- Biomedical Research (2011) Volume 22, Issue 4
Acidic Xylooligosaccharide promotes recovery from iron deficiency anemia by enhancing serum iron level in rats
Yukiko Kobayashi1*, Etsuko Wakasugi1, Takayuki Ohbuchi1, Meiko Yokoyama1, Risa Yasui1, Masa-shi Kuwahata1, Yukihiro Nakabou2, Yasuhiro Kido1.1Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Kyoto 606-8522, Japan
2Graduate School of Health and Welfare, Kawasaki University of Medical Welfare, Kurashiki 701-0193, Japan
- Corresponding Author:
- Yukiko Kobayashi
Kyoto Prefectural University
Shimogamo, Sakyo, Kyoto 606-8522
Japan
Accepted Date: May 11 2011
Abstract
Iron deficiency anemia (IDA) is one of the most serious forms of malnutrition. A promo-tion function of mineral absorption has been reported for neutral oligosaccharides, but not acidic xylooligosaccharide (U-XOS), which is a novel oligosaccharide. We hypothe-sized that U-XOS could promote recovery from IDA by enhancing the serum iron level. Therefore, the aim of this study was to investigate whether or not U-XOS was useful for recovery from IDA in the rat. Twenty-four female Sprague-Dawley rats were randomly divided into a control group fed a control diet (4.0 mg Fe /100 g) and an IDA model group created by employing a low-iron diet (0.4 mg Fe /100 g). The IDA group was further di-vided into three subgroups on day 21: U-XOS-supplemented diet (LI-X, 4.0 mg Fe /100 g), low-iron diet (LI), and control diet (LI-C) groups. No significant differences in the serum iron transferrin saturation levels were demonstrated between the control and LI-X groups on days 26 and 35. The divalent metal transporter 1 and ferroportin mRNA expression levels shown in the first segment of the small intestines showed a significant decrease in the LI-X group, compared with the LI group. A significant decrease in the hepatic hep-cidin mRNA expression level and iron content was also demonstrated in the LI-X group, compared with the control group, but not compared with the LI group. These results sug-gested that U-XOS could promote recovery from IDA by enhancing serum iron at an early stage of the recovery process.
Keywords
Indigestible oligosaccharide; Xylooligosaccharide; Iron deficiency anemic rat; Iron absorption; Prebiotics
Introduction
It is well known that iron deficiency anemia (IDA) is one of the most serious forms of malnutrition. According to the World Health Organization, it is estimated that more than 1.6 billion people are suffering from anemia, and approximately half of these people have IDA [1], showing that IDA has been increasing worldwide.
In order to achieve any improvement in subjects with IDA, dietary management is most important in order to increase the dietary iron intake. However, there are three major factors that affect iron absorption. The first factor is the form of dietary iron. Most dietary iron is in the form of Fe3+, and is usually reduced to Fe2+ by the Fe-reduction factor and imported into the mucosal cells by the divalent metal transporter 1 (DMT1), which is one of the iron transporters in the small intestines [2]. The second factor is the storage iron in the body. If serum iron levels and iron stores are decreased, secretion of the hepcidin from the liver decreases, and then the expression of iron trans-porter ferroportin, which exports Fe2+ from cells on the basolateral membrane, increases. As a result, the amount of iron absorption increases [3-6]. The third factor is the consumption of dietary components. Several food factors have been demonstrated to enhance iron absorption, for example, ascorbic acid [7-9], cysteine-containing peptides [10-12], phosphopeptide [13-15], sugar alcohol [16,17], polysaccharides [18,19], and so on. However, relatively little attention has been paid to the effects of the food ma-trix on iron absorption.
Recently, it was reported that indigestible oligosaccha-rides, such as fructooligosaccharide [20,21], difructose anhydride III [22-24], and xylooligosaccharide [25], pro-mote mineral absorption. Acidic xylooligosaccharide (U-XOS) is a novel oligosaccharide in which glucronic acid is linked to xylooligosaccharide by α-1, 2 bonds [26]. U-XOS has been reported to have an antibacterial effect, inhibitory effects on stress-induced gastric inflammation, preventive effects on contact hypersensitivity, and preven-tive effects on the development of atopic dermatitis [27,28]. However, the promotive effects of U-XOS on serum iron and recovery from IDA have not been examined to date.
Therefore, we hypothesized that U-XOS could promote recovery from IDA by enhancing the serum iron level. Accordingly, the aim of this study was to investigate whether or not U-XOS was useful for recovery from IDA in the rat.
Methods and Materials
The structure and composition of Acidic xylooligosac-charide (U-XOS)
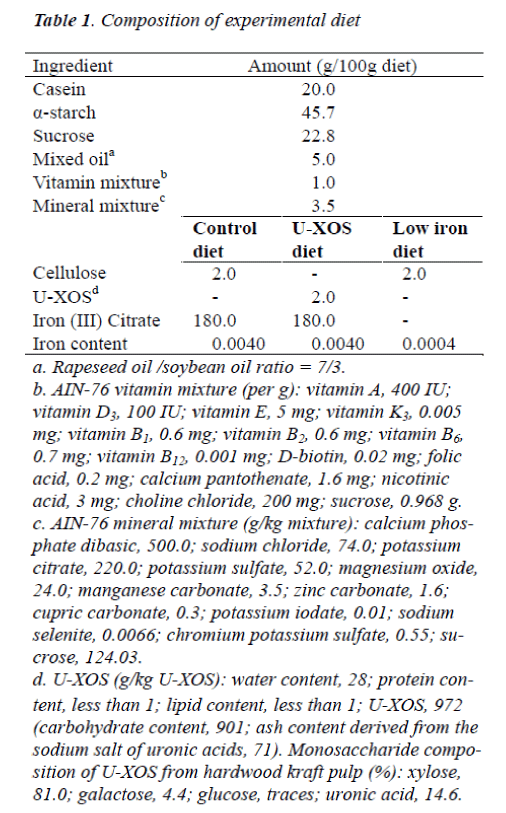
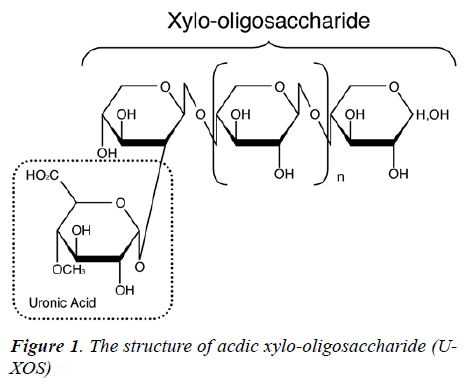
The structure of U-XOS is shown in Figure 1. U-XOS contains one or more 4-O-methyl glucuronic acids as side chains and it is a xylo-oligomer with an average of 10 polymerization units. U-XOS is prepared from hardwood kraft pulp, such as eucalyptus, according to the method described by Izumi et al. [29]. The U-XOS employed in this study was kindly donated by Oji Paper (Tokyo, Ja-pan). The composition of U-XOS was shown in Table 1 [26].
Study design
This study was conducted in accordance with the guide-lines for animal experimentation published by Kyoto Pre-fectural University. Twenty-four female Sprague-Dawley rats aged 4 weeks (Japan SLC, Hamamatsu, Japan) were employed in the study. The rats were individually housed in stainless steel cages at a controlled temperature of 22 – 24°C, a relative humidity of 40 – 60%, and a light cycle of 12 hours with free access to distilled water (the iron content of the distilled water had been measured). The composition of the diets used in the experiment was as shown in Table 1. The rats were randomly divided into two groups. For the first group (C group, n = 6, weighing 106 – 112 g), a control diet was supplied for 35 days. The second group (base LI group, n = 18, weighing 94 – 121 g), fed a low-iron diet for 21 days, was employed to cre-ate IDA rats. IDA rats were then divided three subgroups on the basis of weight and hemoglobin concentration. Each subgroup was fed either a low-iron (LI) diet (LI group, n = 6, weighing 155 – 195 g), a U-XOS–supplemented diet (LI-X group, n = 6, weighing 153 – 177 g), or the control diet (LI-C group, n = 6, weighing 163 – 182 g) for another 14 days. The LI-C and LI-X groups were provided with the diet on the following day, with the same amount of diet freely provided to the LI group during the pair-feeding period. All diets were pre-pared according to the AIN-76 formulation with one modification (addition of choline chloride). The LI diet contained 0.4 mg Fe /100 g without any ferrous citrate in the mineral mixture: U-XOS was added to 2% of the whole diet by substituting for cellulose. Blood samples, all 280 μL, were drawn from the tail vein of all of the animals every 4 days during the last 14 days of the study. Body weight and food intake were recorded at the same time everyday. The rats were euthanized during the early phase of the light cycle in a non-fasting state, by cervical dislocation under ether anesthesia, and blood samples drawn from the inferior vena cava were collected in tubes with heparin. Samples of the liver and the small intestinal mucosa (upper side, 1/4th) were also collected.
Blood constituent analysis
Hemoglobin concentration was measured using Hemo-globin B-test Wako (Wako Pure Chemical Industries, Osaka, Japan). The hematocrit level was measured after centrifugation of the blood at 12,000 rpm for 5 minutes at 4°C. Serum iron levels and unsaturated iron binding ca-pacity were measured using Detaminer Fe and UIBC (Kyowa Medix, Tokyo, Japan) with an automatic bio-chemical analyzer (CL-8000, Shimadzu, Kyoto, Japan).
Total iron binding capacity (TIBC) and serum transferrin saturation were calculated as follows:
TIBC = serum iron + UIBC
Serum transferrin saturation = serum iron / TIBC ×100.
Estimation of gene expression
Total RNA was isolated from the homogenized mucosa and liver samples using Total RNA Isolation mini kit (Agilent Technologies, California, USA). The cDNA was synthesized from isolated RNA using ReverTra Ace (To-yobo, Osaka, Japan), according to the manufacturer’s in-structions. A real-time polymerase chain reaction (PCR) for gene expression analysis was performed using DNA Engine Opticon and Opticon Monitor software (Bio-Rad Laboratories, California, USA). TaqMan primer pairs /probes for gene analysis were obtained using TaqMan Gene Expression Assay (Applied Biosystems, California, USA): Rn00565927_m1: DMT1, Rn00591187_m1: Fer-roportin, Rn00584987_m1: Hepcidin, Rn00667869_m1: β-actin. Reactions were performed with 10 μL of Premix EX Taq (Takara Bio, Ohtsu, Japan), 1 μL of the primer pairs /probes sets and 3 μL of cDNA in a final volume of 20 μL. After heating the test sample at 96°C for 10 sec-onds, 50 PCR cycles were performed as follows: 95°C for 7 seconds, 60°C for 30 seconds and 72°C for 20 seconds. DMT1, ferroportin, and hepcidin mRNA were normalized against the mRNA expression of the housekeeping gene β-actin.
Iron content of hepatic tissue
All liver samples were perfused with saline, and weighed (0.5g), and treated by the wet-ash method using a micro-wave extraction system (Ethos-1000, Milestone, Italy). The samples were diluted to 10 mL with 0.5% hydrochlo-ric acid solution after evaporation to dryness. After suit-able dilution, iron concentrations of the samples were measured by flame Zeeman-effect atomic absorption spectrometry (Z-6100, Hitachi, Tokyo, Japan) using a standard solution (Wako Pure Chemical Industries, Osaka, Japan). We determined that the coefficient of variation was 0.04. Iron concentrations were expressed on a wet-weight basis.
Statistical analyses
Data (C, LI, LI-X and LI-C group, n = 6; base LI group, n = 18) were presented as means ± standard error (SEM). Before assessing the different variables, we carried out a Bartlett test to check the normal distribution of the vari-ables. Data that fit the normal distribution were compared by 1-way analysis of variance (ANOVA) followed by the Tukey-Kramer test (Figs. 2 – 4; all parameters on day 35, Table 2), or Student’s t test (all parameters on day 21, Ta-ble 2). The level of significance was set at P < 0.05.
Results
Effect of U-XOS on recovery from IDA
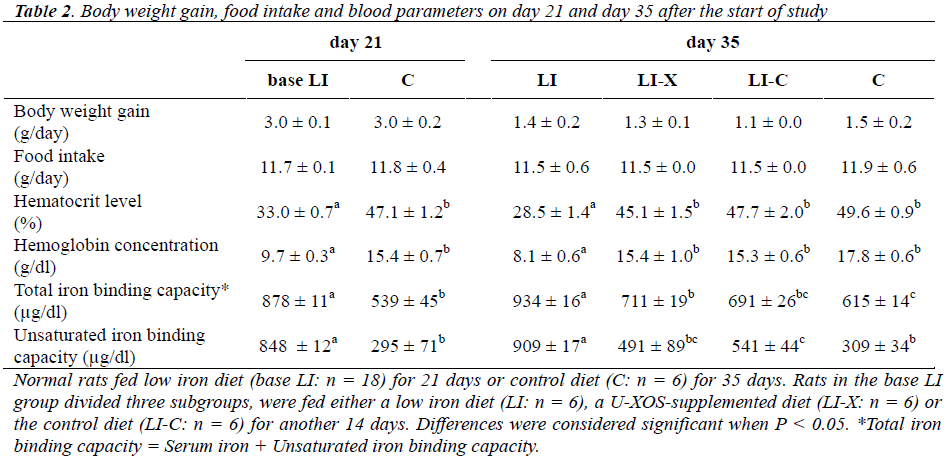
Body weight gain, food intake and blood parameters on day 21 and day 35 after the start of study are shown in Table 2. No significant difference in body weight gain and food intake was observed between the two base groups on day 21 or among the four subgroups on day 35. On day 21, the hematocrit level and hemoglobin concentration of the base LI group (LI, LI-X and LI-C group) were lower than those of the C group, and the total iron binding capacity and unsaturated iron binding capacity of the base LI group increased compared with the C group, confirming that the LI group had developed IDA. On day 35, the he-matocrit level and the hemoglobin concentration of the LI-X group were higher than those of the LI group, but no significant difference was demonstrated between the C and LI-C groups, indicating that the LI-X and LI-C groups showed recovery from IDA. The total iron binding capacity of the LI-X group decreased remarkably com-pared with that of the LI group and increased compared with the C group, and both differences were statistically significant. The unsaturated iron binding capacity of LI-X group decreased significantly compared with the LI group and increased compared the C group, but no statistical difference was shown between the LI-X and C groups.
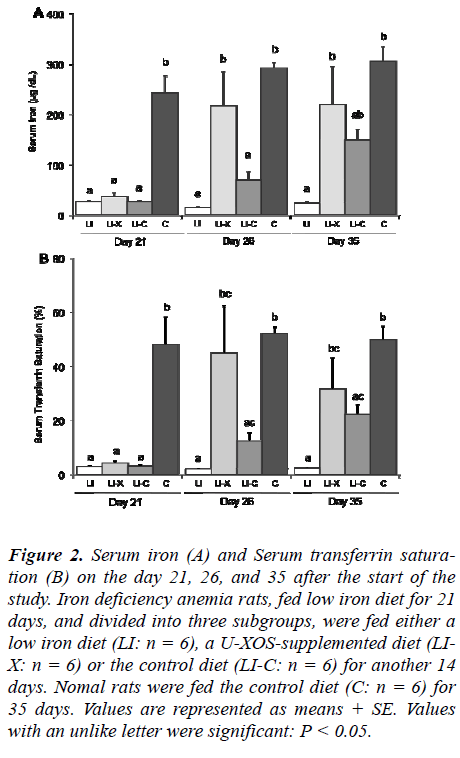
Figure 2 shows the changes in the serum iron and trans-ferrin saturation level of the groups on day 21, 26, and 35 after the start of the study. On day 21, the serum iron and transferrin saturation level of the base LI group (LI, LI-X and LI-C groups) were significantly lower than those of the C group, confirming that the base LI group had devel-oped an iron deficiency state. The serum iron level of the LI-X group drastically increased compared with that of the LI and LI-C groups, but no significant difference was demonstrated compared with the C group on day 26. On day 35, the serum iron level of the LI-X group had been maintained at the same level as that on day 26. Showing a pattern similar to the serum iron level, the serum transfer-rin saturation level of the LI-X group markedly increased compared with the LI and LI-C groups on day 26, but it was comparable with the C group on day 26 and 35.
Figure 2: Serum iron (A) and Serum transferrin satura-tion (B) on the day 21, 26, and 35 after the start of the study. Iron deficiency anemia rats, fed low iron diet for 21 days, and divided into three subgroups, were fed either a low iron diet (LI: n = 6), a U-XOS-supplemented diet (LI-X: n = 6) or the control diet (LI-C: n = 6) for another 14 days. Nomal rats were fed the control diet (C: n = 6) for 35 days. Values are represented as means + SE. Values with an unlike letter were significant: P < 0.05.
Expression of transporter mRNA
Figure 3 shows the iron transporter DMT1 and ferroportin mRNA expression level in first segment of the small in-testines on day 35 after the start of the study. The DMT1 mRNA expression levels of the LI-X group were signifi-cantly lower than those of the LI group, but no statistical difference was shown between the LI-X and the other groups. The ferroportin mRNA expression level of the LI-X group was remarkably decreased compared with that of the LI group, and was lower than that of the LI-C group. However, these decreases were not statistically significant. No significant difference was shown in the ferroportin mRNA expression level between the LI-X and C groups.
Figure 3: Iron transporter DMT1 (A) and Ferroportin (B) mRNA expression in the first segment of small intestines (upper side, 1/4th) on day 35 after the start of the study. Iron deficiency anemic rats, fed a low iron diet for 21 days, and divided into three subgroups, were fed either a low iron diet (LI: n = 6), a U-XOS-supplemented diet (LI-X: n = 6) or the control diet (LI-C: n = 6) for another 14 days. Nomal rats were fed the control diet (C: n = 6) for 35 days. Values are represented as means + SE. Values with an unlike letter were significant: P < 0.05
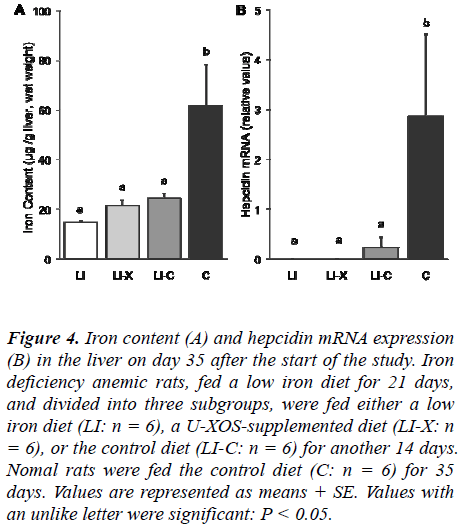
Iron content and hepcidin mRNA in the liver
Iron content and hepcidin mRNA expression levels in the liver on day 35 after the start of the study are shown in Figure 4. On day 35 after the start of the study, the LI-X group showed a remarkable and statistically significant decrease in hepatic iron content compared with the C group, but no statistically significant difference was shown between the LI-X and the other groups. The he-patic hepcidin mRNA expression levels of the LI-X group decreased significantly compared with those of the C group, but no statistically significant difference was shown between the LI-X and the other groups.
Figure 4: Iron content (A) and hepcidin mRNA expression (B) in the liver on day 35 after the start of the study. Iron deficiency anemic rats, fed a low iron diet for 21 days, and divided into three subgroups, were fed either a low iron diet (LI: n = 6), a U-XOS-supplemented diet (LI-X: n = 6), or the control diet (LI-C: n = 6) for another 14 days. Nomal rats were fed the control diet (C: n = 6) for 35 days. Values are represented as means + SE. Values with an unlike letter were significant: P < 0.05.
Discussion
In the present study, we investigated whether or not U-XOS was useful in achieving recovery from IDA using a rat model. Our results demonstrated that U-XOS could promote recovery from IDA by enhancing serum iron and transferrin saturation levels at an early stage of the recov-ery process. It is necessary for dietary iron to be reduced from Fe3+ to Fe2+ ions [30,31]. Therefore, it appeared that soluble Fe2+ ions in the small intestines were increased in quantity by U-XOS. In regard to the mechanisms by which U-XOS dissolves and reduces dietary iron, this may be related to the intermolecular force between U-XOS molecules, which may vary according to the number of uronic acid groups per chain, or related to the average sugar unit of U-XOS.
On the other hand, it has been reported that DMT1 ex-pression is dramatically induced in IDA [32]. These find-ings suggested that the reason the DMT1 mRNA expres-sion level resulting from the ingestion of U-XOS showed no significant difference, compared with the normal con-trols, was because the DMT1 mRNA expression was regulated by recovery from IDA at the end of the experi-ment period. However, DMT1 mRNA expression might increase at the stage where there is an observed rise in the serum iron level due to U-XOS ingestion. On the other hand, the diet with no U-XOS showed that the capability to export iron ions from the epithelial cells by ferroportin was still active at the end of the experiment.
In this study, a decrease in hepatic hepcidin and intestinal ferroportin mRNA expression was shown on day 35 in rats fed the U-XOS diet. The level of hepatic hepcidin mRNA expression decreases in response to iron defi-ciency [33,34]. In the present study, the level of hepatic iron concentration and hepcidin mRNA expression sig-nificantly decreased in the LI group compared with con-trol group on day 35. The level of hepatic iron level and hepcidin mRNA did not increase in the LI-X group com-pared with the control group on day 35. However, the serum iron level of LI-X group increased to almost same level of the control group on day 26 and 35. Moreover, the ferroportin mRNA level of the LI-X group was re-duced to almost the same level as the control group on day 35. There is the possibility that the regulation of fer-roportin mRNA expression under conditions of iron defi-ciency without anemia will be influenced not only by hepcidin, but also by the serum iron or transferrin satura-tion levels. Iron deficiency without anemia is caused pri-marily by asiderosis. Despite the status of the iron defi-ciency, this regulation of hepcidin and ferroportin mRNA expression is disadvantageous for an early recovery from asiderosis. The fact that the treatment of asiderosis re-quires a long-term administration of iron may be the re-sult of such adverse regulation.
The limitations of the current study include the fact that we did not directly examine or measure the iron absorp-tion rate. In order to elucidate the effects of U-XOS on the promotion of iron absorption, further studies should be conducted to investigate the iron balance. In addition, the IDA rats did not show any recovery of storage iron, al-though they were administered a diet containing iron for 14 days. However, it should be possible to examine the effect on storage iron with a U-XOS diet by lengthening the experimental period. Furthermore, from the viewpoint of preventing IDA, we should also examine the efficacy of U-XOS in regard to the difference in the amount of iron intake. Moreover, another limitation of this study was that we did not assay the short chain fatty acids and the organic acid levels. Indigestible oligosaccharides such as U-XOS reach the large intestine without undergoing en-zymatic degradation, are fermented by intestinal bacteria [35], and produce the short chain fatty acid and the or-ganic acid [36]. It has been suggested that the bacterial generation of short-chain fatty acids from oligosaccha-rides increases iron bioavailability [22,37]. U-XOS is also a prebiotic [28,38]. Therefore, one of the items on our agenda for future studies is to investigate the in-volvement of U-XOS in iron bioavailability.
In conclusion, we demonstrated that U-XOS could be employed to promote recovery from anemia by rapidly increasing the serum iron and transferrin saturation levels. The use of U-XOS is likely to provide important clues for dietetic treatment, in combination with dietary carbohy-drates, which will be useful for promoting recovery from IDA.
Acknowledgments
This work was supported by Grant-in-Aids for Young Scientists B (No. 19700593) from the Japan Society for the Promotion of Science. We would like to thank Profes-sor Kenji Sato and Professor Hiroshi Ichikawa for their helpful discussions. We would like to thank Professor Kaoru Yoshida and Kohtaro Ishikawa for their technical support. We would also like to thank Shunsuke Ito, Eriko Tada, Yui Miyazawa, Tomo Fukuda, and Mai Hamada for their technical assistance.
References
- World Health Organization. Worldwide prevalence of anaemia 1993-2005 WHO Global Database on Anae-mia. Geneva, Switzeland: World Health Organization 2008.
- McKie AT, Barrow D, Latunde-Dada GO, et al. An iron-regulated ferric reductase associated with the ab-sorption of dietary iron. Science 2001; 291: 1755-1759.
- Donovan A, Brownlie A, Zhou Y, et al. Positional clon-ing of zebrafish ferroportin1 identifies a conserved ver-tebrate iron exporter. Nature 2000; 403: 776-781.
- Anderson GJ, Frazer DM. Recent advances in intestinal iron transport. Curr Gastroenterol Rep 2005; 7: 365-371.
- Mena NP, Esparza A, Tapia V, et al. Hepcidin inhibit apical iron uptake in intestinal calls. Am J Physiol Gas-trointest Liver Physiol 2008; 294: 192-198.
- Ikuta K. Function of hepcidin in iron metabolism. Rin-sho Ketsueki 2007; 48: 36-45.
- Ballota D, Baynesa RD, Bothwell TH, et al. The effects of fruit juices and fruits on the absorption of iron from a rice meal. Br J Nutr 1987; 57: 331-343.
- Cook JD, Monsen ER. Vitamin C, the common cold, and iron absorption. Am J Clin Nutr 1977; 30: 235-241.
- Bjorn-Rasmussen E, Hallberg L. Iron absorption from maize. Effect of ascorbic acid on iron absorption from maize asupplemented with ferrous sulphate. Nutr Metab 1974; 16: 94-100.
- Taylor PG, Martinez-Torres C, Romano EL, et al. The effect of cysteine-containing peptides released during meat digestion on iron absorption in humans. Am J Clin Nutr 1986; 43: 68-71.
- Martinez-Torres C, Layrisse M. Effect of amino acid on iron absorption from a staple vegetable food. Blood 1970; 35: 669-682.
- Hurrell RF, Reddy MB, Juillerat M,et al. Meat protein fractions enhance nonheme iron absorption in humans. J Nutr 2006; 136: 2808-2812.
- Sato R, Noguchi T, Naito H. Casein phosphopeptide (CPP) enhances calcium absorption from the ligate segment of rat small intestines. J Nutr Sci Vitaminol 1986; 32: 67-76.
- Aît-Oukhatar N, Bouhallab S, Arhan P, et al. Iron tissue storage and hemoglobin levels of deficient rats repleted with iron bound to the caseinophosphopeptide 1-25 of beta-casein. J Agric Food Chem 1999; 47: 2786-2790.
- Bouhallab S, Cinga V, AÃt-Oukhatar N, et al. Influence of various phosphopeptides of caseins on iron absorp-tion. J Agric Food Chem 2002; 50: 7127-7130.
- Goda T, Yamada M, Takase S, et al. Effect of maltitol intake on intestinal calcium absorption in the rat. J Nutr Sci Vitaminol 1992; 38: 277-286.
- Brommage R, Binacua C, Antille S, et al. Intestinal calcium absorption in rats is stimulated by dietary lac-tulose and other resistant sugars. J Nutr 1993; 123: 2186-2194.
- Hara H, Suzuki T, Kasai T, et al. Ingestion of guar-gum hydrolysate partially restores calcium absorption in the large intestine lowered by suppression of gastric acid secretion in rats. Br J Nutr 1999; 81: 315-321.
- Watanabe O, Hara H, Aoyama Y, et al. Increased intes-tinal calcium absorption from the ingestion of a phos-phorylated guar gum hydrolysate independent of cecal fermentation in rats. Biosci Biotechnol Biochem 2000; 64: 613-616.
- Ohta A, Ohtsuki M, Baba S, et al. Effect of fructooligo-saccharides on the absorption of iron, calcium and magnesium in iron-deficient anemic rats. J Nutr Sci Vi-taminol 1995; 41: 281-291.
- Sakai K, Ohta A, Shiga K, et al. The cecum and dietary short-chain fructooligosaccharides are involved in pre-venting post gastrectomy anemia in rats. J Nutr 2000; 130: 1608-1612.
- Afsana K, Shiga K, Ishizuka S, et al. Ingestion of an indigestible saccharide, difructose anhydride III, par-tially prevents the tannic acid-induced suppression of iron absorption in rats. J Nutr 2003; 133: 3553-3560.
- Asvarujanon P, Ishizuka S, Hara H. Promotive effect of non-digestible disaccharides on rat mineral absorption depend on the type of saccharide. Nutrition 2005; 21: 1025-1035.
- Suzuki T, Hara H, Kasai T, et al. Effects of difructose anhydride III on calcium absorption in small and large intestine of rats. Biosci Biotechnol Biochem 1998; 62: 837-841.
- Toyoda Y, Umeyama T, Suwa Y, et al. Mineral absorp-tion promoter composition. Japanese Patent 1995; JP7067575.
- Ohbuchi T, Takahashi T, Azumi N, et al. Structural analysis of neutral and acidic xylooligosaccharides from hardwood kraft pulp, and their utilization by in-testinal bacteria in vitro. Biosci Biotechnol Biochem 2009; 73: 2070-2076.
- Ohbuchi T, Sakaino M, Takahashi T, et al. Oral admini-stration of acidic xylooligosaccharides prevents the de-velopment of atopic dermatitis-like skin lesions in NC/Nga mice. J Nutr Sci Vitaminol 2010; 56: 54-59.
- Ohbuchi T, Sakaino M, Haga I, et al. Prebiotic Effects of Xylooligosaccharide Derived from Hardwood Xylan. J Advd Food Ingred 2010; 13: 10-18.
- Izumi Y, Azumi N, Furujyo A, Kimura T, Uotsu N, Sa-kamoto R. Acidic oligosaccharide composition and the process of manufacture. Japanese Patent 2003; JP183303.
- Andrews NC. Forging a field: the golden age of iron biology. Blood 2008; 112: 219-230.
- Gunshin H, Fujiwara Y, Custodio AO, et al. Slc11a2 is required for intestinal iron absorption and erythropoi-esis but dispensable in placenta and liver. J Clin Invest 2005; 115: 1258-1266.
- Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997; 388: 482-488.
- Nicolas G, Chauvet C, Viatte L, et al. The gene encod-ing the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 2002; 110: 1037-1044.
- Adamsky K, Weizer O, Amariglio N, et al. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol 2004; 124: 123-124.
- Okazaki M, Fujikawa S, Matsumoto N. Effect of xy-looligosaccharide on the growth of bifidobacteria. J Jpn Soc Nutr Food Sci 1990; 43: 395-401.
- Sakata T, Kojima T, Fujieda M, et al. Influence of pro-biotic bacteria on organic acid production by pig caecal bacteria in vitro. Proc Nutr Soc 2003; 62: 73-80.
- Younes H, Demigné C, Rémésy C. Acidic fermentation in the caecum increases absorption of calcium and magnesium in the large intestine of the rat. Br J Nutr 1996; 75: 301-304.
- Okazaki M, Fujikawa S, Matsumoto N. Effect of xy-looligosaccharide on the growth of bifidobacteria. Bifi-dobacteria Microflora 1990; 9: 77-86.