- Biomedical Research (2014) Volume 25, Issue 2
Analysis of meiotic segregation patterns and interchromosomal effects in sperm from a Robertsonian translocation family
Shuqin Xu1#, Dongling Tang2#, Kun Fang3#, Yanzhi Xia3, Jieping Song1, Weipeng Wang1, Maowei Cheng4, Bo Wang1*
1Genetics Laboratory, Hubei Maternal and Child Health Hospital, Wuhan, Hubei, PR China
2Department of Clinical Liboratory, Renmin Hospital?Wuhan University, Wuhan, Hubei, PR China
3College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, Hubei, PR China
4Prevent and Control Disease Center of Hubei Province, Wuhan, Hubei, PR China
#These authors contributed equally to this work.
- *Corresponding Author:
- Bo Wang
Genetics Laboratory
Hubei Maternal and Child Health Hospital
Wuhan, Hubei,PR China
Accepted January 07 2014
Abstract
The objective of the present study is to provide more genetic information about meiotic segregation behavior and the possibility of interchromosomal effects (ICE) in spermatozoa from carriers of Robertsonian (Rob) translocation. Analysis of sperm chromosomes was done by fluorescence in situ hybridization (FISH). In vitro fertilization was conducted in clinic and genetics laboratory in a hospital. Two patients (men) from a Rob translocation family were included in the study. Multicolor FISH was used for probing of chromosomes 14, 15, 18, X, and Y on sperm. Main Outcome Measure: Frequencies of meiotic segregation products in sperm and sperm aneuploidy of chromosomes 14, 15, 18, X, and Y. To Rob Translocation heterozygote of this paper, the rate of normal/balanced spermatozoa resulting from alternate segregation is 79.9%. The frequency of unbalanced spermatozoa resulting from adjacent segregation is 20.1%. The higher frequencies of aneuploidy for sex chromosome were observed. In addition, the increased rates of diploid were found. To Rob translocation homozygosity, the rate of balanced spermatozoa is 99.7%. The frequency of unbalanced spermatozoa is 0.3%. The higher frequencies of aneuploidy for sex chromosome were not observed. Alternate segregation is dominant in the different types of Rob translocations. Carriers may be at an increased risk for ICE. Rob translocation homozygosity could be seen as a potential speciation in humans with 44 chromosomes.
Keywords
Sperm fluorescence in-situ hybridization, interchromosomal effects, meiotic segregation, Robertsonian translocation homozygosity, evolution
Introduction
The frequency of Rob translocation in newborn babies is approximately one in 1,000. Rob translocation is an unusual type of chromosome rearrangement caused by two particular chromosomes joining together. In humans, it occurs in the five acrocentric chromosomes, such as chromosomes 13, 14, 15, 21, and 22. During a Rob translocation, the participating chromosomes break at their centromeres and the long arms fuse to form a single chromosome with a single centromere. The short arms also join to form a reciprocal product, which in the acrocentric chromosomes, typically contains nonessential genes and repetitive sequences such as nucleolar organizing regions, and is usually lost within a few cell divisions.
Translocation between chromosomes 13 and 14 is the most frequent one in humans, estimated to be approximately 75% of all Rob translocations. The t (14; 22) and t (13; 21) are two rare Rob translocations, comprising about only 1.2 and 2% of all detected Rob translocations, respectively [1].
Since Rob translocation carriers have a balanced chromosomal complement, they are healthy and have a normal lifespan, and may be unaware of their unusual chromosome rearrangement. A Rob translocation can be transmitted for many generations without detection.
In Rob translocations, at the end of meiosis I, segregation of the translocated and nontranslocated chromosomes from the two different chromosome pairs implicated leads to the formation of either balanced (alternate segregation mode) or unbalanced (adjacent 1, adjacent 2, and 3:1 segregation modes) gametes [2,3], which can segregate in different ways at anaphase. Only products of alternate segregation have normal/balanced karyotype.
All other segregation modes (adjacent-1, adjacent-2, 3:0) produce unbalanced gametes with disomies and nullisomies of chromosomes involved in Rob translocations. It is well known that meiotic tetravalent configuration tends to segregate in alternate way [4], resulting in preferential production of normal/balanced spermatozoa. However, certain percentages of unbalanced gametes derived from adjacent segregation are also produced, leading to the increased risk of miscarriage and pregnancy with livebirth of chromosomally unbalanced fetus.
Analysis of the chromosomal constitution in sperm of Rob translocation carriers is of great interest for assessing the risk of unbalanced offspring and adapting genetic counseling. Sperm fluorescence in situ hybridization (FISH) using appropriate probes is a useful technique for predicting the rate of each class of segregation modes in sperm from translocation carriers. During the last decade, meiotic segregation in spermatozoa has been repeatedly studied in male carriers of Rob translocations [5-9]. Although it may vary from one translocation to the other, the rate of unbalanced gametes is generally not conclusive enough for genetic counseling, but most of these studies showed strong prevalence of alternate segregation in gametes. However, one recent study gave conflicting results, showing a high percentage of unbalanced spermatozoa in two Rob translocation carriers [10].
More recently, interchromosomal effects (ICE) have been described for several chromosome pairs in Rob translocations. The interchromosomal effect (ICE) refers to a disturbance of meiosis where rearranged chromosomes disrupt disjunction and distribution of chromosome pairs not involved in the rearrangement. This effect was first postulated by Lejeune [11], who noticed an excess of carriers of balanced reciprocal translocations among the fathers of children with Down syndrome. Contradictory data have been reported on the analysis of spermatozoa. Several studies have found such an ICE in male carriers of Rob translocations [12,13], but others did not [14,15].
In this study, we report results of chromosome segregation studies in sperm of a Rob translocation family.
Case report
This case report was occasioned by the ascertainment of a 25-year-old Chinese man (IV-1) married to a nonconsanguineous woman with normal chromosomes (IV-2).
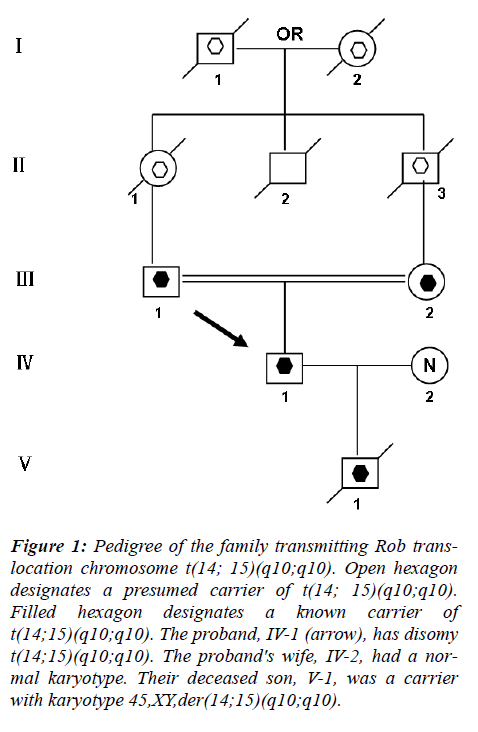
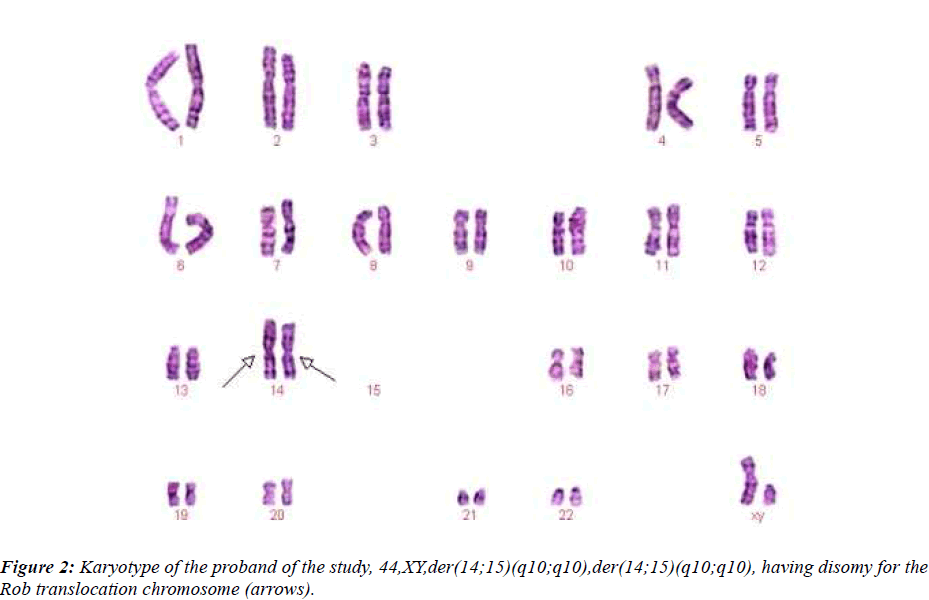
This couple had a son who died at the age of 6 months, buried without an autopsy, but had a chromosome study because of cerebral palsy. The karyotyping analysis of IV-1 was authenticated by the Chinese Academic Committee of the state key laboratory of medical genetics with previously undescribed balanced human karyotype 44XY, der(14;15)(q10;q10), der(14;15)(q10;q10) (see Figure 2.).
The parents of the propositus are phenotypically normal first consins, each a carrier of the same Rob translocation (?-1, ?-2). Their parents, a mutual uncle, and both grandparents are deceased, thus it is not possible to determine whether I-1 or I-2 was the carrier of the translocation (Figure 1).
Figure 1: Pedigree of the family transmitting Rob translocation chromosome t(14; 15)(q10;q10). Open hexagon designates a presumed carrier of t(14; 15)(q10;q10). Filled hexagon designates a known carrier of t(14;15)(q10;q10). The proband, IV-1 (arrow), has disomy t(14;15)(q10;q10). The proband's wife, IV-2, had a normal karyotype. Their deceased son, V-1, was a carrier with karyotype 45,XY,der(14;15)(q10;q10).
Materials and Methods
Patients
Two men were included in the study. IV-1: 25-year-old, Karyotype is 44,XY, der (14;15) (q10;q10), der(14;15)(q10;q10) and ?-1: 48-year-old, Karyotype is 45,XY,der(14;15)(q10;q10)
Sperm preparation, FISH and scoring
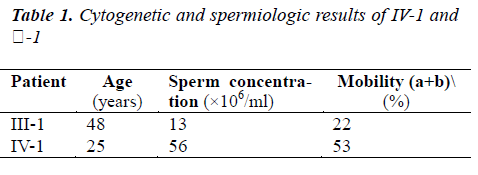
All semen samples were first analyzed to evaluate volume, concentration and motility (Table. 1.), according to the World Health Organization criteria [16]. After semen analysis, sperm with progressive motility was isolated and washed twice in phosphate buffered saline (pH 7.4) by centrifugation at 1500 rpm for 11 min. Final pellets were fixed with 5 ml of acetic acid/methanol mixture (1:3) for at least 30 min at 4 °C. Aliquots (40–50μl) of the resulting suspension of nuclei were smeared on cold pre-cleaned slides. Nuclei decondensation was performed in 1 N NaOH for 2 min. After dehydration in ethanol series (70 %, 90 %, 100 %), denaturation was performed in 0.25 % formamide in 2xSSC followed by overnight hybridization with a combination of commercially available probes.
Two sets of probe mixtures were used in this study: Firstly, for the detection of normal/balanced or unbalanced sperm, dual-color FISH was carried out using locusspecific probes (LSP) and Tel probes from Vysis (Vysis, Downers Grove, IL, USA). For IV-1 and ?-1 ? 14/15 two fluorescent probes were used: TelVysion Probe 14q (D14S1420, Spectrum Red) for 14q32.33 and TelVysion Probe 15q(D15S120, Spectrum Green) for 15q26.3.
Secondly, to investigate the presence of ICE, triple-color FISH was performed using the second probe mixture which consist of commercial satellite (DNA) probes from Vysis, including chromosomes 18, X and Y (CEP 18, Spectrum Blue/CEP X, Spectrum Green/CEP Y, Spectrum Red).
Post-hybridization washes included 2 min in 0.4xSSC/ 0.3%NP-40 (pH=7) at 72 °C, followed by 1 min in 2xSSC/ 0.1%NP-40 (pH=7) at room temperature. Slides were covered with DAPI II (Vysis). Only intact spermatozoa bearing a similar degree of decondensation and clear hybridization signals were scored; disrupted or overlapping spermatozoa were excluded from analysis. Only slides with hybridization efficiency of 99% and more were analyzed. 1,000 sperm nuclei per patient were analyzed.
Statistical analysis
Chi-squared test was used to compare frequencies of segregation products. A probability value of less than 0.05 was considered to be statistically significant.
Results
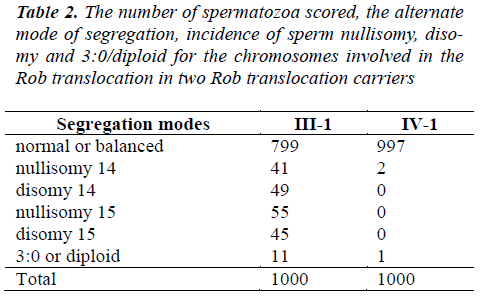
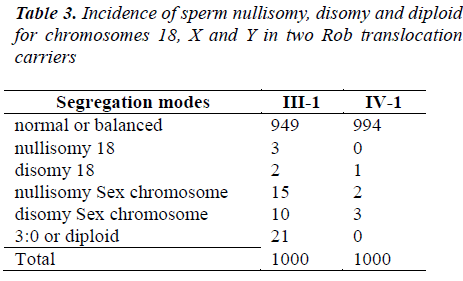
A total of 4,000 sperm nuclei from the two translocation carriers were analyzed for this study. The results of the segregation analysis are detailed in Table 2 and Table 3.
To Rob translocation heterozygote of this paper(-1), the rate of normal/balanced spermatozoa resulting from alternate segregation is 79.9%. The frequency of unbalanced spermatozoa resulting from adjacent segregation is 20.1%. To Rob translocation homozygosity (IV-1), the rate of balanced spermatozoa is 99.7%. The frequency of unbalanced spermatozoa is 0.3% (see Table 2). In ?-1, the frequency of unbalanced spermatozoa was significantly higher compared to that of IV-1 (P<0.05). In IV-1, the frequency of unbalanced spermatozoa was similarity to some controls [17-21].
The nullisomy, disomy and diploid rates for chromosomes 18, X and Y in ?-1 and IV-1 are summarized in Table 3. In ?-1, the higher frequencies of aneuploidy for sex chromosome were observed. In addition, the increased rates of diploid were found. The incidence of spermatozoa with nullisomy?disomy and diploid for the sex chromosomes of ?-1 was significantly higher compared to that of IV-1 (P<0.05). But, compared IV-1 with controls, the incidence was not significant [17-21].
Discussion
Fusion of human spermatozoa with zona-free hamster egg has been the only available method for the study of structural chromosomal aberrations in human sperm until the occurrence of FISH [22]. Multicolor FISH on decondensed sperm nuclei allows for a rapid analysis (less than 1 hour) of meiotic segregation in sperm of translocation carriers, providing information on the exact amount of normal/balanced sperm.
Accumulation of such information is undoubtedly important not only for basic cytogenetic research but also for reproductive counseling of Rob translocation carriers. The t(14;15) are rare Rob translocations. Our data showed that alternate segregation was largely dominant over adjacent segregations in the uncommon Rob translocations carriers. This finding is consistent with results from previous studies [20,21].
To Rob translocation heterozygote of this paper (?-1), the rate of normal/balanced spermatozoa resulting from alternate segregation is 79.9%. The frequency of unbalanced spermatozoa resulting from adjacent segregation is 20.1%. To Rob translocation homozygosity (IV-1), the rate of balanced spermatozoa is 99.7%. The frequency of unbalanced spermatozoa is 0.3% (Table 2). In ?-1, the frequency of unbalanced spermatozoa was significantly higher compared to that of IV-1 (P<0.05). In IV-1, the frequency of unbalanced spermatozoa had similarity to some controls. This finding is consistent with previously published data [5-7]. The high prevalence of the alternate segregation had been presumed to occur because cisconfiguration of the trivalent during meiosis favored an alternate segregation in all Rob translocations [23,24].
Variability in frequencies of unbalanced sperm in different studies can be related to technical aspects, such as FISH protocols, probes or scoring criteria used [25].
ICE first described by Lejeune [11], but the hypothesis of an ICE associated with carriers of Rob translocations has always been a controversial issue [26]. Some reports supported the possibility of ICE in Rob translocations [12,13]. However, others demonstrated no evidence of this phenomenon [9,18].
The interchromosomal effect could be explained by the formation of heterosynapses between chromosomes involved in the translocation and the sex vesicle, which could also involve other chromosomes [11,26].
In the present study, the significant increased rates of nullisomy for the sex chromosomes were observed. In ?-1, the higher frequencies of aneuploidy for sex chromosome were observed. In addition, the increased rates of diploid were found. The incidence of spermatozoa with nullisomy?disomy and diploid for the sex chromosomes of ?-1 was significantly higher compared to that of IV-1 (P < 0.05). But, compared IV-1 with controls [17-21], the incidence was not significant.
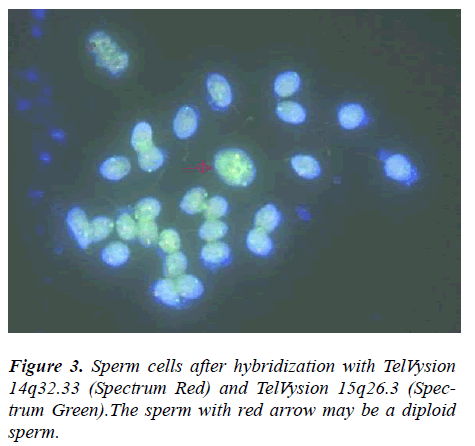
The propositus (IV-1) is healthy and has a balanced chromosomal complement. Assessment of a semen sample from the propositus (IV-1) showed normal sperm number and morphology. Given his karyotype of 44,XY,der(14;15)(q10;q10),der(14;15)(q10;q10), we assumed that the person’s sperm karyotype to be consistently 22,X ,der(14;15)and 22,Y,der(14;15), then, our assumption was proved by this research. From Table 2 and 3, we can see most of sperms of IV-1 are balanced haploid (not normal haploid). From Figure 3, we can see a sperm (with arrow) has four fluorescence signals (two red and two green). This means the sperm has two derivative chromosomes of der(14;15), but we can not tell the sperm is disomy of derivative chromosome or diploid. In our future research, we will employ tripl-color FISH (for example: the probe mixture consist of chromosomes 14?15 and 18) for the detection of normal/balanced or unbalanced sperm to distinguish between disomy and diploid.
Rob rearrangements are common chromosomal changes that can lead to rapid and efficient reproductive isolation between karyotypically similar populations, especially when many rob metacentric chromosomes display monobrachial homologies [27]. In the case of Muntjac, little or no measurable genetic or morphological differences have been found [28].
Homozygosity for Rob translocations in man has been described before. A fetus with two t(14;21) chromosomes was found by Dallapiccola et al. [29]. The related parents were heterozygous for the same translocation. Martinez et al. [30] described three adult sibs homozygous for t(13;14). Their parents were first-cousins and both were heterozygous carriers. Rajangam et al. [31] found a unique DS karyotype 45, XY, der(14;21)pat, der(14;21)- mat, +21mat. Whereas translocation heterozygosity is associated with meiotic disturbances that cause infertility and subfecundity. Translocation homozygosity should not have any effect on meiosis, at least in theory [30].
In conclusion, this present work supports that alternate segregation is largely dominant over adjacent segregations in spermatozoa from Rob translocation carriers. Furthermore, the higher incidences of aneuploidy for sex chromosomes in spermatozoa found in Rob translocation carriers indicated that the ICE on sex chromosome is likely in some male carriers of Rob translocations.
Taking into consideration relatively high levels of unbalanced sperm and unbalanced embryos, PGD is justified for Rob translocation carriers to reduce the risk of miscarriage and to increase the chances of pregnancy achievement [32-35].
Since the propositus is phenotypically normal with normal fertility, we consider the chromosomal rearrangement of the person to be a balanced polymorphism [36].The aberration can provide material for evolution. The establishment of a new human subspecies with a diploid complement of 44 chromosomes could occur if a small population with the karyotype of the propositus undergoes long-term reproductive isolation [37].
Acknowledgments
We thank Barry Starr and John M. Opitz for their help. This work was supported by a grant 81102141 from National Science Foundation of China
References
- Therman E, Susman B, Denniston C. The nonrandom participation of human acrocentric chromosomes in Robertsonian translocations. Ann Hum Genet 1989; 53: 49-65.
- Luciani JM, Guichaoua MR, Mattei A, Morazzani MR. Pachytene analysis of a man with a 13q;14q transloca- tion and infertility. Cytogenet Cel Genet 1984; 38: 14-22.
- Nino Guy Cassuto, Nathalie Le Foll, Sandra Chantot- Bastaraud, Balet R, Bouret D, Rouen A,et al. Sperm fluorescence in situ hybridization study in nine men carrying a Robertsonian or a reciprocal translocation: relationship between segregation modes and high- magnification sperm morphology examination. Fertil Steri 2011; 96 : 826-832.
- Sybenga J. Chromosome structural variants. Amster- dam: North-Holland Publishing Company 1975; p. 165-212.
- Roux C, Tripogney C, Morel F, Joanne C, Fellmann F, Clavequin MC, et al. Segregation of chromosomes in sperm of Robertsonian translocation carriers. Cytoge- net Genome Res 2005; 111: 291-296.
- Rives N, Ravel C, Duchesne V, Siffroi JP, Mousset- Simeon N, Mace B. Molecular cytogenetics analysis with whole chromosome paint probes of sperm nuclei from a (13;15) Robertsonian translocation carrier. J Hum Genet 2005; 50: 360-364.
- Anahory T, Hamamah S, Andreo B, Hedon B, Claus- tres M, Sarda P, et al. Sperm segregation analysis of a (13;22) Robertsonian translocation carrier by FISH: a comparison of locus-specific probe and whole chromo- some painting. Hum Reprod 2005; 20: 1850-1854.
- Moradkhani K, Puechberty J, Bhatt S, Vago P, Janny L, Lefort G, et al. Meiotic segregation of rare Robertso- nian translocations: sperm analysis of three t(14q;22q) cases. Hum Reprod 2006; 21: 1166-1171.
- Anton E, Vidal F, Blanco J. Role of sperm FISH stu- dies in the genetic reproductive advice of structural re-organization carriers. Hum Reprod 2007; 22: 2088-2092.
- Liu Y, Zhu H. Detection of sperm chromosomes in Robertsonian translocation carriers by dual-color fluo- rescence in situ hybridization. Zhonghua Nan Ke Xue 2004; 10: 90-93.
- Lejeune J. Autosomal disorders. Pediatrics 1963; 32:326-337.
- Douet-Guilbert N, Bris MJ, Amice V, Marchetti C, Delobel B, Amice J, et al. Interchromosomal effect in sperm of males with translocations: report of 6 cases and review of the literature. Int J Androl 2005; 28: 372- 379.
- Ogur G, Van Assche E, Vegetti W, Verheyen G, Tour- naye H, Bonduelle M, et al. Chromosomal segregation in spermatozoa of 14 Robertsonian translocation carri- ers. Mol Hum Reprod 2006; 12: 209-215.
- Estop AM, Cieply K, Munne S, Surti U, Wakim A, Feingold E. Is there an interchromosomal effect in re- ciprocal translocation carriers? Sperm FISH studies. Hum Genet 2000; 106: 517-524.
- Acar H, Yildirim MS, Cora T, Ceylaner S. Evaluation of segregation patterns of 21; 21 Robertsonian translo- cation along with sex chromosomes and interchromo- somal effects in sperm nuclei of carrier by FISH tech-nique. Mol Reprod Dev 2002; 63: 232-236.
- World Health Organisation. WHO laboratory manual for the examination and processing of human semen. 5 ed. Geneva, Switzerland; 2010.
- Nadejda Machev, Philippe Gosset, Stéphanie Warter, Treger M, Schillinger M, Viville S. Fluorescence in si- tu hybridization sperm analysis of six translocation car- riers provides evidence of an interchromosomal effect. Fertility and Sterility 2005; 84 (2): 365-373.
- Mahjoub M, Mehdi M, Brahem S, Elghezal H, Ibala S, Saad A. Chromosomal segregation in spermatozoa of five Robertsonian translocation carriers t(13;14). J As- sist Reprod Genet. 2011; 28: 607-613.
- Rouen A, Pyram K, Pollet-Villard X, Hyon C, Dorna M, Marques S, et al. Simultaneous cell by cell study of both DNA fragmentation and chromosomal segregation in spermatozoa from chromosomal rearrangement car- riers. J Assist Reprod Genet. 2013. doi:10.1007/s10815-012-9915-7.
- Cassuto NG, Foll NL, Chantot-Bastaraud S, Balet R, Bouret D, Rouen A, et al. Sperm fluorescence in situ hybridization study in nine men carrying a Robertso- nian or a reciprocal translocation: relationship between segregation modes and high-magnification sperm mor- phology examination. Fertil Steril. 2011; 96: 826-832.
- Ferfouri F, Selva J, Boitrelle F, Gomes DM, Torre A, AlbertM, et al. The chromosomal risk in sperm from heterozygous Robertsonian translocation carriers is re- lated to the sperm count and the translocation type. Fertil Steril. 2011; 96: 1337-1343.
- Benet J, Oliver-Bonet M, Cifuentes P, Templado C, Navarro J. Segregation of chromosomes in sperm of reciprocal translocation carriers: a review. Cytogenet Genome Res 2005; 111: 281-290.
- Luciani JM, Guichaoua MR, Mattei A, Morazzani MR. Pachytene analysis of a man with a 13q;14q transloca- tion and infertility. Behavior of the trivalent and non- random association with the sex vesicle. Cytogenet Cell Genet 1984; 38: 14-22.
- Navarro J, Vidal F, Benet J, Templado C, Marinas Egozcue J. XY-trivalent association and synaptic ano- malies in a male carrier of a Robertsonian t(13;14) translocation. Hum Reprod 1991; 6: 376-381.
- Morel F, Douet-Guilbert N, Le Bris MJ, Herry A, Amice V, Amice J, et al. Meiotic segregation of trans-locations during male gametogenesis. Int J Androl 2004; 27: 200-212.
- Lindenbaum RH, Hulten M, McDermott A, SeabrightM. The prevalence of translocations in parents of child- ren with regular trisomy 21: a possible interchromo- somal effect? J Med Genet 1985; 22: 24-28.
- Britton-Davidian J, Catalan J, da Graça Ramalhinho M, Ganem G, Auffray JC, Capela R, et al. Rapid chromo- somal evolution in island mice. Nature. 2000; 403: 158.
- Wang W, Lan H, Rapid and parallel chromosomal number reductions in muntjac deer inferred from mito- chondrial DNA phylogeny. Mol Biol Evol. 2000; 17: 1326-1333.
- Dallapiccola B, Ferranti G, Altissimi D, Colloridi F,Paesano R, First-trimester prenatal diagnosis of homo- zygous (14;21) translocation in a fetus with 44 chro- mosomes. Prenat Diagn. 1989; 9: 555-558.
- Martinez-Castro P, Ramos MC, Rey JA, Benitez J, Sanchez Cascos A. Homozygosity for a Robertsonian- translocation (13q14q) in three offspring of heterozyg- ous parents. Cytogenet. Cell Genet. 1984; 38: 310-312.
- Sayee Rajangam, Ron C. Michaelis, GopalRao V.N. Velagaleti, Shavanthi Lincoln, Sridevi Hegde, Sanjeev Lewin, et al. Down Syndrome With Biparental Inherit- ance of der(14q21q) and Maternally Derived Trisomy 21: Confirmation by Fluorescent In Situ Hybridization and Microsatellite Polymorphism Analysis. Am J Med Genet Part A. 1997; 70: 43-47.
- Bint SM, Makie Ogilvie C, Flinter FA, Khalaf Y, Scri- ven PN. Meiotic segregation of Robertsonian transloca- tions ascertained in cleavage-stage embryos— implications for preimplantation genetic diagnosis. Hum Reprod. 2011: 1-10.
- Bernicot I, Schneider A, Mace A, Hamamah S, Hedon B, Pellestor F, et al. Analysis using fish of sperm and embryos from two carriers of rare rob(13;21) and rob(15;22) robertsonian translocation undergoing PGD. Eur J Med Genet. 2012; 55: 245-251.
- Fischer J, Colls P, Escudero T, Munne S. Preimplanta- tion genetic diagnosis (PGD) improves pregnancy out- come for translocation carriers with a history of recur- rent losses. Fertil Steril 2010; 94: 283-289.
- Jin H, Ping L, Jie Q, Ying L, Jongjian C. Translocation chromosome karyotypes of the Robertsonian transloca- tion carriers’ embryos. Fertil Steril. 2010; 93: 1061-1065.
- John B, Chromosome change and evolutionary change: A critique. In: Atchley WR, Woodruff DS, editors. Evolution and specification: Essays in honor of M.J.D. White, Chapter 2. London, 1981, New York: Cam- bridge University.
- Bo Wang, Yanzhi Xia, Jieping Song, Weipeng Wang, Yanping Tang. Potential Speciation in Humans Involv- ing Robertsonian Translocations. Biomed Res 2013; 24: 171-174