Research Article - Biomedical Research (2017) Volume 28, Issue 8
Adipose derived mesenchymal stem cells improve diabetic wound healing in mouse animal model: extracellular matrix remodeling maybe a potential therapeutic usage of stem cells
Hori Ghaneialvar1, Abbas Sahebghadam Lotfi1*, Sareh Arjmand2, Masoud Soleimani3 and Fatemeh Mashhadi Abbas4
1Department of Clinical Biochemistry, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
2Protein Research Center, Shahid Beheshti University, Tehran, Iran
3Department of Hematology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
4Department of Oral Pathology, School of Dentistry, Shaheed Beheshti University of Medical Sciences, Tehran, Iran
- *Corresponding Author:
- Lotfi AS
Department of Clinical Biochemistry
Faculty of Medical Sciences
Tarbiat Modares University, Iran
Accepted on January 11, 2017
Abstract
Skin wounds cause damage to the body's first layer of protection. This, disclosed a person to further injury. Wounds normally heal in a very orderly and efficient process. However, activation of this efficient process is sometimes lost in pathologic conditions such as diabetes. The objective of the present study was to evaluate the expression of some genes in Adipose Derived Mesenchymal Stem Cells (ADSCs) that were used for the healing of the diabetic wound of mouse. ADSCs were separated from adipose tissue of mice, confirmed by surface markers CD34, CD105, CD44 and bone or fat cells differentiation. Then 10 × 105 stem cells were immediately injected in four areas around the wound that previously were created on the dorsal skin of each diabetic mouse. TIMP-1, MMP-2, MMP-9 and uPA genes expression folds in the wound area were examined on 3rd, 7th, 14th and 21st days, using q-PCR. Three groups’ mice were evaluated: non-diabetic, diabetic without any treatment and diabetic with ADSCs treatment. The expression level of uPA and MMP-9 genes were decreased in the stem cell-treated group but TIMP1 and MMP2 genes folds were increased significantly (p<0.05) compared to the diabetes group without any treatment. The results of this study suggest that stem cell transplantation maybe applicable in diabetic wounds healing via changing the content of tissue gene expressions.
Keywords
Adult stem cells, Wound healing, Extracellular matrix, Matrix metalloproteinase.
Abbreviations
MSCs: Mesenchymal Stem Cells; CD: Cluster of Differentiation; STZ: Streptozotocin; ADSCs: Adipose- Derived Mesenchymal Stem Cells; uPA: Urinary Plasminogen Activator; MMPs: Matrix Metalloproteinase; IP: Intraperitoneally; ECM: Extracellular Matrix; MMP9: Matrix Metalloproteinase 9; MMP2: Matrix Metalloproteinase 2; TIMPs: Metalloproteinase Tissue Inhibitor; FBS: Fetal Bovine Serum; GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase; DMEM: Dulbecco's Modified Eagle Medium; F12: Ham's F12 Medium; DPBS: Dulbecco's Phosphate-Buffered Saline.
Introduction
Diabetes is a metabolic disorder as well as a serious health threat [1]. Incidence of diabetes has increased significantly in recent decades. According to the report of World Health Organization in 2016, more than 350 million people worldwide are affected by the disease [2]. Although various physiological and genetic factors have a role in emergence and progression of the disease, the main mechanisms that cause the condition are still not fully understood [3,4]. One of the most common and serious complications of diabetes mellitus is extremely slow, or complete lack of the body to heal wounds.
Identification of this healing defect in terms of the pathophysiology of diabetes is still unclear [5,6]. Despite significant advances in new therapies and wound care, in many cases, wounds in diabetic patients do not heal and become chronic, leading to amputation or death [7]. Peripheral neuropathy and peripheral vascular disease are among factors affecting formation and lack of healing of diabetic wounds, which leads to reduced fibroblast growth, increased expression of extracellular matrix metalloproteinase degradation and reduced extracellular matrix synthesis [8]. Wound healing is a complex biological process. It has four stages; haemostasis, inflammation, proliferation and reconstruction [9]. The process requires precise organization by signals generated by growth factors and cytokines and is set through complex molecular and biological processes including cell migration, cell proliferation and extracellular matrix remodeling [10-13]. Wounds are grouped according to acute and chronic classification based on healing time. The wound healing process is often incomplete in diabetic wounds and several important factors of the process are impaired, which prolongs the healing process. It is usually during the stage of inflammation that a wound becomes chronic [14,15]. Since Friedenstein and his colleagues separated the first mesenchymal stem cells (MSCs) from rat bone marrow half a century ago, these cells have been used in tests on several diseases [16]. While in early studies, stem cells were separated from bone marrow, in subsequent decades these cells were also separated and tested from many adult tissues such as adipose tissue, synovial fluid, gum tissue and such like. Stem cells have the special feature of asymmetric proliferation, so with each proliferation they create one stem cell with proliferation ability and another cell that is directed into the differentiation [17,18]. The therapeutic potential of stem cells is largely attributed to their ability for production of resuscitation cytokines. This has made the stem cells suitable for treating many disorders including chronic wounds. Up until now stem cells have been separated from various sources of the body and have been evaluated in preclinical and clinical tests for wound healing and tissue regeneration [19]. The urinary plasminogen activator (uPA) is a serine protease protein that activates conversion of plasminogen to plasmin. The plasminogen/plasmin activation system starts the proteolytic pathway and leads to fibrin degradation and extracellular matrix metalloproteinase activity [20,21]. Some studies have demonstrated proteolytic degradation of the extracellular matrix using simultaneous activity of plasmin and matrix metalloproteinase (MMPs). MMPs have a key role in a variety of physiological and pathological processes of tissue regeneration and cell migration [22-24]. uPA is naturally secreted by endothelial, epithelial, monocytes and neutrophils cells [25]. It has been observed that a lack of precursor plasminogen plasmin or protease responsible for the conversion of plasminogen to plasmin reduces plasmin levels and this eventually leads to fibrin deposition and disruption of inflammation and wound healing processes [25,26]. Studies have shown that inhibition of uPA activity reduces activity of MMP9 in wound healing which confirms the start of proteolytic way with uPA protein [21]. MMPs are a group of endopeptidases related to zinc and calcium which have the ability to destruction and restructure all kinds of extracellular matrix (ECM) [27]. These enzymes are secreted by keratinocytes migrated to the edge of a wound and activity of these metalloproteinase enzymes is inhibited by metalloproteinase tissue inhibitor (TIMPs) [28]. TIMPs act as MMPs local inhibitor and thereby control degradation of extracellular matrix. TIMP is a 29 kDa glycoprotein which is synthesized by fibroblast cells, smooth muscle cells, endothelial cells, and keratinocytes [29]. TIMPs react from the N-terminal domain with active site of MMPs [30]. Although the matrix metalloproteinase play an important role in wound healing, but a failure of expression reduction causes a reversal role. An abnormal increase of the metalloproteinase enzymes such as Matrix Metalloproteinase 2 (MMP2) and Metalloproteinase Matrix 9 (MMP9) and reduction of TIMP have been observed in other studies on chronic wounds [31,32]. Since MMPs have been identified as the main group of enzymes to destroy the extracellular matrix, TIMPs control the rate of metabolism of an extracellular matrix during the natural process of tissue regeneration [33]. TIMPs also have activity independent of MMPs inhibition including an effect on cell growth and differentiation, cell migration, angiogenesis antioxidant activity, these activities have significant role in wound healing [34]. The objective of this research was to examine the ability of ADSCs in adjustment of MMP2, MMP9, TMP1 and uPA genes which play a role in degradation and degeneration of tissue and wound healing process in diabetic mouse.
Materials and Methods
All chemicals were purchased from Sigma-Aldrich (USA); otherwise, they are specified within the text.
Mesenchymal stem cell isolation from mice adipose tissue
The cells used in this study were extracted from inguinal adipose tissue of BALB/c male mice under completely sterile conditions [35]. For this purpose, adipose tissue was fragmented into tiny pieces using a scalpel after removing connective tissue and blood. Then, fragmented tissues were incubated for 45 minutes at 37°C with 150 rpm rotation rate and in exposure to collagenase I enzyme .Then, an equal volume of medium containing 10% FBS was added to neutralize collagenase I. The solution was centrifuged for 10 minutes at 4000 rpm at 4°C and a pellet containing stem cells was transferred into the cell culture flask containing DMEM: F12 medium (DMEM: F12, 3: 1 (V/V)) with 15% FBS, penicillin (100 IU/ml), streptomycin (100 μg/ml). Then, the flasks were incubated in 37°C with 5% CO2 and 90% humidity. Culture medium was changed after 24 h and then every three days; cells were sub-cultured when they reached confluence of 75-80%.
MSCs differentiation into the adipocyte and osteocyte lineages
In order to evaluate differentiation potential of cells isolated from adipose tissue, initially the number of 10 × 103 cell/ml were seed, then medium of cells was replaced with adipocyte differentiation medium containing DMEM, 10% FBS, LAscorbic- acid 2-phosphate, Dexamethasone, Indomethacin after 48 h. The cell culture medium was changed every 3 days. The differentiated cells were washed with DPBS after completion of the course of differentiation and were fixed using 4% paraformaldehyde, adipocyte differentiated cells were then stained with Oil Red for 10-15 minutes. Ultimately differentiated cells were observed by inverted microscope after washing to make the cytoplasmic lipid droplets visible [35]. Differentiation into osteocytes was performed using osteogenic medium containing DMEM, 10% FBS, L-Ascorbic-acid 2- phosphate, Dexamethasone, β-glycerol phosphate for 21 days. Visualization of calcium deposits was done using Alizarin Red staining and after fixation according to the previously described method [35].
Flow cytometry characterization of surface markers of stem cells
For confirmation of isolated stem cells and evaluation of contamination with blood cells, expression of specific cell surface markers was evaluated using flow cytometry. For this purpose, CD44 and CD105, as specific surface markers of mesenchymal stem cells, and CD34 as a surface marker of blood cells were evaluated. The cells were initially isolated from the flasks using Trypsin/EDTA treatment and centrifuged for 5 minutes at room temperature and at 1000 rpm. Then, the supernatant was removed and the resulting pellet was dissolved in 1 ml DPBS. 1 × 106 cells/ml were counted. After mixing, 100 μl cell containing PBS solution was poured into test tubes and 2 μl of specific anti-bodies was added to it in the dark status. Used antibodies were FITC-Conjugated rat anti-mouse CD44 (BD Bioscience, 553133), PE-conjugated rat anti-mouse CD105 (BioLegend, 120407), FITC-Conjugated rat anti-mouse CD34 (eBioscience, 11-0341-82). Further, 1 ml of the isotype control antibody (Rat Isotype IgG2a kappa) was added to the control tube. All tubes were placed at 4°C for 30 min in the dark. After incubation, contents of the tubes were initially vortexed for 1-2 seconds and the expression percentages of surface markers were measured with a counter CyFlow.
Diabetic wound animal model
In this study, three groups of BALB/c mice were examined; a control group (without diabetes), a diabetic group without treatment and a group treated with stem cells. Examination of wounds in each of these groups was made at 3rd, 7th, 14th and 21st days and three mice were considered for each group at each day. For induction of diabetes, after 12 h of fasting, a dose of 200 mg/kg streptozotocin was used intraperitoneally (IP) [36]. Blood glucose level of each mouse was measured 48 to 72 hours after administration of STZ using a glucometer (Bionime) and also polydipsia and polyuria were examined. After confirmation that mice were diabetic, each one was anesthetized using a solution of anaesthetic ketamine/xylazine and a wound with diameter 8 mm was created in the dorsal skin of mice using punch biopsy (Kai Biopsy Punch). Then, mice were kept separately in sterile conditions in type A cages.
Treatment of diabetes wounds using mesenchymal stem cells
Immediately after creation of the wound, one million MSCs were isolated from adipose tissue at the third passage that had been dissolved in 80 μl DPBS were injected into four points of the dermis around the wound using 31-gauge 1 cc insulin syringe with a short needle (BD Ultra-Fine™ II) [37].
RNA extraction and cDNA synthesis
RNA extraction from tissue of wound area was done by (Sigma, St. Louis, Missouri) reagent based on the protocol [35]. Then, the extracted RNA was treated with DNase I (Fermentas EN0525) in order to eliminate contamination with DNA. The synthesis of cDNA was done with cDNA synthesis kit (Bioneer, K-2046) and according to the kit instructions.
Real-time PCR
The real time PCR method was used to study gene expression. GAPDH gene expression was measured in each test to normalize the results. Forward and reverse primers were designed with Oligo7 software for specific amplification of each gene (Table 1). Real time PCR was done using SYBR green (Takara, RR420B) and Rotor Gene 6000 (Corbett, Australia). Real time PCR programme was performed as follows: initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 15 sec, annealing at the temperature of 61°C for 15 sec and elongation at 72°C for 20 sec. All reactions were performed in triplicate and results were analysed by the method 2-ΔΔ CT. The obtained data were analysed using SPSS software and ANOVA test and mean comparison was done using the LSD method. Data were shown based on mean ± SD and p<0.05 was considered statistically significant.
| Product size (bp) | Annealing temperatures (Tm, °C) | Sequences of primers | Name of genes |
|---|---|---|---|
| 233 | 61 | F: GGTGAAGGTCGGTGTGAACG | GAPDH |
| R: CTCGCTCCTGGAAGATGGTG | |||
| 137 | 61 | F:CACTGCTTCATTCAACTCCCAAAG | uPA |
| R:CTGTCTTCCCTGTAGTATTCGTGC | |||
| 162 | 61 | F: GCAAAGGCGTCGTGATCC | MMP9 |
| R: TGCCGTCCTTATCGTAGTCAG | |||
| 130 | 61 | F: TGTGTTCTTCGCAGGGAATGAG | MMP2 |
| R: ACTCCAGTTAAA GGCAGCATCTAC | |||
| 152 | 61 | F: TCTTGGTTCCCTGGCGTACTC | TIMP1 |
| R: GACCTGATCCGTCCACAAACAG |
Table 1. Forward and reverse primers designed for genes analyses by real time PCR, annealing temperatures and their expected product sizes.
Results
Following the 48 h incubation period, the primary mesenchymal stem cells were isolated from adipose tissue; these single cells were attached to the surface area of the flask. The majority of these cells showed elongated, spindle and fibroblast-like appearance when observed under phase contrast microscopy (Figure 1).
Characterization of isolated mouse ADSCs
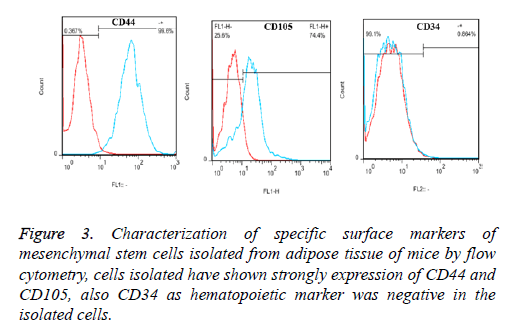
The results of flow cytometry analysis of Adipose Derived Mesenchymal Stem Cells (ADSCs) showed that expression percentage of specific surface markers of mesenchymal stem cells of mouse including CD44, CD105 and CD34 were evaluated as 99.6%, 74.4% and 0.86%, respectively (Figure 2). This result indicates isolated high purity of ADSCs and lack of contamination of these cells with blood cells.
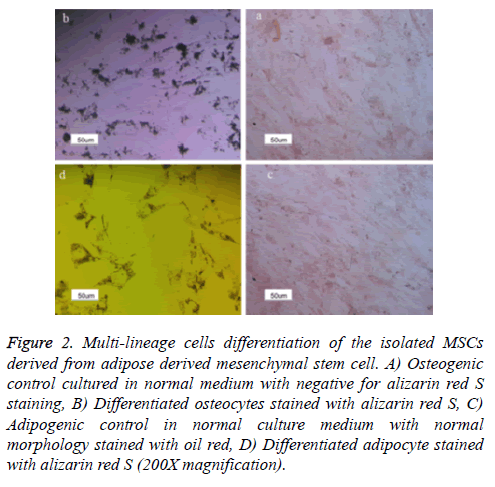
Figure 2: Multi-lineage cells differentiation of the isolated MSCs derived from adipose derived mesenchymal stem cell. A) Osteogenic control cultured in normal medium with negative for alizarin red S staining, B) Differentiated osteocytes stained with alizarin red S, C) Adipogenic control in normal culture medium with normal morphology stained with oil red, D) Differentiated adipocyte stained with alizarin red S (200X magnification).
Formation of the cytoplasmic lipid droplets was observed in isolated MSCs which had been exposed to adipogenic differentiation medium for 21 days after staining with Oil Red (Figures 3a and 3b), also Alizarin Red staining showed the presence of calcium and mineralization for cell receiving osteogenic differentiation medium for 21 days (Figures 3C and 3D).
Verification of diabetes in mice after STZ injection
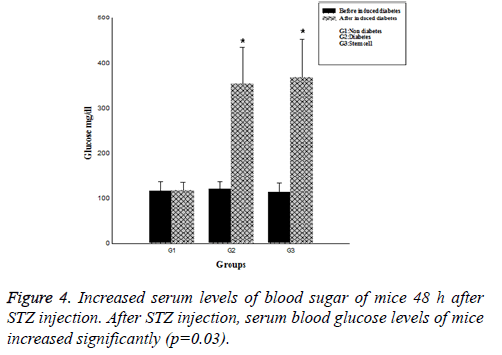
After 48 h, serum blood sugar level of the mice that had received a single high dose of STZ was more than 250 mg/dl, which was a significant increase compared to the control group (p=0.03). (Figure 4), Polyuria and polydipsia were also observed in these animals.
Evaluation of expression of genes using qPCR
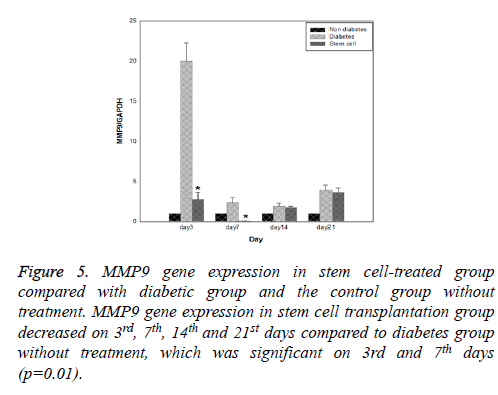
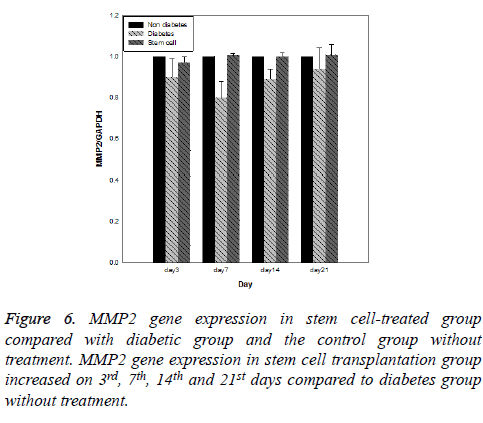
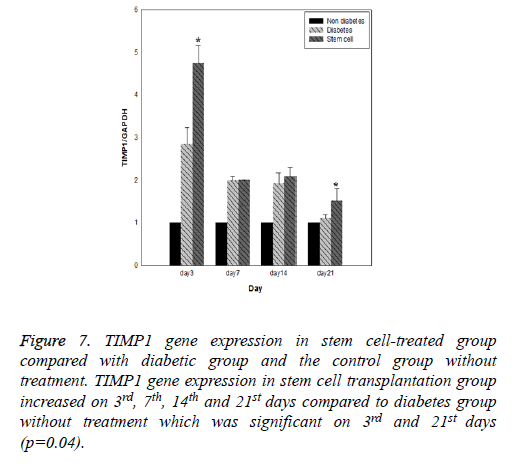
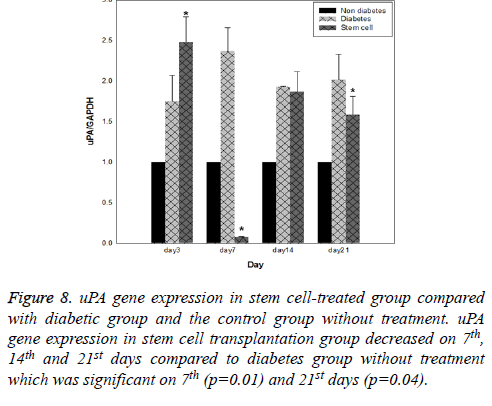
Results of expression of MMP9, MMP2, TIMP1 and uPA genes at intervals of the 3rd, 7th, 14th and 21st days in three evaluated groups are shown in Figures 5-8, respectively. Results of evaluation of gene expression of MMP9 in the area of the wound in the group treated with stem cells using the RT-qPCR technique showed reduction at 3rd, 7th, 14th and 21st days compared to the diabetic group (Figure 5). In diabetes group treated with ADSCs, MMP2 and TIMP1 genes showed higher expression at all days compared to the diabetic groups, gene expression of MMP-2 was the same in both groups; those treated with stem cells and non-diabetes (Figures 6 and 7). uPA gene expression had normal process on 3rd, 7th, 14th and 21st days at the wound area. Expression of this gene increased at the early stages of the repair process, an indication of uPA activity in the inflammatory phase that activates MMP enzymes in the wound; while expression of uPA decreased on 3rd, 7th, 14th and 21st days compared to the diabetic group without treatment (Figure 8). These results show that stem cells had an effective role in healing diabetes wound through expression of MMPs, TIMPs and uPA genes.
Figure 7: TIMP1 gene expression in stem cell-treated group compared with diabetic group and the control group without treatment. TIMP1 gene expression in stem cell transplantation group increased on 3rd, 7th, 14th and 21st days compared to diabetes group without treatment which was significant on 3rd and 21st days (p=0.04).
Figure 8: uPA gene expression in stem cell-treated group compared with diabetic group and the control group without treatment. uPA gene expression in stem cell transplantation group decreased on 7th, 14th and 21st days compared to diabetes group without treatment which was significant on 7th (p=0.01) and 21st days (p=0.04).
Discussion
Several studies to date have reported therapeutic effects of stem cells in wound healing. Many mechanisms have been reported for healing potency such as secretion of growth factors including PDGF, VEGF, TGF-ß and FGF. These Growth factors have a role in skin tissue regeneration with different properties such as anti-apoptotic, being angiogenic, increasing oxygenation, increasing migration of fibroblasts and increasing expression of extracellular matrix proteins [38-45]. Also, researchers showed that these cells have paracrine effects on adjacent cells in the skin and its role in healing wounds [46]. Other known mechanisms for the effect of stem cells in wound healing are related to the reduction of matrix metalloproteinase enzymes and in turn increased amounts of collagen and elastin, increased epidermal thickness and increased effect of dermal appendages in skin regeneration [47,48]. Considering the multilateral application of MSCs in the treatment of diseases, including wound healing, these cells are separated from various sources of the human body and are evaluated in related clinical and para-clinical trials [28]. MSCs can be isolated from various sources; adipose tissue is easily accessible and is considered as a source of stem cells that can be used to obtain a high number by a non-invasive method. Beneficial effects of these cells have been reported on skin regeneration. These cells secrete a variety of growth factors that can lead to restoration and replacement of defective cells. These factors are useful for skin regeneration, wound healing and wrinkle treatment [40-43]. In the present study, isolated MSCs from mouse adipose tissue were characterized by adipogenic and osteogenic differentiation, as well as CD44, CD105 and CD34 cell surface markers. Similar our study, regarding characterization of mice mesenchymal stem cells, Zhu et al,. Shen et al., Sagi observed positive expression of CD90 and CD105 as well as differentiated into adipocyte and osteocyte lineage [49-51].
Studies have shown that one of the most important factors in chronic wounds such as diabetic wounds is imbalance of protease and protease inhibitors that favor an increase in protease. Madhyastan et al. showed that uPA protein dissolved fibrin, increased migration, created cell proliferation and adhesion and healed the wound and a critical role in the early phase of wound healing [52]. Similarly, in our study, stem cells therapy in diabetic group significantly was increased expression of uPA gene compared with diabetic group in early stages of wound healing. Stem cells maybe cause increase expression of uPA in the wound area by increasing keratinocyte proliferation. Vaalamo et al. showed that MMPs and uPA proteins are secreted in acute and chronic wounds by leukocytes and keratinocytes, while TIMPs are not secreted in chronic wounds and balance between MMPs and TIMPs is disrupted in chronic wounds [53]. Also, results of this study showed that level of expression of uPA diabetic wounds without treatment was significantly increased compared to the level in normal mice (p<0.05). Studies have shown that abnormality in the inflammatory phase, formation of fibrin deposition, and ultimately impaired wound healing can be caused by a deficiency of plasmin due to decreased expression of TIMPs [25,26]. We have shown that, injection of mesenchymal stem cells in wound after induced diabetes increased expression of TIMP1. This increased expression of TIMP1 maybe accelerates healing process. Similarly, in a study done by Brew et al. showed that TIMPs have a role in wound healing activities such as cell growth and differentiation, cell migration and extracellular matrix synthesis process [34]. Annette showed that if tPA activity is inhibited then activity of some of MMPs including MMP9 decreases in wound healing [21] and greater activity of some of MMPs such as MMP9 disrupts cell migration and breaks down some essential matrix proteins and growth factors [12]. In this study, one of the most important changes observed after injection of the stem cells was decreased expression of MMP9 gene in diabetic wound area. These result indicated that stem cells can inhibit abnormal increase of MMP9 through increased expression of TIMP1. MMPs and their specific inhibitor have activity in some wound healing processes such as haemostasis, formation of epithelium and granulation tissue formation [53]. Results similar to those reported by Salo et al. was related to MMP2 gene expression, MMP2 expression remained constant during the wound healing process in group treated with stem cell and group without diabetes who had normal wound healing [54]. In this study, the expressions of MMP2, MMP9, TIMP1 and uPA after mesenchymal stem cells therapy in diabetic mice adjusted. This adjustment of expression affected on healing process.
Conclusion
Although presence of MMPs, uPA and TIMPs in the wound is required for the normal wound healing but an increase or decrease of each of these important factors could disrupt the healing process. According to the results obtained in this study, it seems that stem cells through expression of genes related to extracellular matrix remodeling at the wound area can have a significant role in improve diabetic wound healing of animal model.
Acknowledgements
This work was supported by Tarbiat Modares University (grant number: 9020192005).
References
- Guay C, Roggli E, Nesca V, Jacovetti C, Regazzi R. Diabetes mellitus, a microRNA-related disease? Transl Res 2011; 157: 253-264.
- Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol 2013; 9: 513-521.
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res ClinPract 2011; 94: 311-321.
- Tang X, Tang G, Ozcan S. Role of microRNAs in diabetes. BiochimBiophysActa 2008; 1779: 697-701.
- Darby IA, Bisucci T, Hewitson TD, MacLellan DG. Apoptosis is increased in a model of diabetes-impaired wound healing in genetically diabetic mice. Int J Biochem Cell Biol 1997; 29: 191-200.
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res ClinPract 2010; 87: 4-14.
- Chen JS, Wong VW, Gurtner GC. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front Immunol 2012; 3: 192.
- Lateef H, Abatan OI, Aslam MN, Stevens MJ, Varani J. Topical pretreatment of diabetic rats with all-trans retinoic acid improves healing of subsequently induced abrasion wounds. Diabetes.2005; 54: 855-861.
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010; 89: 219-229.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9: 283-289.
- Roy S, Sen CK. MiRNA in innate immune responses: novel players in wound inflammation. Physiol Genomics 2011; 43: 557-565.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005; 366: 1736-1743.
- Collier M. Understanding wound inflammation. Nurs Times 2003; 99: 63-64.
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 2009; 37: 1528-1542.
- Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE 2010; 5: e9539.
- Sharma RK, John JR. Role of stem cells in the management of chronic wounds. Indian J PlastSurg 2012; 45: 237-243.
- Zuk PAZM, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7: 211-228.
- Cha J, Falanga V. Stem cells in cutaneous wound healing. ClinDermatol 2007; 25: 73-78.
- Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ. Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review. Gerontology 2016; 62: 216-225.
- Kanno Y, Ishisaki A, Kawashita E, Kuretake H, Ikeda K, Matsuo O. uPA attenuated lps-induced inflammatory osteoclastogenesis through the plasmin/PAR-1/Ca(2+)/CaMKK/AMPK axis. Int J BiolSci 2016; 12: 63-71.
- Wysocki ABKA, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen 1999; 7: 154-165.
- Lund LR, Green KA, Stoop AA, Ploug M, Almholt K, Lilla J. Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J 2006; 25: 2686-2697.
- Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med 1996; 2: 287-292.
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2: 161-174.
- Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol 2004; 25: 450-455.
- Lund LR, Romer J, Bugge TH, Nielsen BS, Frandsen TL. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J 1999; 18: 4645-4656.
- Maral S, Acar M, Balcik OS, Uctepe E, Hatipoglu OF, Akdeniz D. Matrix metalloproteinases 2 and 9 polymorphism in patients with myeloproliferative diseases: a strobe-compliant observational study. Medicine 2015; 94: e732.
- Vaalamo MWM, Puolakkainen P, Kere J, Saarinen P, Lauharanta J. Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 1996; 135: 52-59.
- Salonurmi T, Parikka M, Kontusaari S, Pirila E, Munaut C. Overexpression of TIMP-1 under the MMP-9 promoter interferes with wound healing in transgenic mice. Cell Tissue Res 2004; 315: 27-37.
- Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008; 40: 1334-1347.
- Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen 1999; 7: 433-441.
- McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care (New Rochelle) 2013; 2: 438-447.
- Leco, Apte SS, Taniguchi GT, Hawkes SP, Khokha R. Murine tissue inhibitor of metalloproteinases-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett 1997; 401: 213-217.
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. BiochimBiophysActa 2010; 1803: 55-71.
- Davoodian N, Lotfi AS, Soleimani M, Mowla SJ. MicroRNA-122 overexpression promotes hepatic differentiation of human adipose tissue-derived stem cells. J Cell Biochem 2014; 115: 1582-1593.
- Sakata N, Yoshimatsu G, Tsuchiya H, Egawa S, Unno M. Animal models of diabetes mellitus for islet transplantation. Exp Diabetes Res 2012; 2012: 256707.
- Wang X, Ge J, Tredget EE, Wu Y. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Protoc 2013; 8: 302-309.
- Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 2008; 5: 453-463.
- Liu Y, Dulchavsky DS, Gao X, Kwon D, Chopp M, Dulchavsky S. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res 2006; 136: 336-341.
- Moon KM, Park YH, Lee JS, Chae YB, Kim MM. The effect of secretory factors of adipose-derived stem cells on human keratinocytes. Int J MolSci 2012; 13: 1239-1257.
- Kim WS, Park BS, Sung JH, Yang JM, Park SB. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J DermatolSci 2007; 48: 15-24.
- Baer PC, Geiger H. Adipose derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012; 2012: 812693.
- Niemela S, Miettinen S, Sarkanen JR, Ashammakhi N. Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. Top Tissue Eng 2008; 4.
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004; 109: 1292-1298.
- Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS. Evidence supporting antioxidant action of adipose-derived stem cells: Protection of human dermal fibroblasts from oxidative stress. J DermatolSci 2008; 49: 133-142.
- Lee SH1, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol 2012; 24: 136-143.
- Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther 2016; 7: 37.
- Bishopric NH. Mesenchymal stem cell-derived IL-10 and recovery from infarction: a third pitch for the chord. Circ Res 2008; 103: 125-127.
- Zhu H, Guo ZK, Jiang XX, Li H, Wang XY. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc 2010; 5: 550-560.
- Shen J, Tsai YT, Dimarco NM, Long MA, Sun X. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci Rep 2011; 1: 67.
- Sági B, Maraghechi P, Urbán VS, Hegyi B, Szigeti A. Positional identity of murine mesenchymal stem cells resident in different organs is determined in the postsegmentation mesoderm. Stem Cells Dev 2012; 21: 814-828.
- Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. Curcumin facilitates fibrinolysis and cellular migration during wound healing by modulating urokinase plasminogen activator expression. PathophysiolHaemostThromb 2010; 37: 59-66.
- Vaalamo M, Leivo T, Saarialho-Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol 1999; 30: 795-802.
- Salo T, Makela M, Kylmaniemi M, Autio-Harmainen H, Larjava H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab Invest 1994; 70: 176-182.