Research Article - Biomedical Research (2017) Volume 28, Issue 14
Advanced oxidation protein products induce the apoptosis of vascular endothelial cells via activating Nrf2 pathway in atherosclerosis
Dao-Ri-Na Bao1, Yu-Qin Jia2, Yan-Mei Liu2, Li-Feng Zhang2, Peng-Fei Wu2, Xiao-Xue Wang2, Heng-Bin Li2 and Xing-Yong Wang3*
1Chongqing Medical University, Chongqing, PR China
2Intensive Care Unit, the Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot, PR China
3Emergency Department, Children’s Hospital of Chongqing Medical University, Chongqing, PR China
- *Corresponding Author:
- Xing-Yong Wang
Department of Emergency
Children’s Hospital of Chongqing Medical University, PR China
E-mail: wxy991818@sina.com
Accepted date: February 2, 2017
Abstract
Objective: To explore the influence of Advanced Oxidation Protein Products (AOPPs) on vascular endothelial cells proliferation and apoptosis and the role of Nrf2 pathway in the process.
Methods: Different concentrations of AOPPs were used to treat vascular endothelial cells (HUVEC) for 12, 24 and 48 h. Vascular endothelial cells were treated with high, middle and low concentrations of AOPPs for 24 h and flow cytometry was used to determine the changes of cell cycle, apoptosis and reactive oxygen species. Western blotting was used to determine the protein expression of Caspase 3, Caspase 8, Caspase 9, Nrf2 and its downstream gene HO-1.
Results: The vascular endothelial cell viability decreased gradually with the increase of AOPPs concentrations from 0 ug/ml to 50 ug/ml, and then further decreased with the increase of concentrations from 50 ug/ml to 200 ug/ml, and the time had no influence on cell viability. Therefore, concentrations of 2, 50 and 200 ug/mL and time of 24 h were used for the further study. Vascular endothelial cells mainly focused at G1 stage at high concentration of AOPPs. The number of apoptotic cells, as well as reactive oxygen and expressions of Caspase 3, Caspase 8, Caspase 9, Nrf2 and HO-1, increased with the increase of AOPPs concentrations.
Conclusion: In atherosclerosis, AOPPs could induce the apoptosis of vascular endothelial cells and inhibit the proliferation by activating Nrf2 pathway.
Keywords
Atherosclerosis, Advanced oxidation protein products, Nrf2, Apoptosis
Introduction
Atherosclerosis is nowadays generally accepted as an inflammatory disease. It is known that local inflammation occurs during the formation of the plaques, as macrophages and other immuno-competent cells are present in the lesions from an early stage [1,2]. Recent advances in basic science have established a fundamental role for inflammation in mediating all stages of this disease [3]. The early phase of atherosclerosis involves the recruitment of inflammatory cells from the circulation and their transendothelial migration [4].
Endothelial cells have long been viewed as a passive lining of blood vessels with negative properties such as nonreactive to blood components. They play a critical role in thromboregulation by virtue of a surface-connected fibrinolytic system [5,6]. They produced a Neutrophil Chemotactic Factor (NCF) upon stimulation with Tumor Necrosis Factor-alpha (TNF-alpha), Interleukin-1 beta (IL-1 beta), or Lipopolysaccharide (LPS) [7]. Vascular endothelial cells undergo morphogenesis into capillary networks in response to angiogenic factors [8]. The abnormal proliferation of vascular endothelial cells was closely related with atherosclerosis. Advanced Oxidation Protein Products (AOPPs) are new protein markers of oxidative stress with pro-inflammatory properties [9]. They are derived from oxidation-modified albumin (its aggregates or fragments), but also of fibrinogen, and lipoproteins [10]. The accumulation of AOPPs has been linked to vascular lesions in diabetes, chronic renal insufficiency, and atherosclerosis [11]. Increased level of plasma AOPPs has been found in patients with uremia and nonuremic subjects with coronary artery disease [12]. During the oxidation process, several proteins have attracted scholars’ interest. Transcription factor Nuclear factor erythroid 2 p45 related factor 2 (Nrf2) is a major regulator of genes encoding phase 2 detoxifying enzymes and antioxidant stress proteins in response to electrophilic agents and oxidative stress [13,14]. It regulates antioxidant-responsive element-mediated induction of cytoprotective genes in response to oxidative stress. Heme oxygenase-1 (HO-1) is a stress-inducible rate-limiting enzyme in heme degradation. Studies show that it confers cytoprotection against oxidative injury and provides a vital function in maintaining tissue homeostasis [15]. Though there are lots of studies focusing on mechanisms of apoptosis of vascular endothelial cells, few of them studied the role of advanced oxidation proteins in the process and its relationship with Nrf2 pathway. In this study, we aimed to study the effect of AOPPs on the proliferation and apoptosis of vascular endothelial cells in atherosclerosis and the role of Nrf2 pathway in the process.
Material and Methods
Cell culture
Vascular Endothelial Cells (HUVEC) were obtained from Cell Bank of Type Culture Collection of Chinese Academy of Sciences, Shanghai, China. The cells were routinely cultured in RPMI-1640 supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified cell incubator with an atmosphere of 5% CO2 at 37°C. Cells growing at an exponential rate were used for the experiments.
Influence of AOPPs on vascular endothelial cell proliferation
Vascular endothelial cell lines were treated with different concentrations of AOPPs (0, 1, 2, 5, 10, 20, 50, 100 and 200 μg/ml) for 12, 24 and 48 h, respectively. For proliferation assays, cells were seeded in 96-well plates at 4,000 cells/well using Lipofectamine 2000 Reagent. Cell proliferation was measured using the Cell Counting Kit-8 assay (CCK-8, Boster, Wuhan, China). The proliferation analysis of each group in each individual experiment and the whole experiment process were performed in triplicate.
Flow cytometry
HUVEC cells were treated with 0, 50 and 200 ug/ml AOPPs for 24 h, and then cell cycle, cell apoptosis and reactive oxygen were determined by flow cytometry. Cell lines were selected and plated in a 96-well plate at a density of 2 × 103 cells/well in supplemented RPMI 1640 and incubated for 16 h before the cells were subjected to treatment in triplicate wells. After treatment, the cells were washed twice in phosphate-buffered saline (PBS) (2.68 mM KCl, 1.47 mM KH2PO4, 8 mM Na2KPO4, 136.75 mM NaCl) and counted. Fifty to one hundred thousand cells were selected and centrifuged 5 min at 1000 r/min. Annexin V-FITC mixed liquor of 195 μL was added to resuspend cytotrophoblast cells and 5 μL was added to mix. Centrifugation at 1000 r/min for 5 min was performed after cultivation 10 min. Sample was obtained after discarding supernatant and 10 μL Propidium Iodide (PI) was added. 2’, 7’-Dichlorofluorescein diacetate was used as fluorescent probe to analyse the level of Reactive Oxygen Species (ROS). Afterwards, the sample was stilled in dark for 30 min. Finally, the apoptosis, cycle and reactive oxygen were detected using Flow Cytometry (FCM) on the Moflo (Dako Cytomation, Glostrup, Denmark).
Western blotting
To determine the protein expression of apoptosis proteins Caspase 3, Caspase 8, Caspase 9, and Nrf2 and its downstream gene HO-1, western blot assay was carried out. Total proteins were prepared from cell lines using Radioimmunoprecipitation Assay (RIPA) lysis buffer (Sigma, USA) supplemented with protease inhibitors. An equal amount (50 μg) of cellular lysates was separated on 10% SDS-Polyacrylamide Gel Electrophoresis (PAGE) minigels and transferred to nitrocellulose filter membranes (Hybond, Escondido, CA, USA). The membrane was blocked with Tris-Buffered Saline Tween-20 (TBST) containing 5% skimmed milk powder for 1 h at room temperature, followed by incubation in TBST containing 5% BSA (Sigma, St. Louis, MO, USA) and primary antibodies overnight at 4°C. Primary antibodies were detected using a peroxidase-coupled goat anti-rabbit secondary antibody (1:8000, ZSBio, Beijing, China) and EZ-ECL chemiluminescence Detection kit for HRP (Biological Industries, Beit-Haemek, Israel). The following primary antibodies were used: rabbit mAb Caspase 3, rabbit mAb Caspase 8, rabbit Caspase mAb 9, rabbit mAb Nrf2, rabbit mAb HO-1 (Cell Signalling Technology, Danvers, MA, USA) and rabbit pAb GAPDH (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 18.0. Data are expressed as the mean ± Standard Error of the Mean (SEM) of three independent experiments. Multiple group comparisons were analysed using ANOVA with a post hoc test. P<0.05 was considered to indicate a statistically significant result.
Results
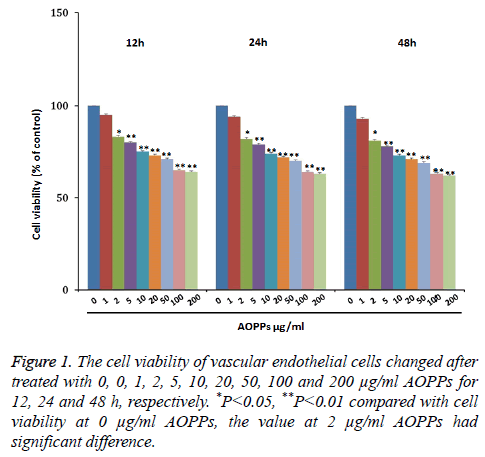
AOPPs increased vascular endothelial cell viability
To explore the effect of AOPPs on vascular endothelial cell proliferation, vascular endothelial cells were treated with 0, 1, 2, 5, 10, 20, 50, 100 and 200 μg/ml AOPPs for 12, 24 and 48 h, respectively. Results were shown in Figure 1. As shown, cell viability decreased with the increase of AOPPs concentrations from 0 μg/ml to 200 μg/ml AOPPs at 12 h, 24 h and 48 h. Moreover, the cell viability under concentrations of 2, 5, 10, 20, 50, 100 and 200 μg/ml was significantly lower than the control group A OPPs.
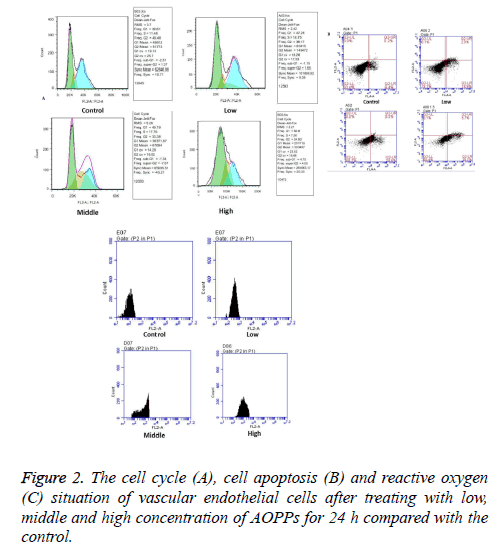
AOPPs increased cell apoptosis
Vascular endothelial cells were treated with 0, 2, 50 and 200 μg/ml AOPPs for 24 h and then cell cycle, cell apoptosis and reactive oxygen situation of vascular endothelial cells were determined by flow cytometry. As shown in Figure 2A, the G1 stage extended with AOPPs concentration increased gradually from 0 μg/ml to 200 μg/ml. The number of apoptotic cells increased with the increase of AOPPs concentration, and the value (Figure 2B). As shown in Figure 2C, fluorescence value for reactive oxygen increased with the increase of AOPPs concentration.
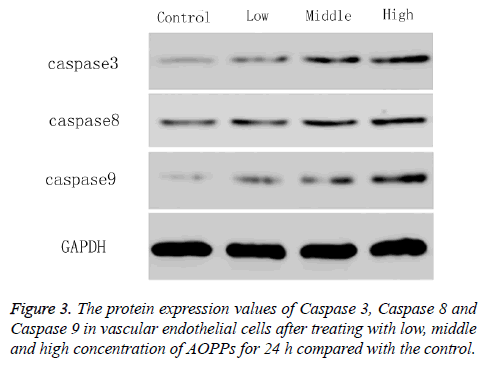
AOPPs increased the level of apoptotic proteins
To explore the molecular mechanism on how AOPPs promote the apoptosis of vascular endothelial cells, the protein expression values of apoptotic protein Caspase 3, Caspase 8 and Caspase 9 were determined by western blotting. As shown in Figure 3, the protein expression of Caspase 3, Caspase 8 and Caspase 9 increased with AOPPs concentration increased from 0 μg/ml to 200 μg/ml. The value in AOPPs concentration of 2 μg/ml was almost the same with that in the control (0 μg/ml).
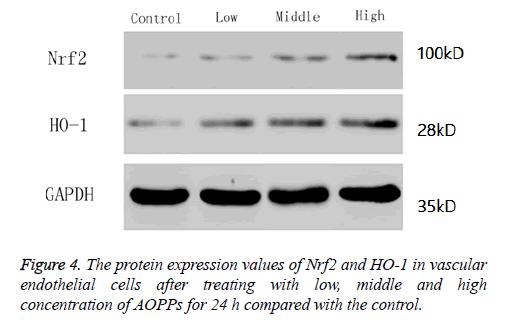
AOPPs increased the level of Nrf2
In this study, we determined the protein expression values of Nrf2 and its downstream gene HO-1 by western blotting. Results showed the levels of Nrf2 and HO-1 increased with the increase of AOPPs concentrations (Figure 4).
Discussion
Atherosclerosis, the principal cause of heart attack, stroke and gangrene of the extremities, is responsible for 50% of all mortality in the USA, Europe and Japan [16,17]. It affects the major elastic and muscular arteries, but some vessels are largely spared while others may be markedly diseased. Atherosclerosis has long been recognized as having an inflammatory component, and this has a particularly important bearing on to its clinical complications as it may result in plaque instability [18]. It is observed in embryonic and injured tissue and is particularly prominent in the vicinity of solid tumors 1, involves the migration and proliferation of capillary endothelial cells [19]. Oxidative stress and expression of the Vascular Cell Adhesion Molecule-1 (VCAM-1) on vascular endothelial cells are early features in the pathogenesis of atherosclerosis and other inflammatory diseases [20]. Endothelial cell damage is one important pathophysiological step of atherosclerosis and restenosis after angioplasty [21]. AOPPs are new protein markers of oxidative stress with proinflammatory properties, accumulated in many pathological conditions [10].
In this study, we aimed to explore the effect of AOPPs on the proliferation of vascular endothelial cells and the related molecular mechanism. First, vascular endothelial cells were treated with 0, 1, 2, 5, 10, 20, 50, 100 and 200 μg/ml AOPPs for 12, 24 and 48 h, respectively, and cell viability was determined. Results showed cell viability decreased with the increase of AOPPs concentrations from 0 μg/ml to 200 μg/ml. Previous studies showed that AOPPs could induce the apoptosis of islet microvascular endothelial cells and enhanced the ROS level via a RAGE-mediated signalling pathway [22,23]. The present study showed similar effect of AOPPs on vascular endothelial cells. Then, effects of AOPPs on cell apoptosis and reactive oxygen were further studied. Concentrations of 2 μg/mL, 50 μg/ml and 200 μg/ml were selected as low, middle and high concentrations of AOPPs for further study. Vascular endothelial cells were then treated with 0, 2, 50 and 200 μg/ml AOPPs for 24 h, and then cell cycle, cell apoptosis and reactive oxygen situation of vascular endothelial cells were determined by flow cytometry. Results showed the cell cycle was mainly focused on G1 stage at high concentration of AOPPs, the number of apoptotic cells was significantly higher in high concentration of AOPPs compared with that in low and middle concentration of AOPPs and the control. It indicated high concentration of AOPPs can promote the apoptosis of vascular endothelial cells. Similar results are found in other studies, which indicate that AOPPs can also induce apoptosis in several kinds of cells like podocyte [24], intestine epithelial cells [25], and chondrocyte [26]. At last we explored the molecular mechanism about how AOPPs promote the apoptosis of vascular endothelial cells, the protein expression values of Caspase 3, Caspase 8, Caspase 9, Nrf2 and HO-1 were determined by western blotting. Results showed the levels of Caspase 3, Caspase 8, Caspase 9, Nrf2 and HO-1 at high concentration of AOPPs were significantly higher than those at low and middle concentration of AOPPs and the control. The levels increased with the increase of AOPPs concentration. Caspases are crucial mediators of programmed cell death (apoptosis). Among them, caspase-3 is a frequently activated death protease, catalysing the specific cleavage of many key cellular proteins [27]. Activated caspase-3 was detected in atherosclerotic [28]. Caspase 8 is a cysteine protease regulated in both a death-receptor-dependent and independent manner during apoptosis [29]. Caspase-9 is a member of caspase family of cysteine proteases that have been implicated in apoptosis and cytokine processing [30]. Caspase-8 and caspase-9 are considered to be two initiator caspases for mediating apoptotic signals from death receptors or from cytochrome c translocation, leading to activation of caspase-3 as a key factor in apoptotic cell death [31]. Transcription factor Nrf2 is a major regulator of genes encoding phase 2 detoxifying enzymes and antioxidant stress proteins which regulates the basal and inducible expression of numerous detoxifying and antioxidant genes [32]. HO-1 is a stress protein induced in response to a variety of oxidative challenges [33]. The Nrf2/HO-1 pathway is reported to be involved in cell death in many diseases [34,35], including some endothelial cells [36]. However, relationship between AOPPs and Nrf2 pathway in apoptosis of vascular endothelial cells has not been reported yet.
In conclusion, in the present study, we investigated role of AOPPs in proliferation and cell apoptosis of vascular endothelial cells and demonstrated that AOPPs could promote the apoptosis of vascular endothelial cells by activating Nrf2/HO-1 pathway in atherosclerosis.
References
- Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis 2003; 169: 203-214.
- Lenglet S, Mach F, Montecucco F. Role of matrix metalloproteinase-8 in atherosclerosis. Mediators Inflamm 2013; 2013: 659282.
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135-1143.
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003; 170: 191-203.
- Hajjar KA, Gavish D, Breslow JL, Nachman RL. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature 1989; 339: 303-305.
- Sena CM, Pereira AM, Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta 2013; 1832: 2216-2231.
- Strieter RM, Kunkel SL, Showell HJ, Remick DG, Phan SH. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science 1989; 243: 1467-1469.
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999; 99: 301-312.
- Krzystek-Korpacka M, Neubauer K, Berdowska I, Boehm D, Zielinski B, Petryszyn P, Terlecki G, Paradowski L, Gamian A. Enhanced formation of advanced oxidation protein products in IBD. Inflammy Bowel Dis 2008; 14: 794-802.
- Piwowar A. Advanced oxidation protein products. Mechanism of the formation, characteristics and property. Pol Merkuriusz Lekarski 2010; 28: 166-169.
- Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N, Nagai R, Lu X, Chen BH, Shan YX, Tian JW, Nagaraj RH, Xie D, Zhang X. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signalling pathway. Antioxid Redox Signal 2008; 10: 1699-1712.
- Liu SX, Hou FF, Guo ZJ, Nagai R, Zhang WR, Liu ZQ, Zhou ZM, Zhou M, Xie D, Wang GB, Zhang X. Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arterioscler Thromb Vasc Biol 2006; 26: 1156-1162.
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cellular Biol 2004; 24: 7130-7139.
- Hayes JD, Chowdhry S, Dinkova-Kostova AT, Sutherland C. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochem Soc Trans 2015; 43: 611-620.
- Lee IT, Wang SW, Lee CW, Chang CC, Lin CC. Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J Immunol 2008; 181: 5098-5110.
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993; 362: 801-809.
- Chadjichristos C, Derouette J, Kwak B. Connexins in atherosclerosis. Ann Rheum Dis 2014; 73: 255-267.
- Lamb DJ, El-Sankary W, Ferns GA. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis 2003; 167: 177-185.
- Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature 1987; 325: 257-259.
- Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, Medford RM. Vascular Cell Adhesion Molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest 1993; 92: 1866.
- Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 2003; 93: 17-24.
- Zhang Z, Yang L, Lei L. Glucagon-like peptide-1 attenuates advanced oxidation protein product-mediated damage in islet microvascular endothelial cells partly through the RAGE pathway. Int J Mol Med 2016; 38: 1161-1169.
- Guo ZJ, Niu HX, Hou FF. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signalling pathway. Antioxidants Redox Signal 2008; 10: 1699-1712.
- Zhou LL, Hou FF, Wang GB. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int 2009; 76: 1148.
- Xie F, Sun S, Xu A. Advanced oxidation protein products induce intestine epithelial cell death through a redox-dependent, c-jun N-terminal kinase and poly (ADP-ribose) polymerase-1-mediated pathway. Cell Death Disease 2014; 5: e1006.
- Wu Q, Zhong ZM, Zhu SY. Advanced oxidation protein products induce chondrocyte apoptosis via receptor for advanced glycation end products-mediated, redox-dependent intrinsic apoptosis pathway. Apoptosis 2016; 21: 1-15.
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999; 6: 99-104.
- Martín-Ventura JL, Blanco-Colio LM, Munoz-García B, Gomez-Hernandez A, Arribas A, Ortega L, Tunon J, Egido J. NF- κB activation and fas ligand overexpression in blood and plaques of patients with carotid atherosclerosis potential implication in plaque instability. Stroke 2004; 35: 458-463.
- Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med 2000; 6: 529-535.
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 1998; 94: 325-337.
- Liu W, Kato M, Akhand AA, Hayakawa A, Suzuki H, Miyata T, Kurokawa K, Hotta Y, Ishikawa N, Nakashima I. 4-hydroxynonenal induces a cellular redox status-related activation of the caspase cascade for apoptotic cell death. J Cell Sci 2000; 113: 635-641.
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Molecular Med 2004; 10: 549-557.
- Le WD, Xie WJ, Appel SH. Protective role of heme oxygenase-1 in oxidative stress-induced neuronal injury. J Neurosci Res 1999; 56: 652-658.
- Tang GH, Chen ZW, Lin TT. Neolignans from Aristolochia fordiana Prevent oxidative stress-induced neuronal death through maintaining the Nrf2/HO-1 pathway in HT22 cells. J Nat Prod 2015; 78: 1894-1903.
- Ansari N, Khodagholi F, Amini M. 2-ethoxy-4, 5-diphenyl-1, 3-oxazine-6-one activates the nrf2/ho-1 axis and protects against oxidative stress-induced neuronal death. EuR J Pharmacol 2011; 658: 84-90.
- Seung El , Seong IJ, Hana Y. Fisetin induces Nrf2-mediated HO-1 expression through PKC-d and p38 in human umbilical vein endothelial cells. J Cell Biochem 2011; 112: 2352-2360.