- Biomedical Research (2016) Volume 27, Issue 3
Age-related EMG responses of the biceps brachii muscle of young adults.
Nizam Uddin Ahamed1*, Mahdi Alqahtani2, Omar Altwijri2, Matiur Rahman3, Kenneth Sundaraj41Department of Mechatronic Engineering, Faculty of Manufacturing Engineering, University Malaysia Pahang, Pekan-26600, Pahang, Malaysia
2Biomedical Technology Department, College of Applied Medical Sciences, King Saud University, Kingdom of Saudi Arabia
3College of Computer and Information System, Najran University, Kingdom of Saudi Arabia
4Department of Electronics & Computer Engineering Technology, Universiti Teknikal Malaysia Melaka (UTeM), Melaka, Malaysia
- *Corresponding Author:
- Nizam Uddin Ahamed
Department of Mechatronic Engineering
University Malaysia Pahang
Malaysia
Accepted date: February 28, 2016
Abstract
Although the effect of an Electromyographic (EMG) signal on the Biceps Brachii (BB) muscle is at the forefront of human movement analysis, there is limited information regarding the importance of the differences in the age-related EMG responses during contraction. The present study aimed to compare the BB muscle activity of three different groups of young adults divided based on age and to find a relationship between surface EMG and endurance time during isometric contraction. The EMG signal was recorded in 30 healthy right-arm-dominant young male subjects during a handgrip force task. The subjects were rationally divided into one of the three age groups (ten in each group): adolescents (‘A’; aged 17.3 ± 1.4 years), vicenarians (‘V’; 24.6 ± 2.1 years), and tricenarians (‘T’; 33.2 ± 1.1 years). The muscle activation during contraction was determined as the root mean square (RMS) EMG signal normalised to the peak RMS EMG signal during a 10-s isometric contraction. The statistical analysis included linear regression to examine the relationship between the EMG amplitude and the endurance time based on five levels of contraction [60%, 70%, 80%, 90% and 100% of the maximal voluntary contraction (MVC)], repeated measures ANOVA to assess differences among the different age groups and the coefficient of variation (CoV) to investigate the steadiness of the EMG activation. The result shows that the early age groups exhibit higher and steadier muscle activity (V: 3.65 ± 0.42 mV, 11.46% and A: 3.12 ± 0.29 mV, 9.29%) compared with the elderly subjects (T: 2.78 ± 0.33 mV, 11.98%). The most important finding is that the linear slope coefficient for the EMG (amplitude) as a function of time for the muscle of the ‘V’ group (r2=0.591, P<0.05) was significantly greater compared those found for the muscles of the ‘T’ (r2=0.152, P>0.05) and ‘A’ groups (r2=0.203, P > 0.05). The results obtained in this study can be used to improve the current understanding of the mechanics and muscle functions of the BB muscle of individuals from different age groups during isometric contraction.
Keywords
EMG, Signal, Age, Contraction, Biceps brachii.
Introduction
The electromyographic (EMG) signal measures the electrical currents that are generated in skeletal muscle during its contraction and represents the neuromuscular activities [1]. Surface EMG is painless, non-invasive, easy to record and always stochastic (random) in nature [2]. The analysis of the amplitude and frequency of surface EMG signals has been used for decades to reveal the exact muscle function during human movement, rehabilitation and exercise [3-5]. In this study, the EMG signal amplitude was recorded and analysed from the dominant biceps brachii (BB) muscle because this is one of the active skeletal muscles in the upper extremity and because EMG signals are repeatedly generated by this muscle during contraction due to the elbow flexion-relaxation phenomenon [6]. Moreover, the BB muscle is one of the longest arm muscles, can easily be activated with good control and shows maximum recruitment of motor units during its contraction. In addition, this muscle is a typical fusiform muscle (with a spindle-like shape that is wide in the middle and tapers at both ends) and has muscle fibres that run parallel to each other as well as to the skin, and it is also easy to locate the fibre direction during electrode placement [7-9]. As a result, the random raw and integrated EMG signals from the contracted BB muscle need to be recorded for further analysis, which may help researchers clarify the functional and clinical significance of the skeletal muscle architecture, develop robotic prosthesis, control exoskeleton robots, design an orthosis system for neuromotor rehabilitation and select the exact subject during experimentation [10,11].
Many studies have attempted to analyse the age-related differences in the EMG activity of different muscles. However, the effect of age on the surface EMG generated by the muscle of young adults remains unclear and has become an important topic of research. A study conducted almost 40 years ago showed the necessity of understanding the influence of age on EMG activity during contraction [12]. Thus, in recent years, there has been increasing interest in studying the changes in the muscle activity of young adults belonging to different age groups through the analysis of surface EMG during voluntary contractions [13]. Previous studies have provided several findings regarding the age-related EMG responses of different muscles using experimental protocols, feature analysis and other factors to compute the signal characteristics. However, these studies mainly focused on comparisons between young and old subjects. For example, Arjunan et al., observed an agerelated reduction in the complexity of the EMG signal of the BB muscle with an increase in age, i.e., in young (20-29 years of age) compared with older subjects (61-69 years of age) [14]. Another study comparing older (mean age 67.2 years) and young (mean age 26.5 years) adults raised concerns regarding the effects of age on the EMG generated by mastication muscles [15]. More recently, Gerasimova et al., analysed the surface EMG signal during graded isometric contraction generated by the load holding task (changing loads) from birth to old age (from 1-month-old to 40-year-old subjects) [16]. Merletti et al. studied the EMG signal to identify muscle fatigue during voluntary isometric contractions of the BB muscle in a group of elderly (67-86 years) and young (23-34 years) male subjects [17]. Plow et al., examined the EMG activity of the non-dominant BB muscle to understand ageinduced weakness between young and old subjects [18]. A previous study compared the RMS EMG activities of the biceps brachii, brachioradialis, and triceps brachii muscles during isometric and dynamic contraction between young adults (adolescents: 18 years; vicenarians: 26 years) and old subjects (66-78 years) [19]. In all of the above-mentioned studies, the researchers used different features or variables to analyse and compare the EMG activity, such as the averagerectified value (ARV), mean absolute value (MAV), root mean square (RMS), integrated EMG (IEMG), median frequency, normalized spectral moments, wavelet transforms, increase in synchronization (IIS) index, and fractal dimension.
However, although a number of studies have identified and characterized the muscle activity between different age groups, no study has compared the differences in the EMG activities between early-adults and mid-adults (i.e., adolescents, vicenarians and tricenarians) through RMS analysis to determine whether the EMG amplitude during voluntary isometric (static) contraction decreases or increases with age. Therefore, a primary objective of this study was to compare the EMG activities during isometric contraction between young adults belonging to three young-age groups. A secondary aim was to compare the relationship (using regression analysis) between the EMG signal and time lags, and the third objective was to determine the steadiness of the EMG signal generated by the muscle based on the coefficient of variation [CoV]. We hypothesized that younger adults (adolescents and vicenarians) would exhibit better muscle activity compared with older (tricenarians) adults.
Thus, these gaps in the present research motivate us to answer several interrelated research questions. Therefore, the study aims were the following: (1) to investigate and compare the EMG activities, (2) to examine the relationship between changes in time lags and the EMG (amplitude), and (3) to quantify the steadiness of the signal. All of these assessments were performed using the following common platforms: i) three different young age groups (‘A’, ‘V’ and ‘T’), ii) dominant biceps brachii muscle, iii) during isometric contractions, and iv) normalized RMS amplitude values of the EMG.
Methodology
Subjects
Thirty healthy male subjects participated in this study. All of the subjects signed an informed consent form prior to the experimental procedures, as required by the Declaration of Helsinki. The thirty subjects were then divided into three age groups (adolescents, 17.3 ± 1.4 years; vicenarians, 24.6 ± 2.1 years; and tricenarians, 33.2 ± 1.1 years) because the main objective of the present study was to identify the EMG activities of individuals belonging to different age groups. These groups were selected and divided according to a previously reported definition and classified based on a previous gerontological study: the ages of the adolescents ranged from 13 to 19 years, the ages of the vicenarians ranged from 20 to 29, and the ages of the tricenarians ranged from 30 to 39 years [20-22]. None of the subjects had a history of musculoskeletal pain or injury in their dominant arm (right) or BB muscle. All of the experimental procedures were approved by the Human Research Ethics Committee at the University.
Familiarization
All of the subjects attended an orientation session approximately one day prior to the final experiment. This familiarization session covered the rules of the activity, the testing protocols and process, and a general discussion regarding surface EMG data collection. Finally, the participants were allowed to practice with a hand dynamometer, received information about the trials and objectives of the study and provided informed consent.
Experimental protocols
The subjects were comfortably seated in a specially designed chair and asked to place their right elbow on a padded support with the joint at a right angle. The subjects then performed an isometric contraction using a digital hand grip dynamometer (Digital Hand Dynamometer, SAEHAN Corporation, Korea). An initial adjustment period allowed the subject to become comfortable with performing isometric contractions with the hand dynamometer. The main task was to apply an upwarddirected force with the wrist using the elbow flexor muscles, primarily the BB muscle. The angle of the elbow joint during data recording was 90º. This angle measurement was from the shoulder to the elbow and from the elbow to the palm and was calculated using a goniometer. The force (kg) exerted by the subject was visible on the dynamometer.
To determine the generated maximal voluntary contraction (MVC), the subject was asked to perform three maximal contractions of 10s with a 5 min rest between each effort. The subject was asked to hold the dynamometer in order to produce the maximum contraction force, and the signal from the muscle was then recorded. The force produced (in kg) was displayed on the dynamometer screen, and the participant was encouraged to apply the maximum muscle force and to steadily maintain the force. The average of three readings was considered the MVC. The sub-contractions at 90%, 80%, 70% and 60% of the MVC were calculated from the generated maximum voluntary contraction (100%). For example, if one subject achieved a MVC of 45 kg (100%), he was asked to maintain a force of 40.5 kg, which is 90% of MVC, for 10 s. The average value from three trials for each contraction level was considered. Figure 1 shows a schematic of the protocol used for recording the surface EMG signal during contraction with a subject from the vicenarian age group.
Data acquisition
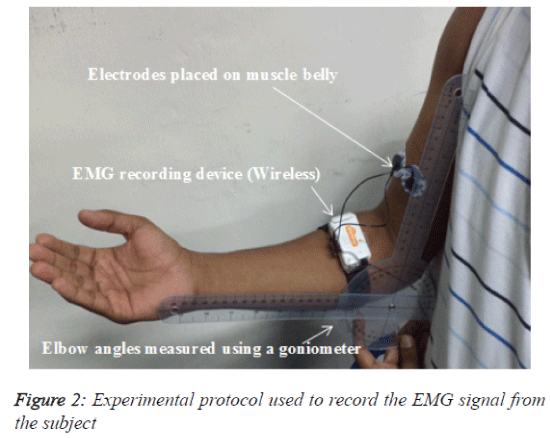
In this study, a wireless, touch-proof and Bluetooth-enabled EMG signal storage device called SHIMMERTM Model SHSHIM- KIT-004 (Real-time Technologies Ltd., Ireland) was used to record the surface EMG signal. This non-invasive device has dual functionality because it has both an IEEE 802.15.4 wireless communication module (contains a Chipcon CC2420 radio transceiver and a giga-Ant 2.4 GHz RufaTM antenna) and a Bluetooth® radio module. The built-in frequency range of the device is 5-482 Hz with an EMG amplifier gain of 682 dB. The two inputs (negative and positive) were used for the EMG recording, and a third was used as the reference channel. Silver-silver chloride (Ag-AgCl) surface electrodes were used to record the EMG signals from the muscle belly and were positioned parallel to the direction of the BB muscle fibres. The belly of the BB muscle was recognized by manual palpation. The centre-to-centre distance between the two sensors was approximately 2 cm (Figure 2). The reference electrode was attached to the lateral epicondyle of the humerus of the right arm (approximately 1 inch from the olecranon of the elbow). Before attaching the electrodes, the skin of the BB was prepared using a skin-cleaning gel (sigma gel) and an alcohol swab.
Data analysis
The raw EMG signals were recorded at a sampling rate of 1 KHz before A-D conversion and stored on a compatible computer. A fourth-order bandpass Butterworth filter was used to remove any skin movement artefacts and high-frequency noise (cutoff frequency between 10 and 500 Hz). The recorded digitized EMG data-sets were processed offline by filtering, windowing, and extraction. The data analysis was performed offline in the MATLAB 2010 software environment (The MathWorks Inc., USA).
Calculated features: The root mean square (RMS) is one of the most commonly accepted features used to describe the signal amplitude of a surface EMG signal. As a result, in this study, the RMS was used to calculate and analyse the surface EMG signals. RMS is the quadratic mean used to statistically investigate the magnitude of a time-varying signal. The mathematical definition of the RMS feature is the following [23,24]:
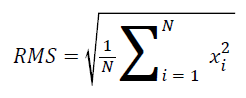
The EMG RMS values were then normalized in terms of the maximum voluntary contraction, i.e., the individual RMS values during the contraction were considered the 100% MVC. The filtered EMG activity was normalized within each subject by dividing the observed EMG value for the BB muscle by the maximum value recorded during the three maximal tests. The mean (RMS) normalized EMG activity was then calculated as the mean of the sum of the normalized EMG percentages from all of the subjects in each age group. The maximum peak-topeak value of the EMG was considered a relative measure of motor activity (the positive portion of the peak is defined as the peak maximum). It should be noted that this normalization procedure only presents information on the level of muscle activity with regard to this peak value (i.e., shape of the EMG pattern). Figure 3 shows a sample waveform obtained during contraction.
Statistical analyses: The statistical analyses were performed using the Minitab™ software (version 13.32). Significant differences in the force and EMG amplitudes (RMS) were detected between the three age groups through repeatedmeasures analysis of variance (ANOVA), and post-hoc tests were applied to test the differences using a significance level of α=0.05 and 95% (P<0.05) confidence intervals for all of the variables. The steadiness of the EMG signal, i.e., the variation in muscle activity, was characterized by the coefficient of variation (i.e., the ratio of the standard deviations divided by the means; coefficient of variation (CoV)=σ/μ). Regression analysis was used to analyse the relationship between the EMG (amplitude) and time (ms) variables after dividing the endurance times into five phases. The null hypothesis of linearity of each regression was tested using the F-test. Finally, each individual dataset from the trial was fit to a linear regression line in the logarithmic form of y=a+bx.
Results
Table 1 summarizes the electromyographic BB muscle activity of the three age groups based on the RMS difference. The information provided in the table also indicates the steadiness of the EMG activation based on the CoV and the linear regression coefficients (r2) based on the F-ratios. Another important result presented in the table concerns the generated average EMG signals for each time lag (between the starting and ending times). The results show that the EMG activity was initially low for all of the age groups; for example, group ‘V’ generated 2.9 ± 0.22 mV during the first 2000 ms. In contrast, the maximum results show that the EMG activity gradually increased until the end of the experimental period; for example; group ‘V’ generated 2.9 ± 0.22 mV during the last 4.1 ± 0.11 ms (at 10000 ms).
| Age groups | Time (ms) | Mean±SD | Total (mV) | CoV (%) | r2 | F-ratio |
|---|---|---|---|---|---|---|
|
‘A’ (n=10) |
2000 | 2.7±0.25 | 3.12±0.29 | 9.29 | 0.203 | 5.86 |
| 4000 | 3.2±0.21 | |||||
| 6000 | 3.3±0.06 | |||||
| 8000 | 3.4±0.11 | |||||
| 10000 | 3.1±0.15 | |||||
|
‘V’ (n=10) |
2000 | 2.9±0.22 | 3.65±0.42 | 11.46 | 0.591* | 33.24 |
| 4000 | 3.8±0.15 | |||||
| 6000 | 3.7±0.22 | |||||
| 8000 | 3.8±0.23 | |||||
| 10000 | 4.1±0.11 | |||||
|
‘T’ (n=10) |
2000 | 2.5±0.42 | 2.78±0.33 | 11.98 | 0.152 | 4.15 |
| 4000 | 3.0±0.12 | |||||
| 6000 | 2.7±0.29 | |||||
| 8000 | 2.8±0.32 | |||||
| 10000 | 3.0±0.14 |
*P<0.05, ‘A’: Adolescents, ‘V’: Vicenarians, ‘T’: Tricenarians, SD: Standard Deviation.
Table 1. Average EMG RMS (mV) for the three age groups during a 10-s contraction
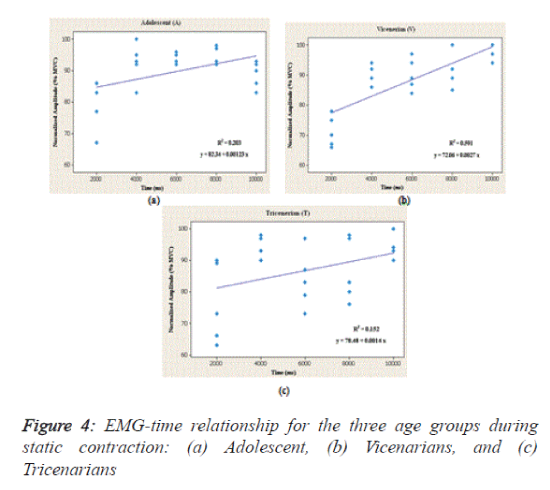
As shown in table 1 and figure 4, the regression between the normalized EMG (%MVC) and the endurance time (ms) showed the relationship of the age groups (subject) to the BB muscle activity during static contraction. Linear regression was used to determine their responses as a function of time. In the figure, the straight lines indicate the linear relationship between the EMG signals and endurance times for the three age groups. In addition, the coefficient of determination (r2) was used to test whether there is an association between the signals and the time lag. Additionally, the slope of the regression relationship was used to test the relationships for the BB muscle of the different subjects during the selected times and static contraction.
The analysis shows that the best regression accuracy was obtained for the Vicenarian age groups compared with the other two groups with an r2 value of 0.591, a level of significance less than 0.05 and an F ratio of 33.23. In contrast, a significant albeit modest correlation was found between EMG and time for the Adolescent group (P=0.024, r2=0.203 and F=5.86), and the linear regression results show a low correspondence between EMG and time in the tricenarian age group (r2=0.152 and F=4.15).
Similarly, no significant difference was found between EMG activity of the BB muscle and time (P>0.05) for this age group.
In conclusion, the results indicate that there is a high level of accuracy between the EMG amplitude and time in the Vicenarian group in terms of the EMG activity of the BB muscle during static contraction. Based on the analysis of the steadiness of the signals, the subjects belonging to the ‘A’ group generated steadier signals than those of the ‘V’ and ‘T’ groups (9.29% 11.46% and 11.98%, respectively).
Discussion
Assessing the age-related muscle function through surface EMG signal analysis is a major unresolved challenge in human ergonomics, biomechanics, biomedicine, and rehabilitation. As a result, the past decade has shown increased efforts to evaluate muscle activity and the relationship between age and the EMG signal. The aims of the present study were to analyse the agerelated changes in the dominant BB muscle of young individuals belonging to three age groups and to assess the relationship between EMG and endurance time. We found that the EMG activity during muscle contraction is influenced by age in young adults and the signals varying according to the endurance time (Figure 4 and Table 1).
The effect of muscle age on EMG signals has been investigated in several studies. A recent study demonstrated the effect of age on EMG variables recorded from lumbar paraspinal muscles [25]. Our findings were similar to those reported by Gerasimova et al., who showed that the EMG amplitude (RMS) decreased with age with individuals in the ‘T’ group showing lower activity than those belonging to the ‘A’ and ‘V’ groups [16]. Similarly, Merletti et al. found that the EMG signal activity is statistically higher in young subjects compared with elderly subjects, but no significant differences in the variance were found between the groups [17]. Yamada et al. showed that the EMG values are lower in older (mean age: 70 years) compared with younger (mean age 21 years) subjects during MVC in the tibialis anterior muscle [26]. These data support our findings, which also show that muscle activity is greater in adolescents (early age) compared with tricenarians (older age). In another study, researchers showed that old men are less physically active (based on the EMG measurement) compared with young men [27].
Other important results of this study are the relationship (r2) between EMG (amplitude) and time (ms) and the steadiness of EMG activation, which explain the characteristics of the agerelated EMG pattern. Few age-related studies have investigated the relationship between EMG and other parameters. For example, Kyoushi et al., examined the effect of age and gender on the muscle fibre conduction velocity (MFVC) and found a significant correlation between MFCV and age. The ages of the subjects age were 20 to 40 years and 50 years [28]. In a previous study, Jansen et al., showed the relationship of the EMG activity of pericranial muscles to human age (between 25 to 64 years) and sex and found a highly significant decrease in the amplitude and frequency with age [29]. Another previous study showed that the time-related EMG parameters associated with mastication muscles while chewing increase from young to elderly subjects [15]. The outcomes of our study partly confirm this hypothesis.
Consequently, several studies have attempted to evaluate the relationship between EMG and other parameters, but the EMG-time relationship in the BB muscle of young adults belonging to three age groups during isometric contraction has not been previously shown. Similarly, one of the striking findings of our study is the EMG signal steadiness among the age groups (subjects). No previous studies have reported the age-related variability in the EMG signal (CoV) during contraction. The current study found that older adults (‘T’) exhibit greater signal variability during isometric contraction compared with younger adults (‘A’). Finally, the findings of this study present consistent evidence supporting the hypotheses that middle-aged young adults (vicenarians) present an improved relationship between EMG amplitude and time during contractions compared with adolescents and tricenarians and that younger adults exhibit increased signals activity and less signal variability compared with older adults.
We hope and expect that the results of this study are valuable to researchers interested in gerontological and electrophysiological analysis in terms of rehabilitation and agerelated muscular studies. Additionally, this research will encourage further investigations of this type of gerontological physiology assessment with EMG. The generated results can be used for different practical applications based on the noninvasive evaluation of the BB muscle and will also aid the development of EMG-assisted biomechanical modelling techniques and prosthetic devices based on different age groups, which is the only possibility for restoration. Surface EMG is commonly applied for the control of prosthetic devices, whereas in most industrial devices, the utilized force is estimated proportionally to the muscle activity [30].
However, further studies are needed to investigate the EMGforce relationship in different muscles during static and dynamic contractions based on the age of the individual. Such studies must include variations in different anthropometric parameters (such as gender, body mass and muscle thickness) during movements of the upper limbs at different angles and various external conditions that influence the signal amplitude (e.g., heat vs. cold).
A number of limitations are associated with this study, and these include some specific technical limitations. First, the strategies used to normalize the EMG data and sensors used throughout the study are not capable of recording the activity from the deep muscle tissues of the BB. Second, all of the subjects were given verbal instructions during the MVC trials and seated in identical testing positions, as discussed in the text, to ensure maximal effort. However, without the use of different methods, such as the superimposed burst technique (short or long period), an inherent constraint in the MVC procedure is that there is no way to determine whether a subject afforded maximal effort [31,32]. Additionally, we did not consider the steadiness of the generated force in our analysis; however, the force may also fluctuate during a period of 10 s to maintain force output, as we will show in our future research work.
Conclusion
This study illustrates the first attempt to compare the EMG signals obtained from young adults belonging to three different age groups (adolescents, vicenarians and tricenarians). The novel findings of this study are the following: (i) younger subjects (‘A’ and ‘V’) exhibit greater muscle activity and less steady signals than older subjects (‘T’) and (ii) the EMG (amplitude) versus time lag relationships were more linear in the ‘V’ group compared with the other two groups (‘A’ and ‘T’). Taken together, these results suggest that the age of young adults is a significant factor for determining the EMG signal activity of the BB muscle. The findings can be applied in biomedical engineering applications for the analysis and control of the neuromuscular system, ergonomics research, rehabilitation engineering, and movement biomechanics. Finally, the results improve our understanding of the mechanics of the upper limbs of individuals belonging to different age groups and open new windows for further research.
Acknowledgment
The authors extend their appreciation to the College of Applied Medical Sciences Research Center and the Deanship of Scientific Research at King Saud University for funding this research.
References
- Farfán FD, Politti JC, Felice CJ. Evaluation of EMG processing techniques using information theory. Biomedical engineering online 2010; 9: 72.
- Komi PV, Viitasalo JHT. Signal Characteristics of EMG at Different Levels of Muscle Tension. ActaPhysiologicaScandinavica 1976; 96:267-276.
- Abdoli-E M, Agnew MJ, Stevenson JM. An on-body personal lift augmentation device (PLAD) reduces EMG amplitude of erector spinae during lifting tasks. ClinBiomech 2006; 21: 456-465.
- Holtermann A, Roeleveld K. EMG amplitude distribution changes over the upper trapezius muscle are similar in sustained and ramp contractions. ActaPhysiol 2006; 186: 159-168.
- Lowery MM, O'Malley MJ. Analysis and simulation of changes in EMG amplitude during high-level fatiguing contractions. Biomedical Engineering 2003; 50: 1052-1062.
- Clark BC, Manini TM, Doldo NA, Ploutz-Snyder LL. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. Journal of Applied Physiol 2003; 94: 2263-2272.
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain research 1976; 219:45-55.
- Bellemare F, Woods J, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J neurophysiol 1983; 50:1380-1392.
- Murray WM, Buchanan TS, Delp SL. The isometric functional capacity of muscles that cross the elbow. Journal of biomechanics 2000; 33: 943-952.
- Veneman JF, Kruidhof R, Hekman EE, Ekkelenkamp R, Van Asseldonk EH, Van Der Kooij H. Design and evaluation of the LOPES exoskeleton robot for interactive gait rehabilitation. Neural Systems and Rehabilitation Engineering 2007; 15: 379-386.
- Jain R, Datta S, Majumder S. Biomimetic Behavior of IPMC Using EMG Signal for Micro Robot. Mechanics Based Design of Structures and Machines 2014; 42: 398-417.
- Visser S, De Rijke W. Influence of sex and age on EMG contraction pattern. European neurology 1974; 12: 229-235.
- Boccia G, Dardanello D, Rosso V, Pizzigalli L, Rainoldi A. The Application of sEMG in Aging: A Mini Review. Gerontology 2014.
- Arjunan SP, Wheeler K, Shimada H, Kumar D. Age related changes in the complexity of surface EMG in biceps: A model based study. In: Biosignals and Biorobotics Conference (BRC) 2013; 1-4.
- Kohyama K, Mioche L, Bourdio P. Influence of age and dental status on chewing behaviour studied by EMG recordings during consumption of various food samples. Gerodontology 2003; 20: 15-23.
- Gerasimova L, Varlamova T, Antonen E, Antropova E, Meigal AY. Age-Related Changes in Turn–Amplitude Characteristics of the EMG Recorded during Graded Isometric Contraction. Human Physiol 2004; 30: 358-363.
- Merletti R, Farina D, Gazzoni M, Schieroni MP. Effect of age on muscle functions investigated with surface electromyography. Muscle & nerve 2002; 25: 65-76.
- Plow EB, Varnerin N, Cunningham DA, Janini D, Bonnett C, Wyant A, Hou J, Siemionow V, Wang X-F, Machado AG. Age-Related Weakness of Proximal Muscle Studied with Motor Cortical Mapping: A TMS Study. PloS one 2014; 9: e89371.
- Yoon T, Schlinder-Delap B, Hunter SK. Fatigability and recovery of arm muscles with advanced age for dynamic and isometric contractions. Experimental gerontology 2013; 48: 259-268.
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child & Adolescent Psychiatry 1994; 33: 809-818.
- Xiaoli L, Weiyuan Z. Effect of maternal age on pregnancy: a retrospective cohort study. Chinese medical journal 2014; 127: 2241-2246.
- Ahamed NU, Sundaraj K, Ahmad RB, Rahman M, Islam MA. Analysis of right arm biceps brachii muscle activity with varying the electrode placement on three male age groups during isometric contractions using a wireless EMG sensor. ProcediaEng2012; 41: 61-67
- Boostani R, Moradi MH. Evaluation of the forearm EMG signal features for the control of a prosthetic hand. Physiological measurement 2003; 24:309.
- Phinyomark A, Phukpattaranont P, Limsakul C. Feature reduction and selection for EMG signal classification. Expert Systems with Applications 2012; 39: 7420-7431.
- Heydari A, Humphrey A, Nargol A, Greenough C. Effect of age on emg variables recorded from lumbar paraspinal muscles. Bone Joint J2008; 90:487.
- Yamada H, Masuda T, Okada M. Age-related EMG variables during maximum voluntary contraction. Perceptual and motor skills 2002; 95: 10-14.
- Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. Journal of applied physiology 2005; 99: 890-897.
- Mase K, Kamimura H, Imura S, Kitagawa K. Effect of Age and Gender on Muscle Function -Analysis by Muscle Fiber Conduction Velocity. Journal of Physical Therapy Science 2006; 18: 81-87.
- Jensen R, Fuglsang-Frederiksen A. Quantitative surface EMG of pericranial muscles. Relation to age and sex in a general population. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section 1994; 93:175-183.
- Kamavuako EN, Rosenvang JC, Bøg MF, Smidstrup A, Erkocevic E, Niemeier MJ, Jensen W, Farina D. Influence of the feature space on the estimation of hand grasping force from intramuscular EMG. Biomedical Signal Processing and Control 2013; 8: 1-5.
- Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 1996; 19: 861-869.
- Norcross MF, Troy Blackburn J, Goerger BM. Reliability and interpretation of single leg stance and maximum voluntary isometric contraction methods of electromyography normalization. Journal of Electromyography and Kinesiology 2010; 20: 420-425.