAC 3’; and glyceraldehyde-3- phosphate dehydrogenase (GAPDH), sense 5’ TGCACCACCAACTGCTTAGC 3’ and anti-sense 5’ GGCATGGACTGTGGTCATGAG 3’. The expression levels of all the genes were normalized to that of the housekeeping gene GAPDH.
Measurement of MCP-1 protein in culture supernatant
The secretary MCP-1 protein levels in the supernatants collected from the samples were measured. The concentration of MCP-1 was determined by quantitative sandwich Enzyme- Linked Immunosorbent Assay (ELISA) using a commercial kit (Biosource Inc., Camarillo, CA, USA), according to the manufacturer's instructions. Each assay was performed in duplicate, and color intensity was measured using an ELISA reader at 450 nm. MCP-1 concentration was expressed relative to the total protein concentration in each sample.
Statistical analysis
We used non-parametric analysis because most of the variables, especially urinary MCP-1, were not normally distributed even after logarithmic transformation. The Mann- Whitney U test was used to compare differences between two groups. Statistical significance was defined as p value<0.05. All statistical analyses were performed using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA). Data have been expressed as mean ± standard error.
Results
Anti-inflammatory effect of rosiglitazone and ATRA on MCP-1 mRNA expression in RMCs
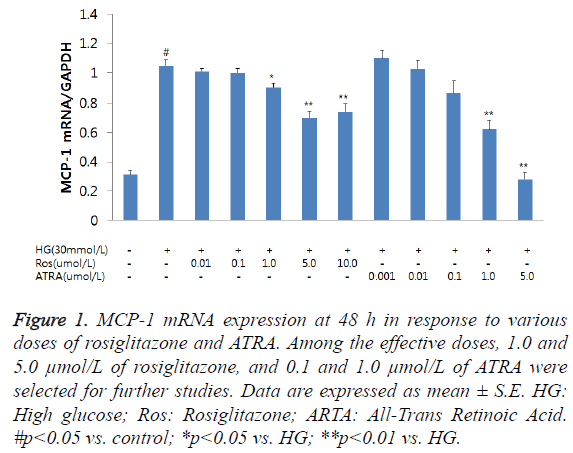
We tested the effect of several doses of rosiglitazone and ATRA on MCP-1 mRNA expression in order to select the optimal doses. The doses that inhibited MCP-1 mRNA expression by 20-40% were selected.
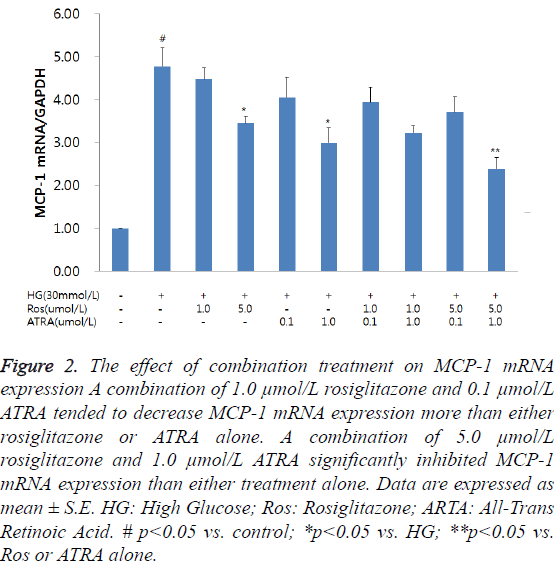
Although MCP-1 mRNA expression was increased at 6 h following incubation of RMCs with HG, it was not significant (data not shown); however, at 48 h, MCP-1 mRNA expression was significantly increased. The combined treatment with rosiglitazone and ATRA resulted in a reduction in MCP-1 mRNA expression in the RMCs in a dose-dependent manner (Figure 1). Among the effective doses, we chose 1.0 and 5.0 μmol/L of rosiglitazone, and 0.1 and 1.0 μmol/L of ATRA for further studies. HG-induced MCP-1 mRNA expression was inhibited by treating the cells with a combination of rosiglitazone and ATRA. A combination of 1.0 μmol/L rosiglitazone and 0.1 μmol/L ATRA tended to decrease MCP-1 mRNA expression compared to either rosiglitazone or ATRA alone. A combination of 5.0 μmol/L rosiglitazone and 1.0 μmol/L ATRA significantly inhibited MCP-1 mRNA expression than either treatment alone (Figure 2).
Figure 1: MCP-1 mRNA expression at 48 h in response to various doses of rosiglitazone and ATRA. Among the effective doses, 1.0 and 5.0 μmol/L of rosiglitazone, and 0.1 and 1.0 μmol/L of ATRA were selected for further studies. Data are expressed as mean ± S.E. HG: High glucose; Ros: Rosiglitazone; ARTA: All-Trans Retinoic Acid. #p<0.05 vs. control; *p<0.05 vs. HG; **p<0.01 vs. HG.
Figure 2: The effect of combination treatment on MCP-1 mRNA expression A combination of 1.0 μmol/L rosiglitazone and 0.1 μmol/L ATRA tended to decrease MCP-1 mRNA expression more than either rosiglitazone or ATRA alone. A combination of 5.0 μmol/L rosiglitazone and 1.0 μmol/L ATRA significantly inhibited MCP-1 mRNA expression than either treatment alone. Data are expressed as mean ± S.E. HG: High Glucose; Ros: Rosiglitazone; ARTA: All-Trans Retinoic Acid. # p<0.05 vs. control; *p<0.05 vs. HG; **p<0.05 vs. Ros or ATRA alone.
Effect of rosiglitazone and ATRA on MCP-1 protein synthesis in RMCs
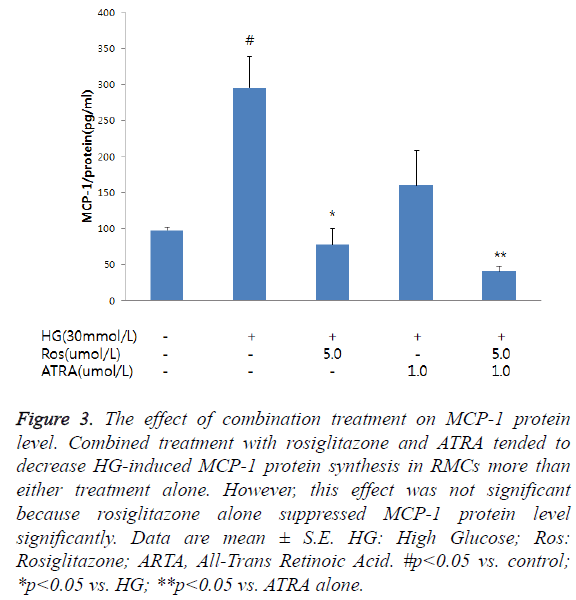
MCP-1 protein levels in the RMCs significantly increased after the 48-h incubation in HG (control: 97.7 ± 3.9 pg/mg protein; HG: 295.5 ± 43.6 pg/mg protein; p<0.01) (Figure 3). The 5- μmol/L dose of rosiglitazone significantly reduced MCP-1 protein synthesis while ATRA decrease MCP-1 expression. Combined treatment with rosiglitazone and ATRA tended to decrease MCP-1 protein synthesis in the RMCs more than either treatment alone. However, the decrease caused by the combination was not significantly higher than that caused by either treatment alone (HG: 295.5 ± 43.6 pg/mg protein; HG + rosiglitazone: 77.3 ± 22.5 pg/mg protein, p<0.01 vs. HG; HG + ATRA: 83.7 ± 48.3 pg/mg protein, p<0.05 vs. HG; HG + rosiglitazone + ATRA: 40.6 ± 7.4 pg/mg protein; p<0.01 vs. HG, p<0.05 vs. HG + rosiglitazone).
Figure 3: The effect of combination treatment on MCP-1 protein level. Combined treatment with rosiglitazone and ATRA tended to decrease HG-induced MCP-1 protein synthesis in RMCs more than either treatment alone. However, this effect was not significant because rosiglitazone alone suppressed MCP-1 protein level significantly. Data are mean ± S.E. HG: High Glucose; Ros: Rosiglitazone; ARTA, All-Trans Retinoic Acid. #p<0.05 vs. control; *p<0.05 vs. HG; **p<0.05 vs. ATRA alone.
Discussion
In this study, we have shown that rosiglitazone and ATRA reduced HG-induced MCP-1 synthesis in RMCs. Moreover, co-treatment with rosiglitazone and ATRA suppressed MCP-1 expression more than either treatment alone. These data show the possibility of a synergistic effect on MCP-1 expression due to combined treatment with PPAR-γ and RXR agonists. PPAR- γ agonists have been widely used as insulin sensitizers in diabetic patients. A meta-analysis has demonstrated potential significant benefits of PPAR-γ agonists in diabetic patients [14]. In addition to having an anti-diabetic effect, PPAR-γ agonists are known to have reno-protective effects. Some studies in animal models of diabetic and non-diabetic kidney diseases have shown that PPAR-γ agonists reduce albuminuria and ameliorate renal injury due to their anti-inflammatory and anti-fibrotic effects [6,15-17]. In mesangial cells, PPAR-γ agonists inhibit fibronectin and collagen syntheses, MCP-1 secretion, and cell growth [18]. Our results also showed that rosiglitazone inhibited MCP-1 expression. Retinoic acid also protects against various renal injuries by inhibiting inflammatory and fibrotic changes. Retinoic acid inhibits MCP-1 expression, inducible nitric oxide synthase, fibronectin, and plasminogen activator inhibitor-1 in mesangial cells [10,19-21]. Retinoic acid has also been shown to reduce glomerulosclerosis and renal injury in animal models of kidney disease as well as in type 2 diabetic rats [22-25]. The results from our study on the anti-inflammatory effects of ATRA agree with those in previous reports.
Heterodimers of PPAR-γ and RXR bind to specific PPAR response elements, thereby regulating numerous gene expressions [11]. There are a few evidences that activation of both PPAR and RXR results in synergistic protection against tissue injuries. Activation of PPAR-γ/RXR induces insulin sensitization in diabetic rats [26], inhibits proliferation of stellate cells [27], blunts pro-inflammatory and pro-invasive phenotypes of tumor-associated fibroblasts [28,29], and inhibits inflammatory mediators such as nuclear factor-kappa B and interleukin 6 in breast cancer cells [30]. Even though PPAR and RXR agonists have beneficial effects, they are associated with well-known side effects that limit their application [31,32]. Therefore, combination therapies can be used to obtain therapeutic efficacy with minimal side effects. Our results showed that combined treatment with PPAR-γ and RXR agonists reduced MCP-1 expression more than using either treatment alone.
There are several limitations of combined treatment with PPAR-γ and RXR agonists. Firstly, gene transcriptions can be activated or repressed by co-factors and ligands due to the treatment. Ligands can affect the activities of PPAR-γ/RXR by acting as co-repressors or co-activators [33,34]. Protein flightless-1, which is a modulator of PPAR-γ, interrupts the formation of the PPAR-γ/RXR complex and results in repression of transcriptional activity [35]. Secondly, several PPAR and RXR isoforms are expressed in a nephron segmentspecific manner [36], which can result in different effects according to different combinations of the isoforms [37]. Thirdly, the effects of RXR agonists are affected by the activation of PPAR-γ. This is because RXR agonists impair arterial monocyte recruitment through PPAR-γ activation [38] and PPAR-γ knockout cell lines have shown blunted responses to RXR agonists [39]. Lastly, unexpected side effects can occur from the combined treatment since some authors found that the combined treatment was harmful in type 1 diabetic rats (data not shown). The beneficial and harmful effects of the combined treatment may depend on the selected dose of each agonist.
Suboptimal doses may constitute a new alternative and effective therapy. Escudero et al. [40] reported that, combined treatment with rosuvastatin and bexarotene at suboptimal doses reduced the serious dose-related adverse effects of the two drugs and controlled vascular inflammation effectively. Our results also revealed that the combined treatment showed synergy between the two agonists in controlling MCP-1 expression. Therefore, combined suboptimal doses of PPAR-γ and RXR agonists may produce reno-protective effects; however, further studies are required to clarify the combined effect of the agonists in pathological conditions.
Acknowledgement
This work was supported by the Korean Society of Nephrology in 2005. We would like to thank GlaxoSmithKline for the generous donation of rosiglitazone for this study.
References
- Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int 2000; 58: 684-690.
- Sassy-Prigent C, Heudes D, Mandet C, Bélair MF, Michel O, Perdereau B, Bariéty J, Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 2000; 49: 466-475.
- Mandard S, Patsouris D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res 2013;2013:613864.
- Gronemeyer H, Miturski R. Molecular mechanisms of retinoid action. Cell MolBiolLett 2001;6:3-52.
- Das BC, Thapa P, Karki R, Das S, Mahapatra S, Liu TC, Torregroza I, Wallace DP, Kambhampati S, Van Veldhuizen P, Verma A, Ray SK, Evans T. Retinoic acid signaling pathways in development and diseases. Bioorg Med Chem 2014; 22:673-683.
- Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabeticglomerulosclerosis in rats. Kidney Int 2001; 59:1899-1910.
- Zhou TB, Ou C, Luo CP, Xu HL. Protective effects of PPARγ agonist in glomerulosclerosis rats induced by adriamycin. Ren Fail 2012; 34:940-941.
- Oseto S, Moriyama T, Kawada N, Nagatoya K, Takeji M, Ando A, Yamamoto T, Imai E, Hori M. Therapeutic effect of all-trans retinoic acid on rats with anti-GBMantibody glomerulonephritis. Kidney Int 2003; 64:1241-1252.
- Vaughan MR, Pippin JW, Griffin SV, Krofft R, Fleet M, Haseley L, Shankland SJ. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int 2005; 68:133-144.
- Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, Kang SW, Han DS, Han JY, Cha DR. Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol 2004; 82:568-576.
- Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 1992; 358:771-774.
- Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, Im SY, Lee JW, Kim TS. Retinoid inhibit interleukin-12 production in macrophages through physical association of retinoid X receptor and NF kappaB. J BiolChem 1999; 274: 7674-7680.
- Giguere V. Retinoic acid receptors and cellular retinoid binding proteins; complex interplay in retinoid signaling. Endocr Rev 1994; 15: 61-79.
- Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis 2010; 55:835-847
- Pistrosch F, Passauer J, Herbrig K, Schwanebeck U, Gross P, Bornstein SR. Effect of thiazolidinedione treatment on proteinuria and renal hemodynamic in type 2 diabetic patients with overt nephropathy. HormMetab Res 2012; 44:914-918.
- Routh RE, Johnson JH, McCarthy KJ. Troglitazone suppresses the secretion of type I collagen by mesangial cells in vitro. Kidney Int 2002;61:1365-1376.
- Ohga S, Shikata K, Yozai K, Okada S, Ogawa D, Usui H, Wada J, Shikata Y, Makino H. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-kappaB activation. Am J Physiol Renal Physiol 2007; 292:F1141-1150.
- Ghosh SS, Gehr TW, Ghosh S, Fakhry I, Sica DA, Lyall V, Schoolwerth AC. PPARgamma ligand attenuates PDGF-induced mesangial cell proliferation: role of MAP kinase. Kidney Int 2003; 64:52-62.
- Lucio-Cazana J, Nakayama K, Xu Q, Konta T, Moreno-Manzano V, Furusu A, Kitamura M. Suppression of constitutive but not Il-1beta-inducible expression of monocyte chemoattractant protein-1 in mesangial cells by retinoic acids: intervention in the activator protein-1 pathway. J Am SocNephrol 2001; 12:688-694.
- Datta PK, Lianos EA. Retinoic acids inhibit inducible nitric oxide synthase expression in mesangial cells. Kidney Int 1999; 56:486-493.
- Liu X, Lü L, Tao BB, Zhu YC. All-trans retinoic acid inhibits the increases in fibronectin and PAI-1 induced by TGF-beta1 and Ang II in rat mesangial cells. ActaPharmacol Sin 2008; 29:1035-1041.
- Hu P, Qin YH, Pei J, Lei FY, Hu B, Lu L. Beneficial effect of all-trans retinoic acid (ATRA) on glomerulosclerosis rats via the down-regulation of the expression of alpha-smooth muscle actin: a comparative study between ATRA and benazepril. ExpMolPathol 2010; 89:51-57.
- Schaier M, Liebler S, Schade K, Shimizu F, Kawachi H, Grone HJ, Chandraratna R, Ritz E, Wagner J. Retinoic acid receptor alpha and retinoid X receptor specific agonists reduce renal injury in established chronic glomerulonephritis of the rat. J Mol Med (Berl) 2004; 82:116-125.
- Lehrke I, Schaier M, Schade K, Morath C, Waldherr R, Ritz E, Wagner J. Retinoid receptor-specific agonists alleviate experimental glomerulonephritis. Am J Physiol Renal Physiol 2002; 282:F741-751.
- Kim CS, Park JS, Ahn CW, Kim KR. All-Trans Retinoic Acid Has a Potential Therapeutic Role for Diabetic Nephropathy. Yonsei Med J 2015; 56:1597-1603.
- Li X, Hansen PA, Xi L, Chandraratna RA, Burant CF. Distinct mechanisms of glucose lowering by specific agonists for peroxisomal proliferator activated receptor gamma and retinoic acid X receptors. J BiolChem 2005; 280:38317-38327.
- Sharvit E, Abramovitch S, Reif S, Bruck R. Amplified inhibition of stellate cell activation pathways by PPAR-γ, RAR and RXR agonists. PLoS One 2013; 8:e76541.
- Papi A, Tatenhorst L, Terwel D, Hermes M, Kummer MP, Orlandi M, Heneka MT. PPAR and RXR ligands act sinergistically as potent antineoplastic agents in vitro and in vivo glioma models. J Neurochem 2009; 109:1779-1790.
- Papi A, Rocchi P, Ferreri AM, Orlandi M. RXR and PPAR Ligands in Combination to Inhibit Proliferation and Invasiveness in Colon Cancer Cells. Cancer Lett 2010; 297:65-72.
- Storci G, Sansone P, Mari S, D’Uva G, Tavolari S, Guarnieri T, Taffurelli M, Ceccarelli C, Santini D, Chieco P, Marcu KB, Bonafè M. TNF up-regulates SLUG via the NF-kB/HIF1 axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physiol 2010;225:682-691.
- Mallipattu SK, He JC. The beneficial role of retinoids in glomerular disease. Front Med (Lausanne) 2015.
- Shah P, Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin Drug Saf 2010; 9:347-354.
- Heikkinen S, Auwerx J, Argmann CA: PPARγ in human and mouse physiology. BiochimBiophysActa 2007; 1771:999-1013.
- Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs:peroxisome proliferator-activated receptors are nuclearreceptors at the crossroads of key cellular functions. Prog Lipid Res 2006; 45:120-159.
- Choi JS, Choi SS, Kim ES, Seo YK, Seo JK, Kim EK, Suh PG, Choi JH. Flightless-1, a novel transcriptional modulator of PPARγ through competing with RXRα. Cell Signal 2015; 27:614-620.
- Yang T, Michele DE, Park J, Smart AM, Lin Z, Brosius FC 3rd, Schnermann JB, Briggs JP. Expression of peroxisomal proliferator-activated receptors and retinoid X receptors in the kidney. Am J Physiol 1999; 277:F966-973.
- Papi A, Guarnieri T, Storci G, Santini D, Ceccarelli C, Taffurelli M, De Carolis S, Avenia N, Sanguinetti A, Sidoni A. Nuclear receptors agonists exert opposing effects on the inflammation dependent survival of breast cancer stem cells. Cell Death Differ 2012; 19:1208-1219.
- Sanz MJ, Albertos F, Otero E, Juez M, Morcillo EJ, Piqueras L. Retinoid X receptor agonists impair arterial mononuclear cell recruitment through peroxisome proliferator-activated receptor-γ activation. J Immunol 2012; 189:411-424.
- Lehman AM, Montford JR, Horita H, Ostriker AC, Weiser-Evans MC, Nemenoff RA, Furgeson SB. Activation of the retinoid X receptor modulates angiotensin II-induced smooth muscle gene expression and inflammation in vascular smooth muscle cells. MolPharmacol 2014; 86:570-579.
- Escudero P, Martinez de Marañón A, Collado A, Gonzalez-Navarro H, Hermenegildo C, Peiró C, Piqueras L, Sanz MJ. Combined sub-optimal doses of rosuvastatin and bexarotene impair angiotensin II-induced arterial mononuclear cell adhesion through inhibition of Nox5 signaling pathways and increased RXR/PPARα and RXR/PPARγ interactions. Antioxid Redox Signal 2015; 22:901-920.