Research Article - Biomedical Research (2021) Current Trends and Challenges in Biomedical Studies
Acetyl-CoA synthetase and isopentyl-diphosphate isomerase inhibition underscore columbin anti trypanosomiasis: Computational studies.
1Center for Bio-Computing and Drug Development, Adekunle Ajasin University, Akungba-Akoko, Nigeria
2Department of Biochemistry, Adekunle Ajasin University, Akungba-Akoko, Nigeria
- Corresponding Author:
- Omotuyi, I.O
Center for Biocomputing and Drug Development
Adekunle Ajasin University, Akungba-Akoko
Ondo state, Nigeria
E-mail: Olaposi.omotuyi@aaua.edu.ng
Accepted date: July 31, 2019
Abstract
Columbin, a plant-derived compound may represent a key frontier in the treatment of blood-stage trypanosomiasis. To be a viable future drug candidate, there is an urgent need to decipher its physiological target along the mevalonate pathway and to modify the original scaffold in order to improve its physicochemical properties and its ability to cross the blood-brain barrier. In this study, homology modeling method was used to generate the 3D models of the putative targets along the mevalonate pathway. Autodock-vina was used to dock columbin into the ligand pockets of the targets. The result showed that acetyl-CoA synthetase (-9.3 kcal/mol Vs -9.3 kcal/mol standard) and isopentyl- diphosphate isomerase (-9.6 kcal/mol vs. -8.5 kcal/mol standard) were the most plausible targets of columbin. In conclusion, acetyl-CoA synthetase and isopentyl-diphosphate isomerase inhibition may underscore the anti-trypanosomal actions of columbin targeting isoprenoid metabolism.
Keywords
Acetyl-CoA synthetase, Trypanosomiasis, Isopentyl-diphosphate isomerase.
Introduction
Trypanosoma brucei transmitted by tsetse flies (Glossina species) is the causative agent of human trypanosomiasis (also referred to as sleeping sickness) [1]. Besides human population, animals such as cattle are also at risk of sleeping sickness caused principally by T. congolense and T. vivax infection [2]. In highly endemic areas, trypanosomiasis constitutes severe socio-economic risk [3]. Human trypanosomiasis has two well-defined stages; the first is trypanosomes within the hemo-lymphatic system and the second stage is the colonization of the central nervous system with attendant disruption of sleep/wake patterns in patients. Metabo-pathologic adaptation response of trypanosomes within the invaded hemo-lymphatic and nervous systems are being studied and key mediators are emerging as drug targets in current research [4-6]. Drugs such as fexinidazoles, a nitroimidazole completely cured CNS mouse model of trypanosomiasis following oral administration for 5 days with no observed mutagenicity [7]. Preliminary data from mouse and monkey models of trypanosomiasis are also promising for diamidine 2, 5-bis (5-amidino-2-pyridyl) furan (CPD-0802), 2, 5-bis [5-(N-methoxyamidino)-2-pyridyl] furan (DB-868) which are dicationic molecules [8]. Yet SCYX-7158, an orally- active benzoxaborole has demonstrated preclinical potency for the treatment of human trypanosomiasis [9,10]. The cost of these drugs and their availability may prove a challenge in low- and-medium resource countries where unfortunately human trypanosomiasis is highly endemic; thus raising a need to develop organic solutions alongside those under current development.
Indeed, extracts prepared from 522 plants collected from various parts of the North America have been screened against blood stage trypamastigote forms of T. brucei and the result had shown that 150 extracts demonstrated>90% inhibition at 20 μg/mL concentration [11]. The only draw-back to this approach is the laborious approach required to elucidate the bioactive compounds in the extracts and their physiological targets. Yet, there is an important aspect of trypanosomal sterol metabolism which had been targeted by plant-derived columbin. It is worthy of note that sterols in trypanosomes are either de novo synthesized or taken up from the host lipoproteins and columbin effectively inhibit de novo sterol synthesis as demonstrated by Nok et al. Demonstrably, columbin has an EC50 value of 50 g/ml, dose-dependently reduced total trypanosomal cholesterol after a 3-day incubation period and cleared blood stage but not cerebrospinal form of trypamastigote forms of T. brucei in mice at 25 mg/kg during a 3-day oral treatment [12,13]. Development of columbin as a full treatment option for trypanosomiasis may require some modification of its chemical scaffold which can only be successfully done with the knowledge of its intracellular target along the mevalonate/isoprenoid/sterol biosynthesis pathway [14]. The computational docking result here demonstrated that out of 11 potential targets evaluated, columbin preferentially targets acetyl-coA synthetase and isopentyl diphosphate isomerase.
Materials and Methods
Homology modeling and docking studies
3D homology model of the proposed targets whose accession numbers are also displayed were generated using the templates whose PDB IDs shown on the same row using the Swiss Model server (Table 1). Prior to docking simulation, all proteins and ligands were reduced using Auto dock tools [15]. The docking simulation was performed using Autodock vina [16]. The coordinates of the co-crystalized ligand from each of the template was used as the binding site in each case. The binding energy of highest ranked pose was recorded. Columbin 2D structure was retrieved from the PubChem database (CID 188289).
| Proposed targets | Accession number (GI) | Template PDB ID |
|---|---|---|
| Acetyl-CoA synthetase | 62175586 | 2P2M |
| Acetyl-CoA acetyltransferase | AC091701.3 | 4UBV |
| HMG-CoA synthase | 1474765381 | 5HWQ |
| HMG-CoA reductase | 62358804 | 2R4F |
| Mevalonate kinase | 1474765200 | 2HFU |
| Phosphomevalonate kinase | CM000207.1 | 2R42 |
| Mevalonate-diphosphate decarboxylase | 1474761691 | 4DU8 |
| Isopentenyl-diphosphate isomerase | 56292021 | 3DH7 |
| Farnesyl-pyrophosphate synthase | 72391330 | 3EGT |
| Protein farnesyltransferase β-subunit | 62176812 | 4L9P |
| Squalene synthase | 1474765427 | 1W6J |
Table 1: Accession number of the query sequences of the proposed targets and the templates for homology modeling.
Results and Discussion
Columbin has multiple targets along the trypanosome mevalonate pathway
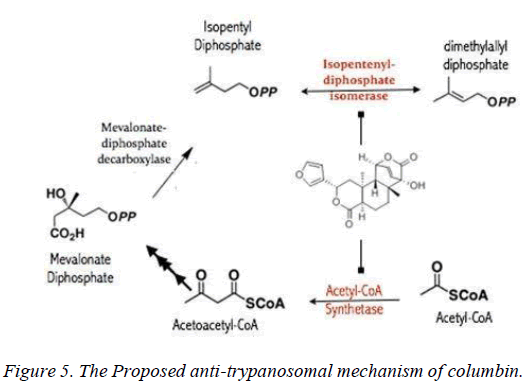
The mevalonate pathway is essential for the de novo synthesis of sterols, dolichols, ubiquinones, carotenoids and prenylated proteins [17], and has served as drug targets for clinically important diseases including cancer [18,19] and neurodegenerative diseases [20]. In trypanosomes just as humans, isoprenoid biosynthesis proceeds from acetyl-CoA substrate and essentially catalyzed by evolutionarily conserved enzymes up till diphosphomevalonate decarboxylase stage which produces isopentyl-diphosphate. Isopentyl-diphosphate isomerizes to dimethyl-allyl diphosphate catalyzed by non- mammalian-type T. brucei pyrophosphate isomerase (type II riboflavin-dependent). Out of 11 potential targets probed for columbin interaction, 18.1% (acetyl-CoA synthetase and isopentyl-diphosphate isomerase) exhibit binding affinity between -9.0 and -11.0 kcal/mol. 63.6% (HMG-CoA synthase, HMG-CoA reductase, Mevalonate kinase, phosphomevalonate kinase, Mevalonate-diphosphate decarboxylase, Farnesyl-pyrophosphate synthase and Protein farnesyltransferase β-subunit) exhibits binding affinity between -7.0 and -8.9 kcal/mol while the rest showed interaction only acetyl-CoA acetyltransferase and squalene synthase showed binding affinity less than -7.0 kcal/mol (Table 2).
| Docking Scores (Kcal/mol) | ||||
|---|---|---|---|---|
| Code | Target | Template ID and Ligand | Co-Cryst Ligand | Columbin |
| AcCS | Acetyl-CoA synthetase | 2P2M (PRX) | -9.3 | -9.3 |
| AAT | Acetyl-CoA acetyltransferase | 4UBV (COA) | -6 | -6.7 |
| HMS | HMG-CoA synthase | 5HWQ (CAA) | -7.4 | -7.1 |
| HMR | HMG-CoA reductase | 2R4F (RIE) | -8 | -8 |
| MK | Mevalonate kinase | 2HFU (MEV) | -5.2 | -8.8 |
| PMK | Phosphomevalonate kinase | 2R42 (FPS) | -5.5 | -8 |
| MDD | Mevalonate-diphosphate decarboxylase | 4DU8 (2PO) | -5.8 | -7.2 |
| IDI | Isopentenyl-diphosphate isomerase | 3DH7 (FMN) | -8.5 | -9.6 |
| FPS | Farnesyl-pyrophosphate synthase | 3EGT (722) | -8.3 | -8.3 |
| PFT | Protein farnesyltransferase β-subunit | 4L9P (FII) | -6.5 | -7.6 |
| SQS | Squalene synthase | 1W6J (R71) | -10.7 | -6 |
Table 2: Shows the binding energies of Columbin to its key proteins along the mevalonate pathway.
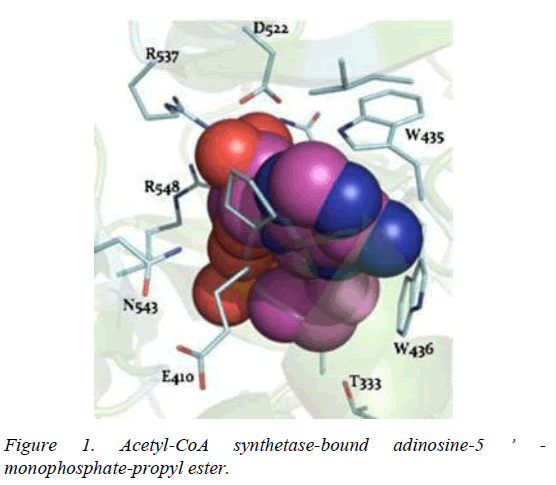
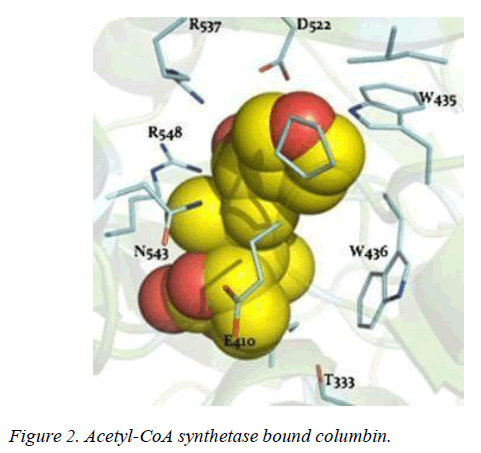
Whilst it is safe to comment that columbin may have multiple targets along this pathway, acetyl-CoA synthetase and isopentyl-diphosphate isomerase are the obvious favorite. Acetyl-CoA synthetase is no stranger as clinical drug target, as celecoxib derivative (AR-12) did show inhibition and currently under development as antifungal drug [21]. Toxicity likely associated with inhibited host acetyl-CoA synthetase may be overcome by a redundant ATP-citrate lyase, in mammalian cells which can perform the same function [22]. Isopentyl- diphosphate isomerase type-II found in trypanosomes is a non- mammalian type under investigation for drug development [23].
Binding poses of columbin bound acetyl-CoA synthetase and isopentyl-diphosphate isomerase
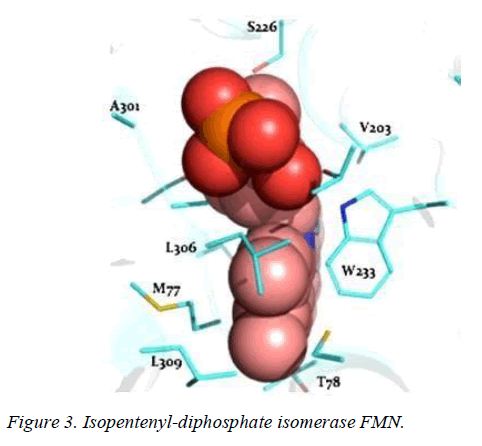
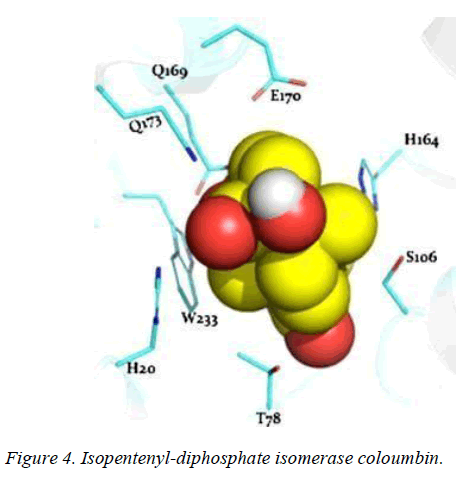
T. brucei acetyl-CoA synthetase is a 673 amino acid which has the active site (acetyl-CoA ligase activity), coenzyme-A (COA) binding site, adenosine monophosphate (AMP) binding site, acetate binding site and acyl-activating enzyme consensus motif. The active site and coenzyme-A binding site spans residues between 195 and 625. AMP and acetate binding sites are contributed by amino acid 330 to 575 and 350 to 425 respectively. Adenosine-5-monophosphate-propyl ester from the template (PDB ID 2P2M) [24] used as standard in this study preferentially interacts with active site residues (E410, W435, W436), and acyl-activating enzyme (AAE) consensus motif residues (R537, R548) which are similar amino acid preference of columbin (Figures 1-5). Isopentenyl-diphosphate isomerase, the second plausible target is a 356 amino acid homotetramer protein. It has a homotetramer interface contributed by amino acids 45-48, 51, 165, 202, 205, 206, 210, 259, 267, 289, 290, 325, and 328. It is a flavin mono nucleotide (FMN)-dependent enzyme. FMN binding site is contributed by amino acid 78, 86 and 87. There is also a homodimer contact (106, 134, 164, 201, 282, 305, and 306) and the active site contributed by amino acid 134, 136 and 164.
Conclusion
In conclusion, columbin, a plant-derived compound may represent a key frontier in the treatment of blood-stage trypanosomiasis. To be a viable future drug candidate, there is an urgent need to decipher its physiological target along the mevalonate pathway and to modify the original scaffold in order to improve its physicochemical properties and its ability to cross the blood-brain barrier.
In this study, it is shown that acetyl-CoA synthase, the first enzyme of the mevalonate pathway and the isopentyl diphosphate isomerase are the most likely targets of columbin (Figure 5). The poses have been well elucidated and may provide clue on how to modify the scaffold in order to improve its ability to cross the blood-brain-barrier.
References
- Simarro PP, Cecchi G, Paone M, Franco JR, Diarra A, Ruiz JA, Fèvre EM, Courtin F, Mattioli RC, Jannin JG. The Atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases. Int J Health Geogr 2010; 9: 57.
- Morrison LJ, Vezza L, Rowan T, Hope JC. Animal African Trypanosomiasis: Time to Increase Focus on Clinically Relevant Parasite and Host Species. Trends Parasitol 2016; 32: 599-607.
- Mwiinde AM, Simuunza M, Namangala B, Chama-Chiliba CM, Machila N, Anderson N, Shaw A. Estimating the economic and social consequences for patients diagnosed with human African trypanosomiasis in Muchinga, Lusaka and Eastern Provinces of Zambia. Infect Dis Poverty 2017; Oct 10; 6: 150
- Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet 2010; 375: 148-159.
- Barrett MP, Boykin DW, Brun R, Tidwell RR. Human African trypanosomiasis: Pharmacological re-engagement with a neglected disease. Br J Pharmacol 2007; 152: 1155-1171.
- Baker CH, Welburn SC. The Long Wait for a New Drug for Human African Trypanosomiasis. Trends Parasitol 2018; 34: 818-827.
- Torreele E, Bourdin Trunz B, Tweats D, Kaiser M, Brun R, Mazue G, Bray MA, Pecoul B. Fexinidazole-a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl Trop Dis 2010; 4: e923.
- Thuita JK, Wolf KK, Murilla GA, Bridges AS, Boykin DW, Mutuku JN, Liu Q, Jones SK, Gem CO, Ching S, Tidwell RR, Wang MZ, Paine MF, Brun R. Chemotherapy of second stage human African trypanosomiasis: Comparison between the parenteral diamidine DB829 and its oral prodrug DB868 in vervet monkeys. PLoS Negl Trop Dis 2015; 9: e0003409.
- Jacobs RT, Plattner JJ, Nare B, Wring SA, Chen D, Freund Y, Gaukel EG, Orr MD, Perales JB, Jenks M, Noe RA, Sligar JM, Zhang YK, Bacchi CJ, Yarlett N, Don R. Benzoxaboroles: A new class of potential drugs for human African trypanosomiasis. Future Med Chem 2011; 3: 1259-1278.
- Jacobs RT, Nare B, Wring SA, Orr MD, Chen D, Sligar JM, Jenks MX, Noe RA, Bowling TS, Mercer LT, Rewerts C, Gaukel E, Owens J, Parham R, Randolph R, Beaudet B, Bacchi CJ, Yarlett N, Plattner JJ, Freund Y, Ding C, Akama T, Zhang YK, Brun R, Kaiser M, Scandale I, Don R. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl Trop Dis 2011; 5: e1151.
- Jain S, Jacob M, Walker L, Tekwani B. Screening North American plant extracts in vitro against Trypanosoma Brucei for discovery of new antitrypanosomal drug leads. BMC Complement Altern Med 2016; 16: 131.
- Nok AJ, Sallau BA, Onyike E, Useh NM. Columbin inhibits cholesterol uptake in bloodstream forms of Trypanosoma Brucei-A possible trypanocidal mechanism. J Enzyme Inhib Med Chem 2005; 20: 365-368.
- Smith TK, Butikofer P. Lipid metabolism in Trypanosoma Brucei. Mol Biochem Parasitol 2010; 172: 66-79.
- Cosentino RO, Aguero F. Genetic profiling of the isoprenoid and sterol biosynthesis pathway genes of trypanosoma cruzi. PLoS One 2014; 9: e96762.
- El-Hachem N, Haibe-Kains B, Khalil A, Kobeissy FH, Nemer G. Auto dock tools for protein-ligand docking: Beta-site amyloid precursor protein cleaving enzyme 1(BACE1) as a case study. Methods Mol Biol 2017; 1598: 391-403.
- Trott O, Olson AJ. Autodock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010; 31: 455-461.
- Kuzuyama T. Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci Biotechnol Biochem 2002; 66: 1619-1627.
- Andela VB, Pirri M, Schwarz EM. The mevalonate synthesis pathway as a therapeutic target in cancer. Clin Orthop Relat Res 2003; 5: 59-66.
- Iannelli F, Lombardi R, Milone MR, Pucci B, De Rienzo S, Budillon A, Bruzzese F. Targeting mevalonate pathway in cancer treatment: Repurposing of statins. Recent Pat Anticancer Drug Discov 2018; 13: 184-200.
- Jiao X, Ashtari N, Rahimi-Balaei M, Chen QM, Badbezanchi I, Shojaei S, Marzban A, Mirzaei N, Chung S, Guan T, Li J, Vriend J, Mehr SE, Kong J, Marzban H. Mevalonate cascade and neurodevelopmental and neurodegenerative diseases: Future targets for therapeutic application. Curr Mol Pharmacol 2017; 10: 115-140.
- Koselny K, Green J, Favazzo L.. Antitumor/antifungal celecoxib derivative AR-12 is a non-nucleoside inhibitor of the ANL-family adenylating enzyme acetyl CoA synthetase. ACS Infect Dis 2016; 2: 268-280.
- Verschueren KHG, Blanchet C, Felix J, Dansercoer A, De Vos D, Bloch Y, Van Beeumen J, Svergun D, Gutsche I, Savvides SN, Verstraete K. tructure of ATP citrate lyase and the origin of citrate synthase in the krebs cycle. Nature 2019; 568: 571-575.
- de Ruyck J, Wouters J, Poulter CD. Inhibition studies on enzymes involved in isoprenoid biosynthesis: Focus on two potential drug targets: DXR and IDI-2 enzymes. Curr Enzym Inhib 2011; 7.
- Reger AS, Carney JM, Gulick AM. Biochemical and crystallographic analysis of substrate binding and conformational changes in acetyl-CoA synthetase. Biochemistry 2007; 46: 6536-6546.