- Biomedical Research (2014) Volume 25, Issue 3
An experimental Study on the mechanism for IL-8 promoting migration of A549 cells.
Jiang Yuequan*, Cai Huarong, Zhou Hong, Zhang ZhiDepartment of Thoracic Surgery, Chongqing Cancer Institute, Sha Ping Ba district, Han Yu Street 181#, Chongqing 400030, China
- *Corresponding Author:
- Jiang Yuequan
Department of Thoracic Surgery Chongqing Cancer Institute
Sha Ping Ba District
Han Yu Street 181#, Chongqing 400030 China
Accepted March 17 2014
Abstract
The effect and mechanism of IL-8 on A549 cells migration were investigated. The appropri-ate concentration of IL-8 was chosen by MTT assay. The Western-blot assay, Scratch test and Transwell assay were used in this study to analyse the effect and mechanism of IL-8 on lung adenocarcinoma A549 cells migration. The results of Western-blot assay showed that: (1) IL-8 can promote the expression of MMP-2 protein and has no effect on the expression of MMP-9. (2) IL-8 can promote the expression of JNK/SAPK phosphorylated protein. (3) In-hibitor (SP600125) can block the effect of IL-8 on the expression of MMP-2 protein. Scratch test proved from the opposite side that the low expression of MMP-2 could inhibit the mi-gration of A549 cells. These results suggest that IL-8 can regulate MMP-2 protein expression through the JNK/SAPK signaling pathway and further promote the migration of lung ade-nocarcinoma A549 cells.
Keywords
.IL-8; MMPs; SP600125; A549 cell; cancer cell migration
Introduction
Lung cancer is one of the most common malignancies. The early invasion and metastasis of tumor cells is the major obstacle to achieving successful lung cancer treat- ment. It is also the most important reason for the death of patients with lung cancer [1]. Early invasion and metasta- sis of tumor cells are associated with the degradation and destruction of extracellular matrix and basement mem- brane, which requires the involvement of the correspond- ing lytic enzyme. During the matrix metalloproteinase (MMPs) family members [2,3], especially MMP-2 and MMP-9 play an important role. Interleukin-8 (IL-8) is a glycoprotein with molecular weight of about 8KD, which was extracted by Yoshimural from mononuclear cell cul- ture medium stimulated with lipopolysaccharide (LPS) and phytohemagglutin in (PHA) [4]. As an inflammatory factor, IL -8 can be involved in the immune and inflam- matory responses. In addition, IL-8 hasa chemotactic effect that can promote cell migration. A growing number of studies have showed that IL-8 is closely related to tu- mor cell migration. Luca et al. [5] found that IL-8 can up regulate the expression of MMP-2 in human melanoma cells and further promote cell migration. Wang et al. [6]. Reported that IL-8 secreted by ovarian cancer cells pro- motes malignant behavior of these cells via inducing in- tracellular molecular signaling. Rafrafi A et al. [7] also demonstrated that the IL-8 promoter polymorphism is associated with NSCLC risk. Although there are many studies showed that IL-8 is related to tumor cell migration, the effect and mechanism of IL-8 on lung cancer cell mi- gration is unclear. Therefore, in this study the specific effects and possible mechanism of IL-8 on lung cancer cell migration were investigated. This may provide valua- ble experimental data for the clinical treatment of lung cancer.
Material and Methods
Materials
Main reagents
Recombinant human interleukin-8 (IL-8) was from Hang- zhou Sijiqing Biological Engineering Materials Co, Ltd.
(China). Anti-MMP-2 antibody, anti-MMP-9 antibody, anti-Phospho-JNK/SAPK (c-Jun NH2-terminal kinase/-
stress-activated protein kinase) antibody and anti-β-actin
antibody were obtained from Beyotime Institute of Bio- technology (China). C-JNK inhibitor (SP600125) was purchased from Invitrogen (USA).
Cell culture
Human lung adenocarcinoma A549 cell line was obtained from the Cell Bank of Institute of Life Sciences, Chong- qing Medical University. RPMI-1640 medium containing 10% inactivated FBS, 100 U/ml penicillin and 100 mg/L streptomycin was cultured at 37°C in a 5 % CO2 incubator. Cells were digested with 0.25% trypsin (containing 0.02% EDTA) and passaged every 2 to 3 days. Cells in logarith- mic growth phase were used.
Methods
Cell growth experiments
A549 cells in logarithmic growth phase were seeded in the 96-well plate (1.0×104 cells/well) and incubated for 24 hours. In each experimental group, 120 µl different con- centrations of IL-8 (25, 50, 100, 500 µg/L) were added respectively. The same volume of normal medium was added in the control group. To each well was then added 20 µl of 5 mg/ml MTT and 80 µl of normal medium at 37ºC to culture for 3 hours. Then to each well was added with 100 µl of DMSO, and shaken at the shaking table for 10 minutes until the blue crystal was completely dis- solved. The absorbance value (D) was measured at 492 nm using an ELISA reader. Each group was given five wells, and the experiment was done in triplicate. The ap- propriate concentration of IL-8 was evaluated by cell via- bility. The value of cell viability was calculated by the following formula: cell viability value=(average D value of experimental group/average D value of control group)× 100 %.
Scratch test
A549 cells in logarithmic growth phase were seeded in the 24-well plate at 1.0×105 cells/well. After the cells grow up to 80%~90%, 10 µl of sterile pipette was used to draw a straight line in the center of each well. To each well was added 400 µl IL-8 of different concentrations prepared by serum-free medium as the experimental group (40 µl serum-free medium was added alone as the control group). Both the experimental and control groups were then incubated for 24 h. Under TE2000-U invert fluorescence microscope (Nikon, Japan), images were taken to observe the remaining distance after the cells in each group migrated from the scratch edge to the scratch center.
Transwell migration assay
The logarithmic growth phase A549 cell were taken to adjust cell concentration with serum-free medium to 1.0×105 cells/ml, and inoculated at 100 µl/well in the upper Transwell chamber (polycarbonate membrane with a pore size of 8µm). Six hundred µl of IL-8 at different concentrations was added in the lower Transwell chamber as experimental group (600 µl of normal medium was added as the control group) and cultured at 37ºC for 24 hours. The cells on the top of filter membrane were re- moved with a cotton swab and washed with PBS twice. The upper chamber was placed in 4% paraformaldehyde fixed for 15 minutes, and stained with hematoxylin for 20 minutes. Under TE2000-U inverted fluorescence micro- scope (Nikon, Japan), the averaged numbers of cells pene- trating the membrane were measured from 5 different fields of view.
Western blot assay
The cells of each experimental group were collected, cen- trifuged at 1000 r/min for 10 minutes, lyzed with CST ly- sate, and centrifuged at 4ºC at 12000 r/min for 15 minutes. The supernatants were collected to measure protein con- centration with BCA method. Protein samples were diluted with 5×loading buffer and boiled in water for 10 minutes at 100°C. Polyacrylamide gel electrophoresis (carrying 70 µg protein) was conducted, and the membrane was translocat- ed with 0.45 µm PVDF membrane and sealed with 5% defatted dry milk for 1 hour. The primary antibodies of MMP-2 (1:800), MMP-9 (1:800), p-JNK/SAPK (1:1000) and ß-actin (1:300) were incubated respectively at 4ºC overnight and rinsed with PBS three times. The horseradish peroxidase conjugated secondary antibodies (1: 2500) were incubated for 1 hour and rinsed. The results were analyzed with ECL (Electrochemiluminescence) reagent under ChemiScope 2650 Fluorescence/Chemiluminescence imag- ing system (Bio-Rad, USA).
Statistical analysis
SPSS12.0 software was used for statistical analysis. Val- ues were expressed as mean±SD. The results were com- pared by t test analysis between the two groups. P values of < 0.05 were regarded as significant.
Results
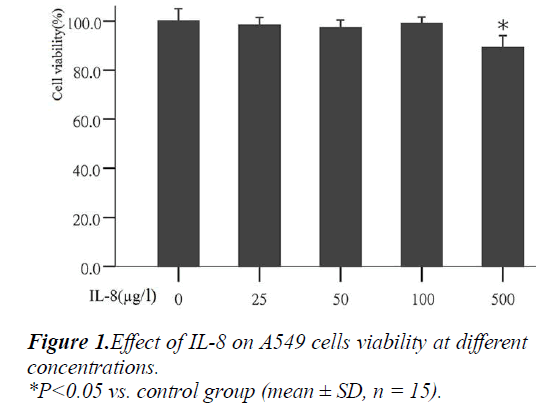
Effect of IL-8 on A549 cell growth
To determine the most appropriate concentration of IL- 8on cells in the experiment, the effect of different concen- trations of IL-8 on cell growth was analyzed first. The results showed that after the action with different concen- trations of IL-8 (25, 50, 100 and 500μg/L) for 24 hours, the mean values of cell vitality were: 98.18%, 97.24%, 99.05% and 89.55% respectively (Fig. 1). Compared with the control group, IL-8 at concentration of 25, 50 and 100 μg/l had no significant effects on cell growth, but at con- centration of 500g/l can significantly inhibited cell growth (P<0.05). Therefore, the three different concentrations of IL-8 (25, 50, 100 μg/L) were used in the following exper- iments.
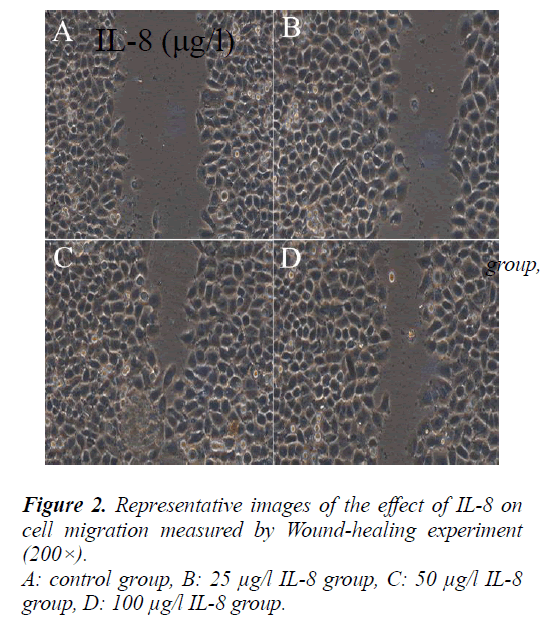
Effect of IL-8 on A549 cell migration
To evaluate the effect of IL-8 on A549 cell migration, a straight line were scratched in the center of each well seeded the cells and treated with different concentrations of IL-8 (25, 50, 100 μg/l) for 24 hours. After the cells in the control group and different IL-8 concentrations group migrated from the scratch edge to the scratch center. The remaining distances were (13 ± 1.95) mm, (7.3 ± 1.1) mm, (3.3 ± 0.5) mm and (4.8 ± 0.72) mm respectively.
Compared with the control group, the migration distance of cells in different IL-8 concentrations group from the scratch edge to the scratch center increased significantly (P<0.05).
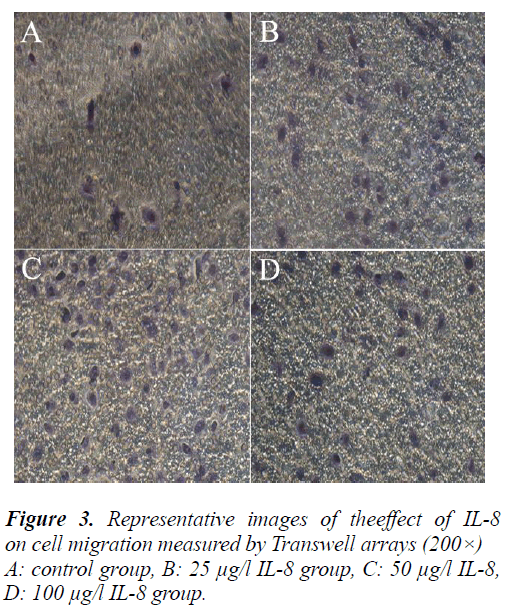
To further validate the effect of IL-8 on the migration of A549 cell, the cells were treated by the same method and determined with Transwell migration assay. Finally, the average counts of cells penetrating the Transwell chamber in the control group and different concentrations in the IL-8 group were 12 ± 1.8, 48 ± 7.2, 108 ± 16.2, 60 ± 9 cells/field respectively. Compared with the control group, the counts of cells penetrating the Transwell chamber in different concentrations of IL-8 increased significantly (P<0.05). This result was consistent with the result of the scratch test and further demonstrates that IL-8 can pro- mote the migration of A549 cells.
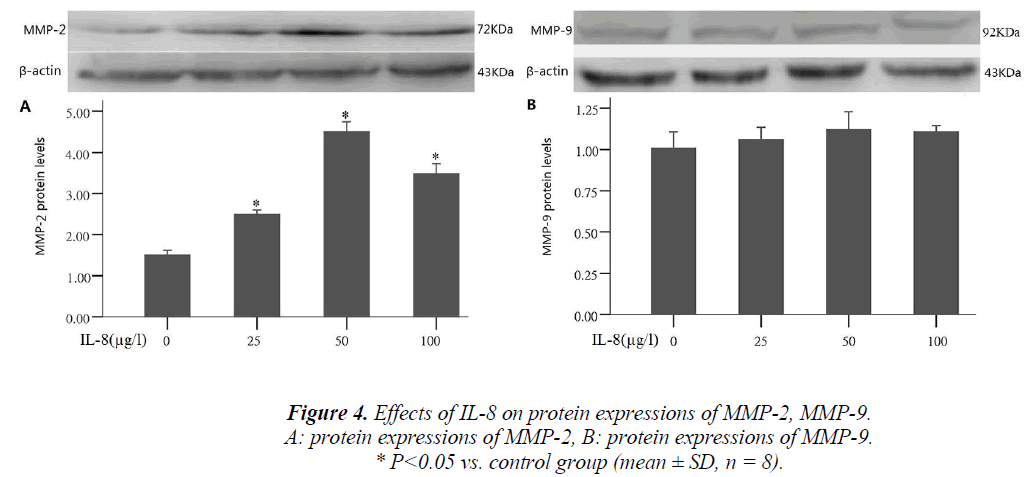
Effect of IL-8 on MMP-2 and MMP-9 protein expression
Tumor cells producing hydrolase is an important step in the process of tumor invasion and metastasis. During the process, MMP-2 and MMP-9 were often highly expressed, and could promote tumor cell migration by extracellular matrix degradation. To study the mechanism of IL-8 in- ducing A549 cell migration, the cells were treated with different concentrations of IL-8 (25, 50, 100 μg/l) for 24 hours, and the proteins were collected to detect MMP-2 and MMP-9. After treatment by different concentrations of IL-8, compared with the control group, MMP-2 ex- pression significantly increased 1.6, 3.1 and 2.4 times that of the control group respectively (P <0.05), and the results were consistent with those of IL-8 on A549 cell migration. By comparing different IL-8 concentrations group with the control group, MMP-9 expression was shown to have no significant change (Fig. 4)
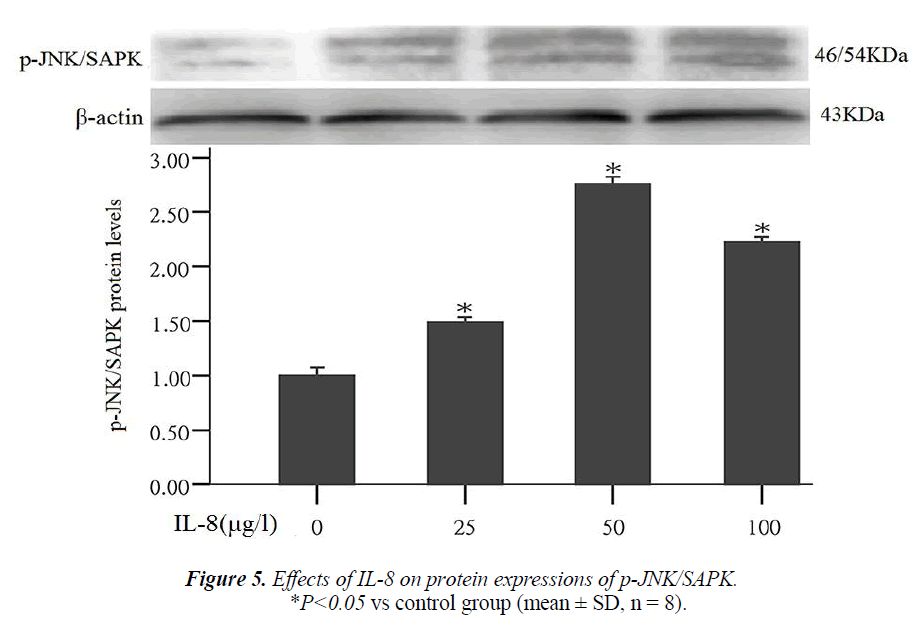
Effect of IL-8 on phosphorylated protein expression in signal pathway JNK/SAPK
Studies have shown that the nuclear transcription factor activator protein-1 (AP-1) can regulate the expression of MMPs. JNK/SAPK is an upstream signal of AP-1. This experiment also studied that whether JNK/SAPK can reg- ulate the expression of MMPs. After the cells were treated by different concentrations of IL-8 (25, 50, 100 μg/l) for 24 hours, the results of Western blot assay showed that compared with the control group, JNK/SAPK phosphory- lated protein expression significantly increased 1.5, 2.8 and 1.9 times that of the control group respectively (P < 0.05) (Fig. 5).
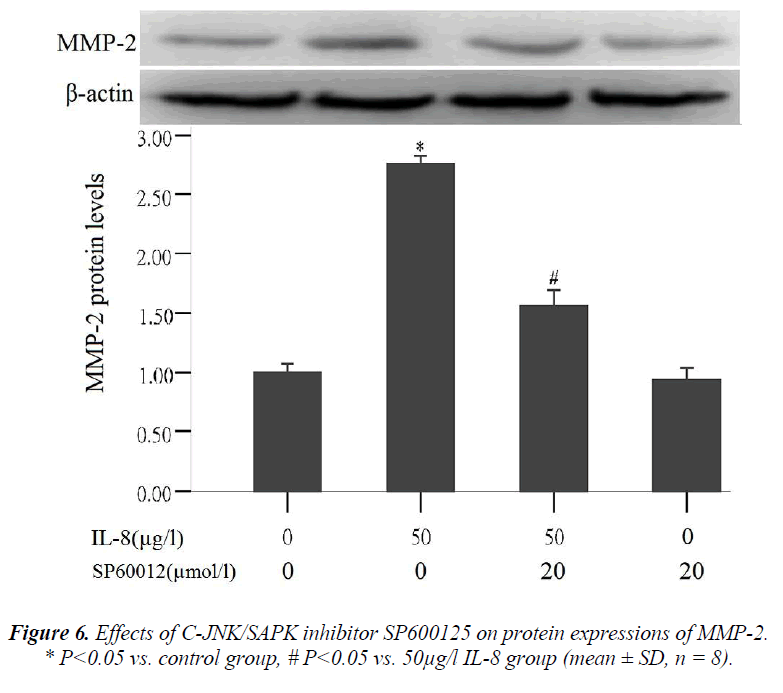
Effect of C-JNK/SAPK inhibitor (SP600125) on MMP-2 protein expression
To further validate that signal pathway JNK/SAPK can regulate MMP-2 expression, the cells were treated in the following way: (1) the cells were not treated with any reagent in the control group, (2) treated with 50 μg/l of IL-8 for 24 hours in 50 μg/l IL-8 group, (3) pre- treated with 20 μmol/l of C-JNK/SAPK signal pathway inhibitors (SP600125) for 1 hour and then treated with IL-8 (50 μg/l) for 24 hours in C-JNK/SAPK inhibitor (SP600125)
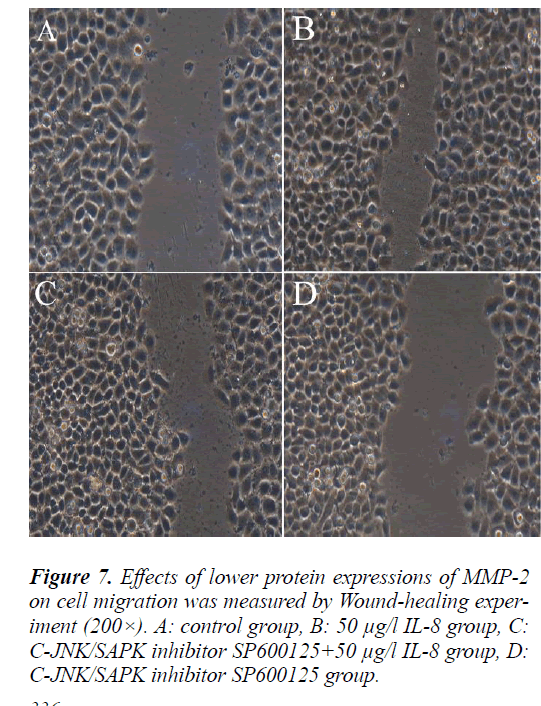
Effect of MMP-2 protein expression on lung adenocar- cinoma A549 cell migration
To further validate the relationship between MMP-2 pro- tein expression and lung adenocarcinoma A549 cell mi- gration, the cells were treated in the same way as above and followed with scratch test. The scratch test results showed that the cells in each group migrated from the scratch edge to the scratch center.The remaining distance was (10.5±1.575) mm, (4±0.6) mm, (7.5±1.125) mm and (12±1.8) mm respectively. Compared with MMP-2 pro- tein high expression group (50 μg/l IL-8 group), MMP-2 protein low expression group (C-JNK/SAPK inhibitor SP600125+50 μg/l IL-8 group) can significantly inhibit the migration of A549 cells (P<0.05).
In the tests about effect of IL-8 on MMP-2 and MMP-9 protein expression and on phosphorylated protein expres- sion in signal pathway JNK/SAPK, it was the 50µ g/l of IL-8 that always produced the highest results.In the later tests about effect of C-JNK/SAPK inhibitor (SP600125) on MMP-2 protein expression and effect of MMP-2 pro- tein expression on lung adenocarcinoma A549 cell migra- tion, only the 50µ g/l of IL-8were used.
Discussion
In recent years, the incidence of lung cancer has increased significantly in China. Among all the cancers leading to death, lung cancer ranks first in the mortality rate. Early tumor cell invasion and metastasis is the main cause of death for cancer patients. Therefore, investigating the mechanism of tumor invasion and metastasis will contrib- ute to the treatment of tumors. Thisstudy demonstrated that IL-8 might promote the migration of lung adenocar- cinoma A549 cells through regulating MMP-2 expression in signal pathway JNK/SAPK.
During tumor growth and progression, a variety of cells like lymphocytes and neutrophils in the tumor tissues and its surroundings can secrete IL-8. Several studies had shown that IL-8 is associated with tumor angiogenesis, invasion and metastasis. For example, Desai S et al. [8] reported that IL-8/VEGF induced JNK/p38-ATF-2 as a novel pro-invasive pathway, which may be explored as potential therapeutic target to circumvent the invasiveness of lung malignancies. Lin et al [9] reported that the high expression of IL-8 in breast cancer cells was highly relat- ed to tumor invasion and angiogenesis. Chen et al [10] re- ported that the high IL-8 mRNA expression in non-small cell lung cancer was highly correlated with angiogenesis. However, the effect of IL-8 on lung cancer cell migration is still unclear.
Tumor metastasis is a complex multi-step process, in which one of the most critical steps is the degradation of extracellular matrix. In this study, it was found that IL-8 could promote lung cancer cell migration.
For MMP expression mechanism, Storz [12] believed that oxidative stress can promote MMP expression by activat- ing Ras or the MAPK family members (ERK1/2, P38, JNK). Takagi et al [13] indicated that the activation of AP- 1 signal pathway could promote MMP expression. For IL- 8 up-regulating MMP expression mechanism, Reich, et al [14] reported that IL-8 could induce MMP-2 production by activating phospholipase D (PLD)-mediated signal trans- duction pathway. However, Luca et al [5] held that IL-8 could promote the combination of MMP-2 promoter and chloramphenicol acetyltransferae (CAT) gene, thus en- hancing the expression of MMP-2. Based on the previous studies, we investigated the effect of IL-8 on JNK/SAPK signal pathway in MAPK family, and the results con- firmed that IL-8 could regulate MMP-2 expression by increasing JNK/SAPK phosphorylation. JNK/SAPK sig- nal pathway is one of the MAPK signal transduction pathways. The effect of other MAPK signal transduction pathways like ERK1/2 and P38MAPK signal transduction pathways on IL-8 induced MMP-2 expression shall be studied further.
IL -8 is both a chemotactic factor and a pro-angiogenic factor. The results of several studies showed that IL-8 can promote tumor angiogenesis, invasion and metastasis [10,15]. The current study showed that anti-IL-8 monoclonal antibody could inhibit growth, angiogenesis and metasta- sis of melanomain confirmation of a previous study by Huang et al. [16]. Anti-IL-8 monoclonal antibody can also inhibit tumor growth of bladder-transplantedcells by down-regulating the expression of MMP and NF-κB [17]. Although there are still many unresolved problems in the study of IL-8 as the therapeutic target, investigating the mechanism of IL-8 on lung cancer might provide a new target for comprehensive treatment of lung cancer.
References
- Wardwell NR and Massion PP. Novel strategies for the early detection and prevention of lung cancer. Semi- nOncol 2005; 32: 259-268.
- Sato H, Takino T, Okada Y, et al. A matrix metallopro-teinase expressed on the surface of invasive tumor cells. Nature 1994; 370: 61-65.
- Deryugina EI and Quigley JP. Matrix metalloproteinas- es and tumor metastasis. Cancer Metastasis Rev 2006; 25: 9-34.
- Baggiolini M and Clark-Lewis I:. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett1992; 307: 97-101.
- Luca M, Huang S, Gershenwald JE, et al. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metas-tasis. Am J Pathol1997; 151: 1105-1113.
- Wang Y, Xu RC, Zhang XL, et al. Interleukin-8 secre- tion by ovarian cancer cells increases anchorage- independent growth, proliferation, angiogenic potential, adhesion and invasion.Cytokine 2012; 59: 145-155.
- Rafrafi A, Chahed B, Kaabachi S, et al. Association of IL-8 gene polymorphisms with non small cell lung can- cer in Tunisia: A case control study. Hum Immunol 2013; 74: 1368-1374.
- Desai S, Laskar S and Pandey BN.Autocrine IL-8 and VEGF mediate epithelial-mesenchymal transition and invasiveness via p38/JNK-ATF-2 signalling in A549 lung cancer cells. Cell Signal 2013; 25: 1780-1791.
- Lin Y, Huang R, Chen L, et al. Identification of inter- leukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer 2004; 109: 507-515.
- Chen JJ, Yao PL, Yuan A, et al. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages:its correlation with tumor angiogenesis and patient surviv- al in non-small cell lung cancer. Clin Cancer Res 2003; 9: 729-737.
- Cheng GJ, Ling L, Fu-RL, et al. Down regulation of connective tissue growth factor inhibits the growth and invasion of gastric cancer cells and attenuates peritone- al dissemination. Molecular Cancer2011; 10: 122-128.
- Storz P. Reactive oxygen species in tumor progression Front Biosci2005; 10: 1881-1896.
- Takagi S, Simizu S, Osada H. RECK negatively regu- lates matrix metalloproteinase-9 transcription. Cancer Res 2009; 69: 1502-1508.
- Eich R, Blumenthal M, Liscovitch M. Role of phospho- lipase D in laminin-induced production of gelatinase A (MMP-2) in metastatic cells. Clin Exp Metastasis 1995; 13: 134-140.
- Chen Y, Ying L, Mei-SC, et al. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer 2007; 121: 1949-1957.
- Huang S, Mills L, Mian B, et al. Fully humanized neu- tralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol 2002; 161: 125-134.
- Mian BM, Dinney CP, Bermejo CE, et al. Fully human anti-interleukin-8 antibody inhibits tumor growth in or- thotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res 2003; 9: 3167-3175.